Abstract
Despite the increasing use of high-flow nasal cannulas (HFNCs) to treat critically ill patients, data on their effectiveness for sepsis patients remains very limited. We studied a prospective cohort of sepsis patients from the Korean Sepsis Registry (18 intensive care units (ICUs)). Patients started on HFNC therapy for hypoxemia within the first three ICU days were enrolled. HFNC failure was defined as intubation or ICU death, and the primary outcome was early HFNC failure occurring within 72 h of HFNC initiation. Of 901 patients with sepsis admitted to the ICU, 206 who received HFNC therapy were finally included (117 with pneumonia vs. 89 with non-pneumonia sepsis; median age, 71.0 (63.0–78.0) years; PaO2/FiO2 ratio, 160.2 (107.9–228.2) mm Hg; septic shock, n = 81 (39.3%)). During HFNC therapy, 72 (35.0%) patients were intubated and 51 (24.8%) died. HFNC failure developed in 95 (46.1%) patients, and among them, early failure rate was 85.3% (81/95). On multivariate analysis, an immunocompromised state (odds ratio (OR) = 2.730), use of a combination of antibiotics (OR = 0.219), and the PaO2/FiO2 ratio (OR = 0.308) were significantly associated with early HFNC failure in pneumonia sepsis patients. However, in non-pneumonia sepsis patients, lactate levels (OR = 1.532) were significantly associated with early HFNC failure. In conclusion, a high proportion of sepsis patients experience HFNC failure, usually within 72 h after therapy initiation, which emphasizes the importance of close monitoring. Furthermore, unlike in pneumonia sepsis, organ failure (i.e., lactate) might serve as a prognostic marker in non-pneumonia sepsis (i.e., type IV respiratory failure).
1. Introduction
Sepsis is a life-threatening infectious condition and imposes a substantial global health burden [1,2,3]. With regard to the initial treatment, in addition to hemodynamic resuscitation and early antibiotics, oxygen therapy is important for sepsis patients exhibiting hypoxemia. Recently, as a noninvasive strategy for acute hypoxemic respiratory failure (AHRF), use of high-flow nasal cannulas (HFNCs) has become popular, as their use can decrease the need for intubation [4,5]. It may also reduce the work required to breathe and improve cardiovascular dynamics in critically ill patients [6,7,8].
A recent multicenter randomized study showed that HFNC was associated with lower mortality and a lower risk of intubation compared to noninvasive ventilation or standard oxygen therapy in AHRF patients, including those who were immunocompromised [4,5]. The respiratory rate–oxygenation (ROX) index was also developed based on a large cohort study to predict the success of HFNC therapy in patients with pneumonia [9]. However, most previous studies examined its role in primary respiratory failure (i.e., type I respiratory failure) and not in sepsis or septic shock, where there are issues of oxygen delivery and oxygen utilization at tissue level, as well as lung injury, secondary to the uncontrolled inflammatory process (i.e., type IV respiratory failure). Hence, we hypothesized that HFNC outcomes and their risk factors would be different in sepsis patients, compared to those with primary respiratory failure.
In the present study, we investigated the rates of HFNC failure and analyzed the risk factors associated with HFNC failure in a prospective cohort of pneumonia and non-pneumonia sepsis patients.
2. Methods
2.1. Study Population
This prospective cohort study analyzed data from the Korean sepsis registry. Eighteen ICUs of 17 tertiary or university-affiliated hospitals that run educational programs on sepsis bundles participated in the study. We analyzed data obtained over the 6-month period from September 2019 to February 2020. To verify data quality, regular audits were conducted by research committee members. All consecutive patients admitted to ICUs with diagnoses of sepsis or septic shock were screened for eligibility. All patients were followed-up until the date of death or hospital discharge. The inclusion criterion was HFNC therapy to treat hypoxemia during the first 3 ICU days. For all patients, the fraction of inspired oxygen (FiO2) and flow rate (L/min) were adjusted at the discretion of the physician to maintain an SpO2 > 92%. The exclusion criteria were non-admission to an ICU, initiation of HFNC therapy 72 h after ICU admission, mechanical ventilation (MV) before HFNC therapy (e.g., post-extubation HFNC), incomplete PaO2/FiO2 ratio data on the day of HFNC initiation, and no data on hospital outcomes. The study was approved by the institutional review boards of all participating hospitals, including the Hallym University Institutional Review Board (approval no. 2018-09-004). Given the observational nature of the study, the requirement for written informed consent from patients or their legal surrogates was at the discretion of the ethics committees of the participating hospitals. We followed the STROBE guidelines for reporting of observational cohort studies [10].
