Mutual Arrangements of Coronary Blood Vessels within the Right Atrial Appendage Vestibule
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Dissection and Measurements
- -
- The shortest distance from the margin of the right coronary artery to the endocardial surface of the right atrium;
- -
- The shortest distance from the margin of the right coronary artery to the margin of the RAA orifice;
- -
- The shortest distance from the margin of the right coronary artery to the margin of the RAV annulus;
- -
- The thickness of the whole right atrial at the level of the artery (spanning from the endocardium to the epicardium).
2.3. Statistical Analysis
3. Results
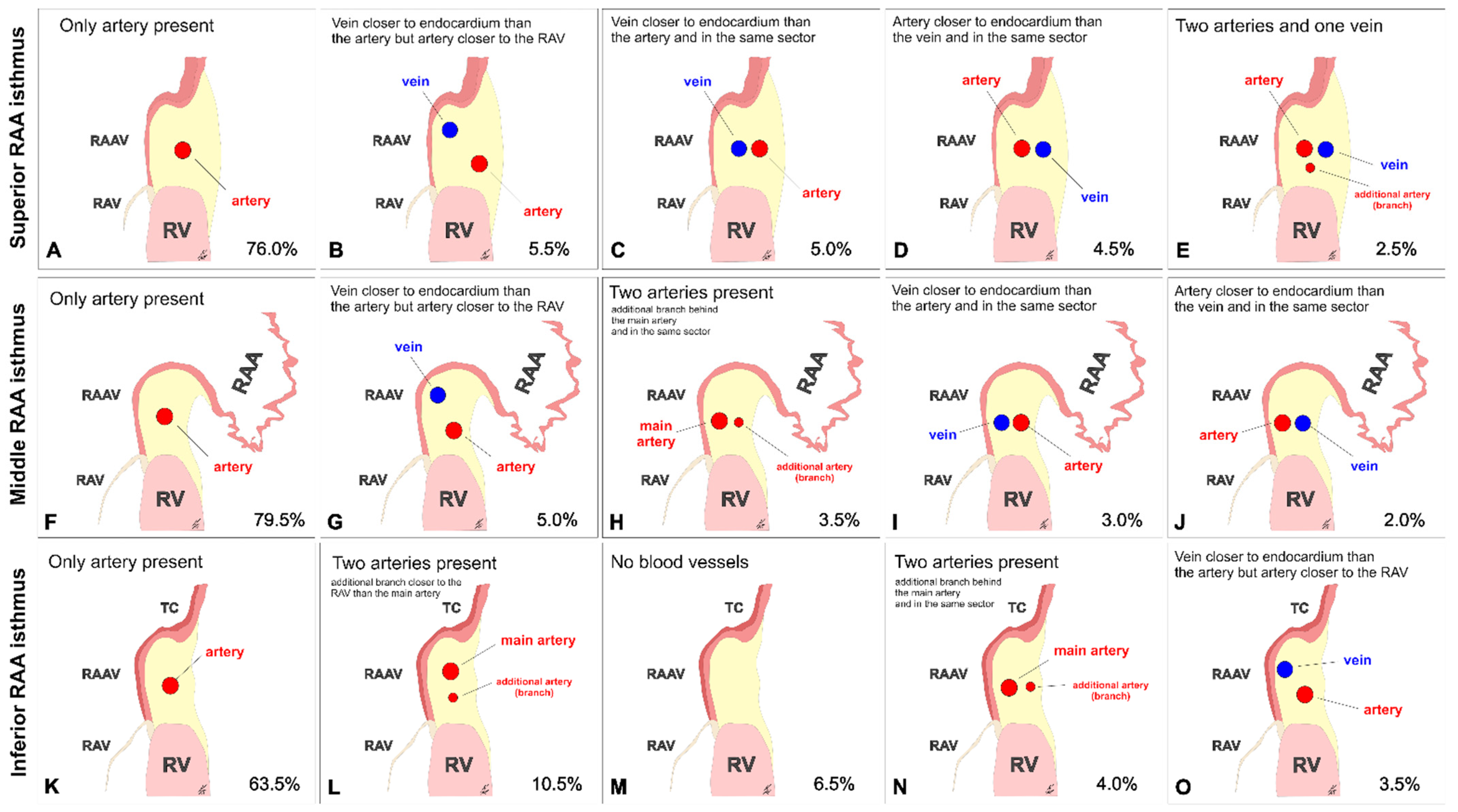
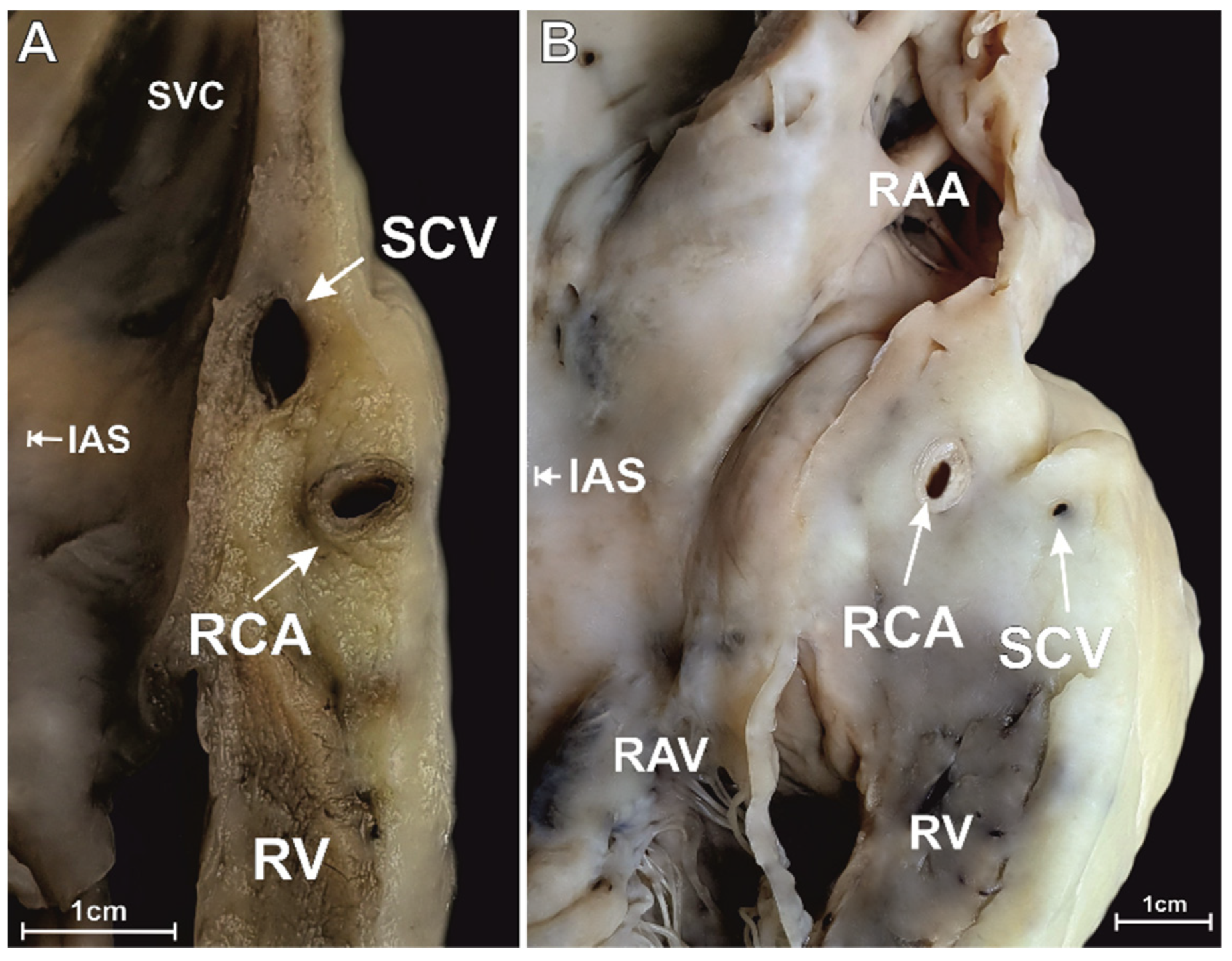
3.1. Superior RAA Isthmus
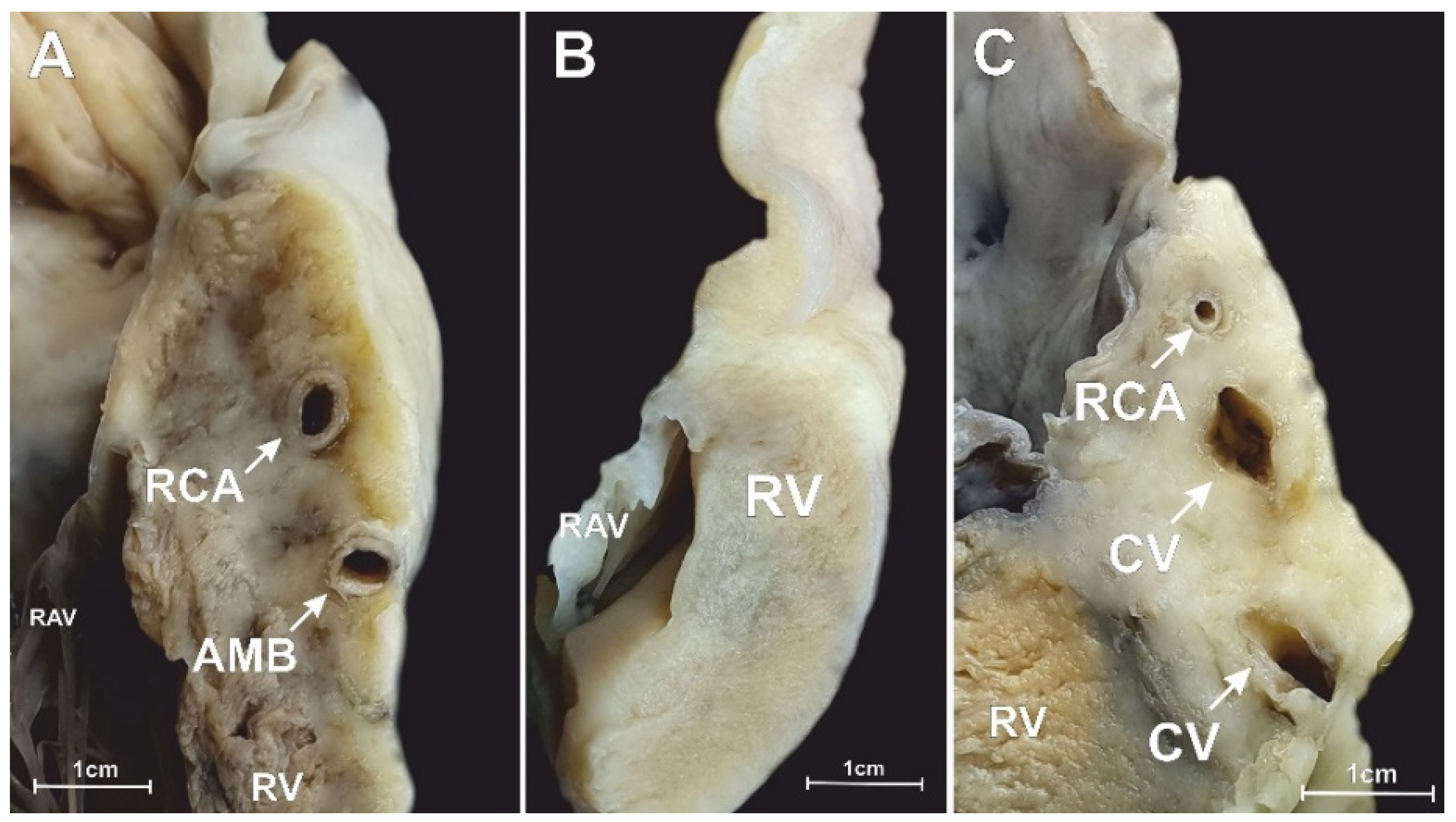
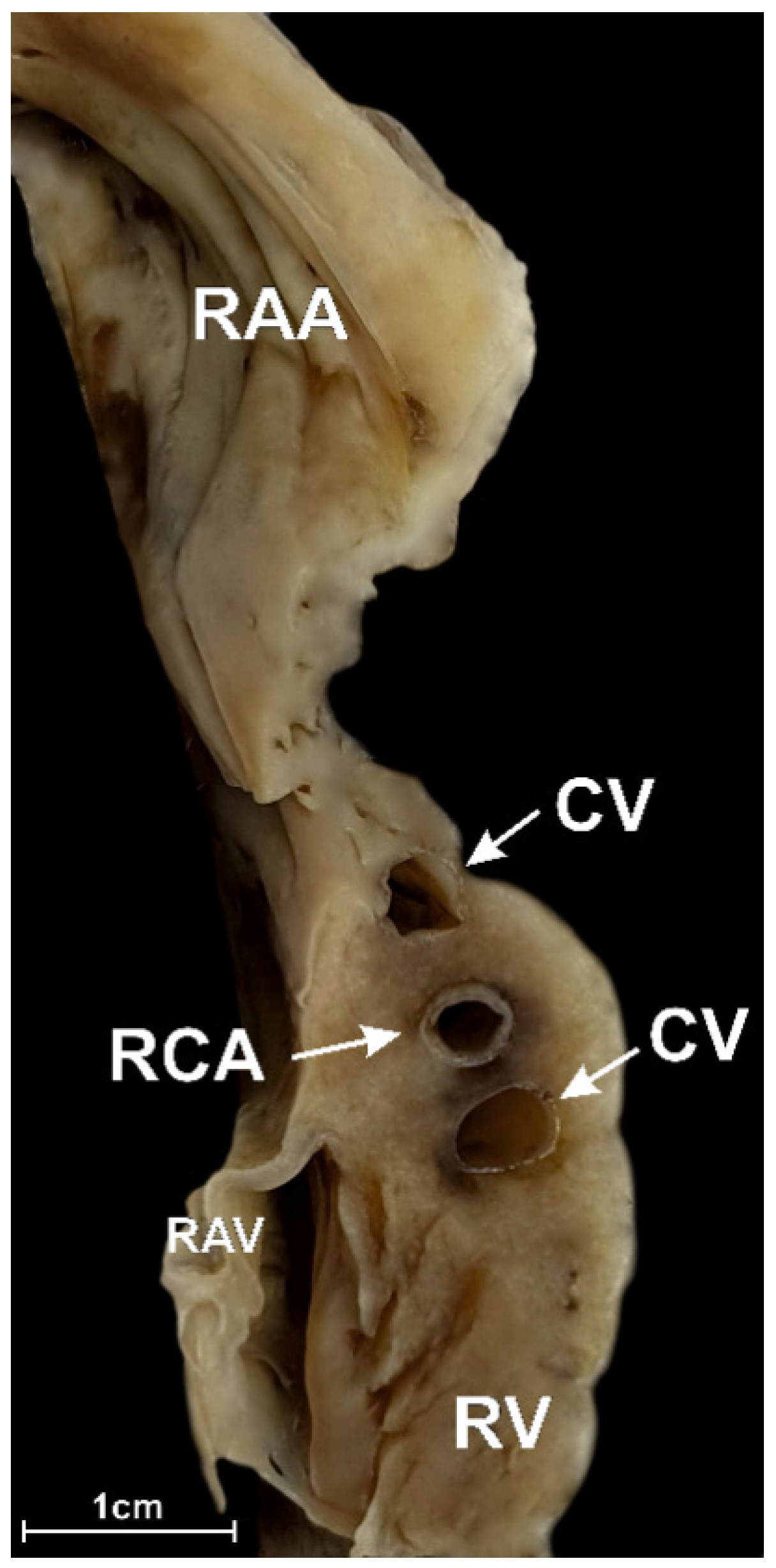
3.2. Middle RAA Isthmus
3.3. Inferior RAA Isthmus
3.4. The Morphometry of the Right Coronary Artery within the Isthmuses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ho, S.Y.; Anderson, R.H.; Sánchez-Quintana, D. Atrial structure and fibres: Morphologic bases of atrial conduction. Cardiovasc. Res. 2002, 54, 325–336. [Google Scholar] [CrossRef]
- Hołda, M.K.; Zhingre Sanchez, J.D.; Bateman, M.G.; Iaizzo, P.A. Right Atrioventricular Valve Leaflet Morphology Redefined. JACC Cardiovasc. Interv. 2019, 12, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Ueda, A.; McCarthy, K.P.; Sánchez-Quintana, D.; Yen Ho, S. Right atrial appendage and vestibule: Further anatomical insights with implications for invasive electrophysiology. Europace 2013, 15, 728–734. [Google Scholar] [CrossRef]