2.2. Data Collection
The study coordinators at each participating hospital prospectively collected data using an electronic case report form (http://sepsis.crf.kr/; accessed on 1 January 2021). The following information was recorded: demographic data (including age, sex, and comorbidities); physiological and laboratory parameters and the Simplified Acute Physiology Score 3 (SAPS3) at ICU admission [11]; the PaO2/FiO2 ratio, lactate levels, and SOFA scores [12] on the day of HFNC initiation (i.e., the pre-HFNC values); infection source and type (i.e., community- or hospital-acquired); multidrug-resistant (MDR) pathogen status in patients with positive culture results; the adequacy of empirical antibiotic therapy; the rate of compliance with the 3-h Surviving Sepsis Campaign bundle [13]; treatments during the first 3 ICU days (transfusion, steroid therapy, noninvasive ventilation, and continuous renal replacement therapy (CRRT)); the net fluid balance during the pre-ICU period and on ICU day 1; and outcome data (including intubation (i.e., MV treatment) and ICU and in-hospital mortality). All information was anonymized.
Community-acquired infection was defined as an infection that occurred in a community setting; hospital-acquired infection was defined as an infection that developed no earlier than 48 h after hospitalization. The culprit pathogen was defined as any agent cultured from samples collected within 48 h or at the time of sepsis diagnosis. The adequacy of empirical therapy was determined based on drug susceptibility testing or the recommendations of relevant guidelines [14,15]. MDR was defined as a microorganism resistant to agents from at least three antimicrobial categories [16].
2.3. Diagnosis of Sepsis and Septic Shock
The Sepsis-3 criteria were used to diagnose sepsis and septic shock [17]. First, we screened patients with suspected infections using the quick SOFA score. If the score was ≥2, organ dysfunction was assessed using the full SOFA score. The criteria for diagnosing sepsis included a probable or confirmed infection, and a change in the total SOFA score of ≥2 after infection. Septic shock was defined as persistent arterial hypotension requiring a vasopressor to maintain a mean arterial pressure of ≥65 mmHg, and a serum lactate level >2 mmol/L, despite adequate volume resuscitation.
Time zero was defined as the time of triage in the emergency department (ED) for patients who presented to an ED, or the time of sepsis diagnosis by a physician or nurse for those in general wards. The 3-h sepsis bundle completion rates (i.e., compliance) were measured based on time zero (Supplemental Materials) [13,18]. Any bolus infusion of crystalloid fluid was considered to indicate compliance with the fluid bundle component.
2.4. Data Analyses
HFNC failure was defined as intubation or death in the ICU (whichever occurred first; the composite outcome). The primary endpoint was the rate of early HFNC failure (within 72 h of initiation of HFNC therapy). We also aimed to identify factors significantly associated with early HFNC failure; this analysis was performed separately for pneumonia and non-pneumonia sepsis patients.
Categorical variables are presented as numbers (%), and continuous variables as medians (interquartile ranges, IQRs). To compare continuous variables, the Mann–Whitney U test was used, and for categorical variables, the chi-square or Fisher’s exact test was used. For multivariable analyses, logistic regression analyses were performed using covariates with a p value of <0.05 on univariable analyses; a backward stepwise selection method based on the likelihood ratio was employed, and variables with overlapping meaning were removed in the model.
A meta-analysis showed that 24.3% of patients with acute hypoxemic respiratory failure experienced HFNC failure (i.e., intubation) [19], and based on our previous retrospective sepsis study [20], the rate of ICU death was expected to be 33.0%. Hence, with a power of 80% and a type I error rate of 5% (two-sided), the calculated sample size was 202 patients in the present study. IBM SPSS for Windows software (ver. 25.0; IBM Corp., Armonk, NY, USA) was used for all statistical analyses. A p value <0.05 was considered significant.
3. Results
3.1. Study Population
Of 2126 patients who met the Sepsis-3 criteria during the 6-month study period, 901 were admitted to ICUs and screened for study eligibility. After excluding patients who met the exclusion criteria, 206 patients (117 with pneumonia sepsis and 89 with non-pneumonia sepsis) were finally enrolled (Figure 1). The median age was 71.0 years (interquartile range: 63.0–78.0 years), and 64 (31.1%) were women (Table 1). The median PaO2/FiO2 at HFNC initiation was 160.2 (107.9–228.2) mm Hg; 81 patients (39.3%) were in septic shock. Diabetes (35.4%) and an immunocompromised state (i.e., a hematological malignancy, solid cancer, or drug-induced immunosuppression; 34.5%) were the most common underlying comorbidities. The prevalence rates of bacteremia and MDR pathogens were 24.8% and 19.4%, respectively; hospital-acquired infections were present in 36.9% of patients. As shown in Table 1, initial disease severity was higher in patients with non-pneumonia sepsis than in those with pneumonia sepsis. The lactate levels and illness severity (SOFA scores and SAPS3), and the rates of septic shock and bacteremia, were higher in the non-pneumonia sepsis patients.