- Hołda, J.; Słodowska, K.; Tyrak, K.; Bolechała, F.; Jasińska, K.A.; Koziej, M.; Hołda, M.K.; Walocha, J.A. Topographical anatomy of the right atrial appendage vestibule and its isthmuses. J. Cardiovasc. Electrophysiol. 2020, 31, 3199–3206. [Google Scholar] [CrossRef]
- Overtchouk, P.; Piazza, N.; Granada, J.; Soliman, O.; Prendergast, B.; Modine, T. Advances in transcatheter mitral and tricuspid therapies. BMC Cardiovasc. Disord. 2020, 20, 1. [Google Scholar] [CrossRef] [Green Version]
- Bateman, M.G.; Quill, J.L.; Hill, A.J.; Iaizzo, P.A. The clinical anatomy and pathology of the human atrioventricular valves: Implications for repair or replacement. J. Cardiovasc. Transl. Res. 2013, 6, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Pothineni, N.V.; Kancharla, K.; Katoor, A.J.; Shanta, G.; Paydak, H.; Kapa, S.; Deshmukh, A. Coronary artery injury related to catheter ablation of cardiac arrhythmias: A systematic review. J. Cardiovasc. Electrophysiol. 2019, 30, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Swissa, M.; Brauner, R.; Shimoni, S.; Paz, O.; Belhassen, B. Late tamponade due to rupture of inferior vena cava-right atrial free wall following multiple radiofrequency ablations of atrial flutter. ISR Med. Assoc. J. 2013, 15, 57–59. [Google Scholar]
- Vloka, C.; Nelson, D.W.; Wetherbee, J. Atriocaval Rupture after Right Atrial Isthmus Ablation for Atrial Flutter. Am. J. Cardiol. 2016, 117, 1856–1857. [Google Scholar] [CrossRef] [PubMed]
- Al-Ammouri, I.; Perry, J.C. Proximity of Coronary Arteries to the Atrioventricular Valve Annulus in Young Patients and Implications for Ablation Procedures. Am. J. Cardiol. 2006, 97, 1752–1755. [Google Scholar] [CrossRef] [PubMed]
- McAlpine, W.A. Heart and Coronary Arteries An Anatomical Atlas for Clinical Diagnosis, Radiological Investigation, and Surgical Treatment; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1975; pp. 179–196. [Google Scholar]
- Díez-Villanueva, P.; Gutirrez-Ibanes, E.; Cuerpo-Caballero, G.P.; Sanz-Ruiz, R.; Abeytua, M.; Soriano, J.; Sarnago, F.; Elízaga, J.; González-Pinto, A.; Fernández-Avilés, F. Direct Injury to Right Coronary Artery in Patients Undergoing Tricuspid Annuloplasty. Ann. Thorac. Surg. 2014, 97, 1300–1305. [Google Scholar] [CrossRef]
- Morrissy, S.J.; Atkins, B.Z.; Rogers, J.H. Iatrogenic right coronary artery stenosis resulting from surgical tricuspid valve replacement: Case report and review of the literature. Catheter. Cardiovasc. Interv. 2014, 84, 1110–1114. [Google Scholar] [CrossRef]
- Varghese, R.; Akujuo, A.; Adams, D.H. Right Coronary Artery Injury After Tricuspid Valve Repair. Semin. Thorac. Cardiovasc. Surg. 2010, 22, 189–190. [Google Scholar] [CrossRef]