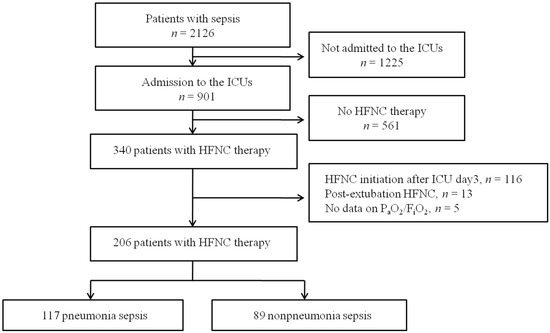
Figure 1.
Flow chart of patient enrolment. HFNC: high-flow nasal cannula; ICU: intensive care unit.

Table 1.
Baseline characteristics and treatments among enrolled patients (n = 206).
3.2. Treatments and Outcomes
The completion rate of the 3-h sepsis bundle was 25.7%, and adequate antibiotics were administered to 88.8% of patients (Table 1). Steroid therapy and CRRT were required by 30.6% and 18.4% of patients, respectively, during the first 3 ICU days. The intubation, ICU, and hospital mortality rates were 35.0%, 24.8%, and 34.0%, respectively (Table 2). In terms of the composite outcome, HFNC failure occurred in 95 (46.1%) patients during the ICU stay. Early HFNC failure (i.e., <72 h after HFNC initiation) occurred in 81 (39.3%) patients, accounting for 85.3% of all HFNC failures (Figure 2). The incidence of early HFNC failure did not differ between the pneumonia and non-pneumonia sepsis patients. Comparisons of baselines characteristics and treatments between patients with HFNC failure and those without are presented in Supplementary Tables (Tables S1 and S2 for pneumonia sepsis; Tables S3 and S4 for non-pneumonia sepsis patients).

Table 2.
Treatment outcomes among enrolled patients (n = 206).
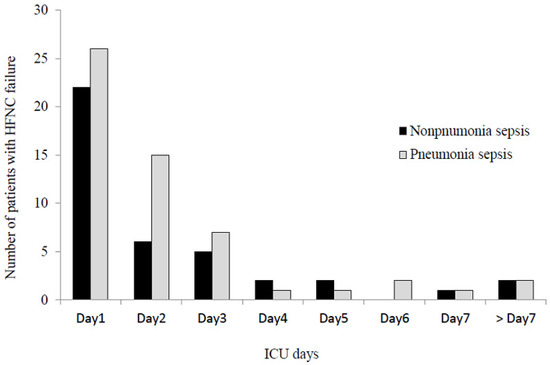
Figure 2.
Daily number of patients experiencing high-flow nasal cannula failure. HFNC: high-flow nasal cannula; ICU: intensive care unit.
3.3. Risk Factors for Early HFNC Failure
In pneumonia sepsis patients, univariable analyses revealed six variables (immunocompromised state, combination of antibiotics, CRRT, net fluid balance on day1, SAPS3, and PaO2/FiO2 ratio) associated with the early HFNC failure (p < 0.05). Of these, five variables were included in multivariable analysis (Table 3), and an immunocompromised state (odds ratio = (OR) 2.730; 95% confidence interval (CI): 1.082–6.889), use of an antibiotic combination (0.219; 95% CI: 0.079–0.605), and the PaO2/FiO2 ratio (0.308; 95% CI: 0.158–0.601) were significantly associated with the risk of early HFNC failure (Figure 3A). However, in non-pneumonia sepsis patients, nine variables (septic shock, steroid therapy, CRRT, transfusion, SAPS3, lactate, pH, PaO2/FiO2, and SOFA score) were associated with the early HFNC failure by univariable analyses (p < 0.05). Among them, six variables were initially included in multivariable analysis, and four variables were finally selected (Table 4). In the model, lactate levels (1.532; 95% CI: 1.218–1.926) were significantly associated with the risk of early HFNC failure (Figure 3B). Additionally, multivariable analysis for all enrolled patients (n = 206) is presented in Supplementary Table S5.

Table 3.
Risk factors for HFNC failure in patients with pneumonia sepsis (n = 117) a.
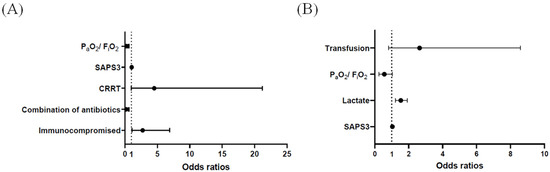
Figure 3.