- Calafiore, A.M.; Iacò, A.L.; Bartoloni, G.; Di Mauro, M. Right coronary occlusion during tricuspid band annuloplasty. J. Thorac. Cardiovasc. Surg. 2009, 138, 1443–1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Rosendael, P.J.; Kamperidis, V.; Kong, W.K.F.; Van Rosendael, A.R.; Van Der Kley, F.; Marsan, N.A.; Delgado, V.; Bax, J.J. Computed tomography for planning transcatheter tricuspid valve therapy. Eur. Heart J. 2017, 38, 665–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wittkampf, F.H.M.; Van Oosterhout, M.F.; Loh, P.; Derksen, R.; Vonken, E.J.; Slootweg, P.J.; Ho, S.Y. Where to draw the mitral isthmus line in catheter ablation of atrial fibrillation: Histological analysis. Eur. Heart J. 2017, 38, 665–674. [Google Scholar] [CrossRef]
- Wong, K.C.; Jones, M.; Sadarmin, P.P.; De Bono, J.; Qureshi, N.; Rajappan, K.; Bashir, Y.; Betts, T.R. Larger coronary sinus diameter predicts the need for epicardial delivery during mitral isthmus ablation. Europace 2011, 13, 555–561. [Google Scholar] [CrossRef]
- Hołda, M.K.; Koziej, M.; Hołda, J.; Tyrak, K.; Pitek, K.; Krawczyk-Ozóg, A.; Klimek-Piotrowska, W. Spatial relationship of blood vessels within the mitral isthmus line. Europace 2018, 20, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Aryana, A.; Tung, R.; d’Avila, A. Percutaneous Epicardial Approach to Catheter Ablation of Cardiac Arrhythmias. JACC Clin. Electrophysiol. 2020, 6, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Pan, Y.; Wang, H.; Li, J.; Nie, P.; Wang, X.; Ma, H.; Huo, F. Assessment of the coronary venous system using 256-slice computed tomography. PLoS ONE 2014, 9, e104246. [Google Scholar] [CrossRef]