Odds ratios and 95% confidence intervals for early high-flow nasal cannula failure by multivariable analysis in (A) pneumonia sepsis and (B) non-pneumonia sepsis patients. CRRT: continuous renal replacement therapy; SAPS3: simplified acute physiology score3.

Table 4.
Risk factors for HFNC failure in patients with non-pneumonia sepsis (n = 89) a.
3.4. Rates of Early HFNC Failure According to PaO2/FiO2 Ratios and Lactate Levels
Figure 4A illustrates the rates of early HFNC failure according to PaO2/FiO2 ratios (≤100 vs. >100 to ≤200 vs. >200 mm Hg). The rate of early HFNC failure increased as the PaO2/FiO2 ratio decreased in both pneumonia and non-pneumonia sepsis patients. However, when lactate levels were examined (≤2 vs. >2 to ≤4 vs. >4 mmol/L; Figure 4B), the rate of early HFNC failure did not differ in patients with pneumonia sepsis by lactate level, but was significantly higher in patients with non-pneumonia sepsis having higher lactate levels.
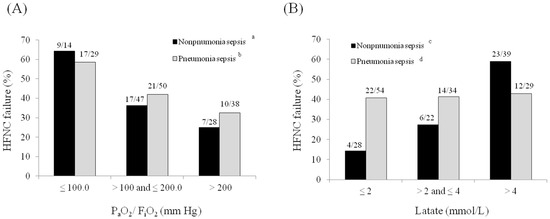
Figure 4.
Rates of early high-flow nasal cannula failure according to (A) the PaO2/FiO2 ratio and (B) lactate level. a p = 0.045, b p = 0.028, c p = 0.001, and d p = 0.988.
4. Discussion
This observational cohort study yielded several interesting findings. First, HFNC therapy failed in 46% of sepsis patients admitted to the ICU, and most HFNC failures occurred early (i.e., within 72 h), thus highlighting the importance of close monitoring in sepsis patients who are connected to a HFNC device (a device without alarms). Second, the predictors of HFNC failure differed between the pneumonia and non-pneumonia sepsis patients. The lactate level was a significant predictor only in the latter group.
HFNC therapy is superior to standard low-flow oxygen therapy for improving respiratory parameters (e.g., dyspnea, the respiratory rate, and oxygenation) [6,7,8], and was shown by electric impedance tomography to improve regional and global lung ventilation [21,22]. In a multicenter randomized trial, HFNC therapy was associated with lower 90-day mortality and a lower risk of intubation than non-invasive ventilation or standard oxygenation therapy in patients with PaO2/FiO2 ratios < 200 mm Hg [5]. Post-hoc analysis also showed that HFNC therapy seemed to be better than non-invasive ventilation or standard oxygenation for immunocompromised patients [4]. However, although beneficial effects have been reported for patients with various medical conditions [23,24,25,26,27], HFNC therapy for sepsis patients at risk of hemodynamic instability has not been sufficiently evaluated. Hence, our study is clinically relevant.
Retrospective studies found that oxygenation [8,28], respiratory rate [8], SOFA score [29,30], SAPS II score [31], and vasopressor use [28,32] were associated with HFNC outcomes. Recently, age, the Glasgow Coma Scale, vasopressor use, and the number of comorbidities were found to significantly predict non-invasive respiratory therapy (non-invasive ventilation and HFNC) failure in patients with coronavirus disease 2019 (COVID-19) [33]. These studies indicate the presence of several factors, other than respiratory variables, affecting HFNC outcomes, which support our findings. We also noted that sicker patients (e.g., higher lactate levels, higher SAPS3 and/or SOFA scores, and lower PaO2/FiO2 ratios) tended to fail more the HFNC therapy. However, the unique feature of our study is that we analyzed patients with sepsis of non-pulmonary origin. Although the underlying cause of hypoxemia was unknown, these patients all suffered type IV respiratory failure, which is rarely a target for clinical studies of HFNC therapy.
We found that 46% of enrolled patients experienced HFNC failure in the ICUs; 85.3% of the failures occurred within 72 h of therapy initiation. This early failure rate seems to be higher than that of the retrospective study by Kang et al. (74.3%) [34]. Although the HFNC failure rate included both intubation and death in our study, the differences may in part be attributable to differences in patient characteristics and disease severity. In the study by Kang et al., a considerable proportion of patients had acute-on-chronic respiratory failure or post-extubation failure as the cause of HFNC therapy; only 8.3% were in septic shock. However, we could only measure HFNC therapy duration on a daily (not hourly) basis; this was a limitation of our study. Nonetheless, given the concerns about delayed intubation [34], our results emphasize that sepsis patients on HFNC should be carefully monitored for HFNC failure during the early period.
The sepsis registry cohort was not designed to investigate the effects of HFNC therapy. Thus, detailed data on HFNC settings (e.g., FiO2 and flow rates) were lacking. Additionally, the criteria for HFNC therapy and intubation were not standardized, so all decisions were at the discretion of each participating hospital. However, we included only severely ill ICU patients for whom pre-HFNC PaO2/FiO2 ratios were available. Although we did not impose an upper limit, only 17 (8.3%) patients had a value of 300–400 mm Hg; the rest had values ≤300 mm Hg, thus meeting the oxygenation criterion of ARDS. However, the main purpose of our study was to investigate the clinical outcomes of HFNC therapy and the risk factors in sepsis patients prone to hemodynamic instability. Although the hospital mortality rate (34.0%) was higher in our study than that from previous sepsis studies (14.5~25.6%) [35,36,37,38], this might be attributable to high proportions of immunocompromised or elderly patients and the inclusion of hospital-acquired infections.
Interestingly, the risk factors for early HFNC failure differed somewhat between the pneumonia and non-pneumonia sepsis groups. In the pneumonia sepsis group, as well as the PaO2/FiO2 ratio, an underlying comorbidity (an immunocompromised state) and use of antibiotic combinations were significant risk factors. Conversely, in the non-pneumonia sepsis group, the lactate level, which reflects sepsis severity, was a significant risk factor for early HFNC failure. Although the PaO2/FiO2 ratio was not significant in the multivariate analysis of non-pneumonia sepsis patients, the HFNC failure rate increased with a decrease in the PaO2/FiO2 ratio (Figure 3A). Therefore, further large-scale studies are needed to confirm this. However, one possible explanation for the difference between pneumonia and non-pneumonia sepsis patients may stem from pneumonia being a primary lung disorder; thus the severity of the disease, given the lower SAPS3/SOFA scores, may be determined by the hypoxemia levels in pneumonia sepsis, whereas in non-pneumonia sepsis, organ failure may be the major determinant of HFNC outcome. Another plausible explanation for the lack of correlation between lactate levels and HFNC failure in pneumonia sepsis is that lactate in this population might be secondary for the work of breathing and less for general tissue oxygenation. Therefore, HFNC, which might lessen the work of breathing, may make the lactate less relevant as a prognostic parameter for this device failure.
In patients who undergo noninvasive therapy for AHRF, the respiratory rate and oxygenation level are important predictors of respiratory failure and the need for intubation [9,27,33,39]. Tracking dynamic changes in these parameters during HFNC therapy is important [9,39]. However, unfortunately, we lacked follow-up data on vital signs and arterial blood gas levels. Additionally, we did not calculate the ROX index [9]. However, our target population was sepsis patients admitted to the ICU with a low PaO2/FiO2 ratio. Our focus was on HFNC outcomes and associated risk factors, and not only on respiratory parameters.
Our study had several limitations. First, it used an observational design and included a small number of patients, so it was underpowered; there may have been unidentified sources of bias. Second, smoking status and hypertension, which are the variables frequently investigated in many studies, were not collected in our study. They may have had an effect on some patients. Third, as mentioned above, the criteria for HFNC and intubation were not standardized. Hence, it is possible that the treatments differed among the patients, which may have affected the HFNC outcomes. Fourth, we lacked data on HFNC settings (i.e., FiO2 and flow rate) and other respiratory parameters (e.g., dyspnea and thoracoabdominal asynchrony); there is a possibility of inadequate settings for some patients. Fifth, all of the patients were from the same country, which limits the generalizability of the results. However, to date, data on the outcomes of HFNC for sepsis patients are very limited. These patients exhibit unique clinical characteristics, and deterioration can be rapid or unpredictable compared to patients with AHRF alone. Thus, our study is clinically relevant, and may aid the design of large-scale trials.
5. Conclusions
In our study, a high proportion of sepsis patients who underwent HFNC therapy in ICUs experienced HFNC failure, usually within 72 h after therapy initiation, thus emphasizing the importance of close monitoring in these patients. Furthermore, in non-pneumonia sepsis, organ failure (i.e., lactate) might serve as a prognostic marker. This is in contrast with primary pneumonia sepsis, where the degree of hypoxemia and immunocompromised state might be the determinant of HFNC failure. Future large-scale studies are warranted.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jcm10163587/s1, Failure of high flow nasal cannula therapy failure in pneumonia, and Definitions for “Time Zero” and Compliance with Sepsis Bundles, Table S1. Baseline characteristics and treatments among patients with pneumonia sepsis (n = 117), Table S2. Severity of illness and laboratory parameters among patients with pneumonia sepsis (n = 117), Table S3. Baseline characteristics and treatments among patients with non-pneumonia sepsis (n = 89), Table S4. Severity of illness and laboratory parameters among non-pneumonia sepsis (n = 89), Table S5. Risk factors for HFNC failure among all enrolled patients (n = 206).