- Igawa, O. Focus on the Atrial Structure—Useful Anatomical Information for Catheter Ablation. J. Arrhythmia 2011, 27, 268–288. [Google Scholar] [CrossRef]
- Hołda, M.K.; Klimek-Piotrowska, W.; Koziej, M.; Piątek, K.; Hołda, J. Influence of different fixation protocols on the preservation and dimensions of cardiac tissue. J. Anat. 2016, 229, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Hołda, M.K.; Hołda, J.; Koziej, M.; Tyrak, K.; Klimek-Piotrowska, W. The influence of fixation on the cardiac tissue in a 1-year observation of swine hearts. Anat. Histol. Embryol. 2018, 47, 501–509. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Superior RAA Isthmus | Middle RAA Isthmus | Inferior RAA Isthmus | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Range (Min–Max) | Median (Q1; Q3) | Mean ± SD | Range (Min–Max) | Median (Q1; Q3) | Mean ± SD | Range (Min–Max) | Median (Q1; Q3) | |
| RCA diameter (mm) | 2.6 ± 0.8 | 0.9–4.8 | 2.0; 3.1 | 2.9 ± 1.1 | 0.7–6.7 | 2.4; 3.8 | 2.7 ± 0.9 | 0.6–6.0 | 2.1; 3.3 |
| RCA to RAVA distance (mm) | 3.9 ± 2.9 | (−0.5)–29.7 | 2.1; 5.0 | 6.2 ± 3.4 | (−6.0)–16.8 | 4.1; 8.4 | 5.0 ± 3.2 | (−3.5)–17.6 | 3.0; 6.7 |
| RCA to RAA orifice distance (mm) | 10.3 ± 3.7 | 0.5–23.3 | 7.7; 12.4 | 5.5 ± 3.8 | 0.0–23.5 | 2.2; 8.0 | 5.7 ± 3.2 | 0.1–14.8 | 3.2; 8.1 |
| RCA to endocardial surface distance (mm) | 9.0 ± 4.0 | 0.5–21.9 | 6.2; 11.3 | 6.2 ± 3.0 | 1.2–18.1 | 4.2; 7.7 | 4.8 ± 2.3 | 0.6–11.9 | 3.1; 6.1 |