Author Contributions
Conceptualization, E.K., S.P., D.K.O., K.J., G.Y.S., C.-M.L.; Software, E.K., S.P., D.K.O., Y.-J.C.; Validation, All authors; Formal Analysis, E.K., S.P., D.K.O., K.J.; Investigation, All authors; Resources, All authors; Data Curation, E.K., S.P., D.K.O., K.J., Y.-J.C., G.Y.S., C.-M.L., S.-B.H., Y.J.L., S.-M.L., M.-H.P.; Writing—Original Draft Preparation, E.K., S.P., D.K.O., K.J.; Writing—Review and Editing, All authors; Visualization, All authors; Supervision, S.-B.H., Y.J.L., S.-M.L., Y.-J.C., G.Y.S.; Project Administration, S.P., S.-B.H., Y.J.L., S.-M.L., M.-H.P., C.-M.L.; Funding Acquisition, C.-M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the 2019 Research Grant (2019E280500) from the Korean Disease Control and Prevention Agency.
Institutional Review Board Statement
This study was approved by the institutional review boards of all participating hospitals, including the Hallym University Institutional Review Board (approval no. 2018-09-004).
Informed Consent Statement
Due to the observational nature of this study, the decision to require (or grant exemption from) written informed consent from patients or their legal surrogates was at the discretion of the ethics committees of the participating hospitals.
Data Availability Statement
Sunghoon Park had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Data can be obtained from the corresponding author: Sunghoon Park (f2000tj@gmail.com).
Acknowledgments
We would like to thank Seong-Sik Cho (Dong-A University Hospital) for his dedication to the statistical analyses in the study. We also would like to thank the Korean Sepsis Alliance (KSA) investigators who participated in this study: Suk-Kyung Hong (Asan Medical Center, University of Ulsan College of Medicine), Woo Hyun Cho (Pusan National University Yangsan Hospital), Jae Young Moon (Chungnam National University Sejong Hospital), Tai Sun Park (Hanyang University Guri Hospital, Guri), Gil Myeong Seong (Jeju National University Hospital), Jeongwon Heo (Kangwon National University Hospital), Youjin Chang (Inje University Sanggye Paik Hospital), Byung Ju Kang (Ulsan University Hospital), Kyung Chan Kim (Daegu Catholic University Hospital), Heung Bum Lee (Chonbuk National University Medical School), Sang Hyun Kwak (Chonnam National University Hospital), and Jae Myung Lee (Korea University Anam Hospital).
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
References
- Angus, D.C.; Linde-Zwirble, W.T.; Lidicker, J.; Clermont, G.; Carcillo, J.; Pinsky, M.R. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001, 29, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Kaukonen, K.M.; Bailey, M.; Suzuki, S.; Pilcher, D.; Bellomo, R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA 2014, 311, 1308–1316. [Google Scholar] [CrossRef]
- Horak, J.; Martinkova, V.; Radej, J.; Matejovič, M. Back to Basics: Recognition of Sepsis with New Definition. J. Clin. Med. 2019, 8, 1838. [Google Scholar] [CrossRef] [Green Version]
- Frat, J.P.; Ragot, S.; Girault, C.; Perbet, S.; Prat, G.; Boulain, T.; Demoule, A.; Ricard, J.D.; Coudroy, R.; Robert, R.; et al. Effect of non-invasive oxygenation strategies in immunocompromised patients with severe acute respiratory failure: A post-hoc analysis of a randomised trial. Lancet Respir. Med. 2016, 4, 646–652. [Google Scholar] [CrossRef]
- Frat, J.P.; Thille, A.W.; Mercat, A.; Girault, C.; Ragot, S.; Perbet, S.; Prat, G.; Boulain, T.; Morawiec, E.; Cottereau, A.; et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N. Engl. J. Med. 2015, 372, 2185–2196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauri, T.; Turrini, C.; Eronia, N.; Grasselli, G.; Volta, C.A.; Bellani, G.; Pesenti, A. Physiologic Effects of High-Flow Nasal Cannula in Acute Hypoxemic Respiratory Failure. Am. J. Respir. Crit. Care Med. 2017, 195, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Roca, O.; Riera, J.; Torres, F.; Masclans, J.R. High-flow oxygen therapy in acute respiratory failure. Respir. Care 2010, 55, 408–413. [Google Scholar]
- Sztrymf, B.; Messika, J.; Bertrand, F.; Hurel, D.; Leon, R.; Dreyfuss, D.; Ricard, J.D. Beneficial effects of humidified high flow nasal oxygen in critical care patients: A prospective pilot study. Intensive Care Med. 2011, 37, 1780–1786. [Google Scholar] [CrossRef]
- Roca, O.; Caralt, B.; Messika, J.; Samper, M.; Sztrymf, B.; Hernandez, G.; Garcia-de-Acilu, M.; Frat, J.P.; Masclans, J.R.; Ricard, J.D. An Index Combining Respiratory Rate and Oxygenation to Predict Outcome of Nasal High-Flow Therapy. Am. J. Respir. Crit. Care Med. 2019, 199, 1368–1376. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [Green Version]
- Moreno, R.P.; Metnitz, P.G.; Almeida, E.; Jordan, B.; Bauer, P.; Campos, R.A.; Iapichino, G.; Edbrooke, D.; Capuzzo, M.; Le Gall, J.R. SAPS 3—From evaluation of the patient to evaluation of the intensive care unit. Part 2: Development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005, 31, 1345–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, F.L.; Bota, D.P.; Bross, A.; Melot, C.; Vincent, J.L. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 2001, 286, 1754–1758. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit. Care Med. 2017, 45, 486–552. [Google Scholar] [CrossRef]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratala, J.; et al. Management of Adults with Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef]
- Mandell, L.A.; Wunderink, R.G.; Anzueto, A.; Bartlett, J.G.; Campbell, G.D.; Dean, N.C.; Dowell, S.F.; File, T.M., Jr.; Musher, D.M.; Niederman, M.S.; et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 2007, 44 (Suppl. 2), S27–S72. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [Green Version]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.M.; Evans, L.E.; Rhodes, A. The Surviving Sepsis Campaign Bundle: 2018 update. Intensive Care Med. 2018, 44, 925–928. [Google Scholar] [CrossRef] [Green Version]
- Rochwerg, B.; Granton, D.; Wang, D.X.; Helviz, Y.; Einav, S.; Frat, J.P.; Mekontso-Dessap, A.; Schreiber, A.; Azoulay, E.; Mercat, A.; et al. High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: A systematic review and meta-analysis. Intensive Care Med. 2019, 45, 563–572. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Jeon, K.; Oh, D.K.; Choi, E.Y.; Seong, G.M.; Heo, J.; Chang, Y.; Kwack, W.G.; Kang, B.J.; Choi, W.I.; et al. Normothermia in Patients with Sepsis Who Present to Emergency Departments Is Associated with Low Compliance with Sepsis Bundles and Increased In-Hospital Mortality Rate. Crit. Care Med. 2020, 48, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Corley, A.; Caruana, L.R.; Barnett, A.G.; Tronstad, O.; Fraser, J.F. Oxygen delivery through high-flow nasal cannulae increase end-expiratory lung volume and reduce respiratory rate in post-cardiac surgical patients. Br. J. Anaesth. 2011, 107, 998–1004. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.H.; Kim, E.Y.; Seo, G.J.; Suh, H.J.; Huh, J.W.; Hong, S.B.; Koh, Y.; Lim, C.M. Global and Regional Ventilation during High Flow Nasal Cannula in Patients with Hypoxia. Acute Crit. Care 2018, 33, 7–15. [Google Scholar] [CrossRef]
- Epstein, A.S.; Hartridge-Lambert, S.K.; Ramaker, J.S.; Voigt, L.P.; Portlock, C.S. Humidified high-flow nasal oxygen utilization in patients with cancer at Memorial Sloan-Kettering Cancer Center. J. Palliat. Med. 2011, 14, 835–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, S.G.; Holets, S.R.; Gay, P.C. High-flow nasal cannula therapy in do-not-intubate patients with hypoxemic respiratory distress. Respir. Care 2013, 58, 597–600. [Google Scholar]
- Makdee, O.; Monsomboon, A.; Surabenjawong, U.; Praphruetkit, N.; Chaisirin, W.; Chakorn, T.; Permpikul, C.; Thiravit, P.; Nakornchai, T. High-Flow Nasal Cannula versus Conventional Oxygen Therapy in Emergency Department Patients with Cardiogenic Pulmonary Edema: A Randomized Controlled Trial. Ann. Emerg. Med. 2017, 70, 465–472.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thille, A.W.; Muller, G.; Gacouin, A.; Coudroy, R.; Decavele, M.; Sonneville, R.; Beloncle, F.; Girault, C.; Dangers, L.; Lautrette, A.; et al. Effect of Postextubation High-Flow Nasal Oxygen with Noninvasive Ventilation vs. High-Flow Nasal Oxygen Alone on Reintubation Among Patients at High Risk of Extubation Failure: A Randomized Clinical Trial. JAMA 2019, 322, 1465–1475. [Google Scholar] [CrossRef]