| RCA to epicardial surface distance (mm) | 6.4 ± 4.4 | 0.5–27.8 | 3.4; 8.6 | 4.3 ± 3.1 | 0.4–16.7 | 1.7; 6.4 | 2.2 ± 1.6 | 0.2–7.9 | 0.9; 2.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hołda, J.; Słodowska, K.; Strona, M.; Malinowska, K.; Bolechała, F.; Jasińska, K.A.; Koziej, M.; Piątek-Koziej, K.; Walocha, J.A.; Hołda, M.K. Mutual Arrangements of Coronary Blood Vessels within the Right Atrial Appendage Vestibule. J. Clin. Med. 2021, 10, 3588. https://doi.org/10.3390/jcm10163588
Hołda J, Słodowska K, Strona M, Malinowska K, Bolechała F, Jasińska KA, Koziej M, Piątek-Koziej K, Walocha JA, Hołda MK. Mutual Arrangements of Coronary Blood Vessels within the Right Atrial Appendage Vestibule. Journal of Clinical Medicine. 2021; 10(16):3588. https://doi.org/10.3390/jcm10163588
Chicago/Turabian StyleHołda, Jakub, Katarzyna Słodowska, Marcin Strona, Karolina Malinowska, Filip Bolechała, Katarzyna A. Jasińska, Mateusz Koziej, Katarzyna Piątek-Koziej, Jerzy A. Walocha, and Mateusz K. Hołda. 2021. "Mutual Arrangements of Coronary Blood Vessels within the Right Atrial Appendage Vestibule" Journal of Clinical Medicine 10, no. 16: 3588. https://doi.org/10.3390/jcm10163588
APA StyleHołda, J., Słodowska, K., Strona, M., Malinowska, K., Bolechała, F., Jasińska, K. A., Koziej, M., Piątek-Koziej, K., Walocha, J. A., & Hołda, M. K. (2021). Mutual Arrangements of Coronary Blood Vessels within the Right Atrial Appendage Vestibule. Journal of Clinical Medicine, 10(16), 3588. https://doi.org/10.3390/jcm10163588