- Bae, S.; Han, M.; Kim, C.; Lee, H.; Ahn, J.J.; Kim, J.H.; Kang, B.J. High-Flow Nasal Cannula Oxygen Therapy Can Be Effective for Patients in Acute Hypoxemic Respiratory Failure with Hypercapnia: A Retrospective, Propensity Score-Matched Cohort Study. J. Korean Med. Sci. 2020, 35, e67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rello, J.; Perez, M.; Roca, O.; Poulakou, G.; Souto, J.; Laborda, C.; Balcells, J.; Serra, J.; Masclans, J.R.; CRIPS Investigators. High-flow nasal therapy in adults with severe acute respiratory infection: A cohort study in patients with 2009 influenza A/H1N1v. J. Crit. Care 2012, 27, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Koga, Y.; Kaneda, K.; Mizuguchi, I.; Nakahara, T.; Miyauchi, T.; Fujita, M.; Kawamura, Y.; Oda, Y.; Tsuruta, R. Extent of pleural effusion on chest radiograph is associated with failure of high-flow nasal cannula oxygen therapy. J. Crit. Care 2016, 32, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.Y.; Sung, H.; Hong, S.B.; Lim, C.M.; Koh, Y.; Huh, J.W. Predictors of high flow nasal cannula failure in immunocompromised patients with acute respiratory failure due to non-HIV pneumocystis pneumonia. J. Thorac. Dis. 2017, 9, 3013–3022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messika, J.; Ben Ahmed, K.; Gaudry, S.; Miguel-Montanes, R.; Rafat, C.; Sztrymf, B.; Dreyfuss, D.; Ricard, J.D. Use of High-Flow Nasal Cannula Oxygen Therapy in Subjects with ARDS: A 1-Year Observational Study. Respir. Care 2015, 60, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Roca, O.; de Acilu, M.G.; Caralt, B.; Sacanell, J.; Masclans, J.R. Humidified high flow nasal cannula supportive therapy improves outcomes in lung transplant recipients readmitted to the intensive care unit because of acute respiratory failure. Transplantation 2015, 99, 1092–1098. [Google Scholar] [CrossRef]
- Liu, L.; Xie, J.; Wu, W.; Chen, H.; Li, S.; He, H.; Yu, Y.; Hu, M.; Li, J.; Zheng, R.; et al. A simple nomogram for predicting failure of non-invasive respiratory strategies in adults with COVID-19: A retrospective multicentre study. Lancet. Digit. Health 2021, 3, e166–e74. [Google Scholar] [CrossRef]
- Kang, B.J.; Koh, Y.; Lim, C.M.; Huh, J.W.; Baek, S.; Han, M.; Seo, H.S.; Suh, H.J.; Seo, G.J.; Kim, E.Y.; et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med. 2015, 41, 623–632. [Google Scholar] [CrossRef]
- Peake, S.L.; Delaney, A.; Bailey, M.; Bellomo, R.; Cameron, P.A.; Cooper, D.J.; Higgins, A.M.; Holdgate, A.; Howe, B.D.; Webb, S.A.; et al. Goal-directed resuscitation for patients with early septic shock. N. Engl. J. Med. 2014, 371, 1496–1506. [Google Scholar]
- Yealy, D.M.; Kellum, J.A.; Huang, D.T.; Barnato, A.E.; Weissfeld, L.A.; Pike, F.; Terndrup, T.; Wang, H.E.; Hou, P.C.; LoVecchio, F.; et al. A randomized trial of protocol-based care for early septic shock. N. Engl. J. Med. 2014, 370, 1683–1693. [Google Scholar] [PubMed] [Green Version]
- Mouncey, P.R.; Osborn, T.M.; Power, G.S.; Harrison, D.A.; Sadique, M.Z.; Grieve, R.D.; Jahan, R.; Harvey, S.E.; Bell, D.; Bion, J.F.; et al. Trial of early, goal-directed resuscitation for septic shock. N. Engl. J. Med. 2015, 372, 1301–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.I.; Park, S. Sepsis: Early Recognition and Optimized Treatment. Tuberc. Respir. Dis. 2019, 82, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Frat, J.P.; Ragot, S.; Coudroy, R.; Constantin, J.M.; Girault, C.; Prat, G.; Boulain, T.; Demoule, A.; Ricard, J.D.; Razazi, K.; et al. Predictors of Intubation in Patients with Acute Hypoxemic Respiratory Failure Treated with a Noninvasive Oxygenation Strategy. Crit. Care Med. 2018, 46, 208–215. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).