2. Materials and Methods
All patients undergoing surgical exploration and liver resections at our center are registered in a prospective institutional database. Patients who underwent liver surgery for intrahepatic cholangiocarcinoma (iCCA) from 2008 to 2020 were eligible for this analysis.
The diagnosis of iCCA was based on histology obtained either by pre- or intra-operative biopsy or by the resected specimen. Patients with hilar cholangiocarcinoma, gallbladder carcinoma, mixed hepatocellular/cholangiocarcinoma, or bile duct carcinoma not clearly attributable to the intrahepatic biliary tree as well as patients with severe parenchymal damage (cirrhosis, fibrosis > F2 or steatosis > 50%) were excluded from our analysis.
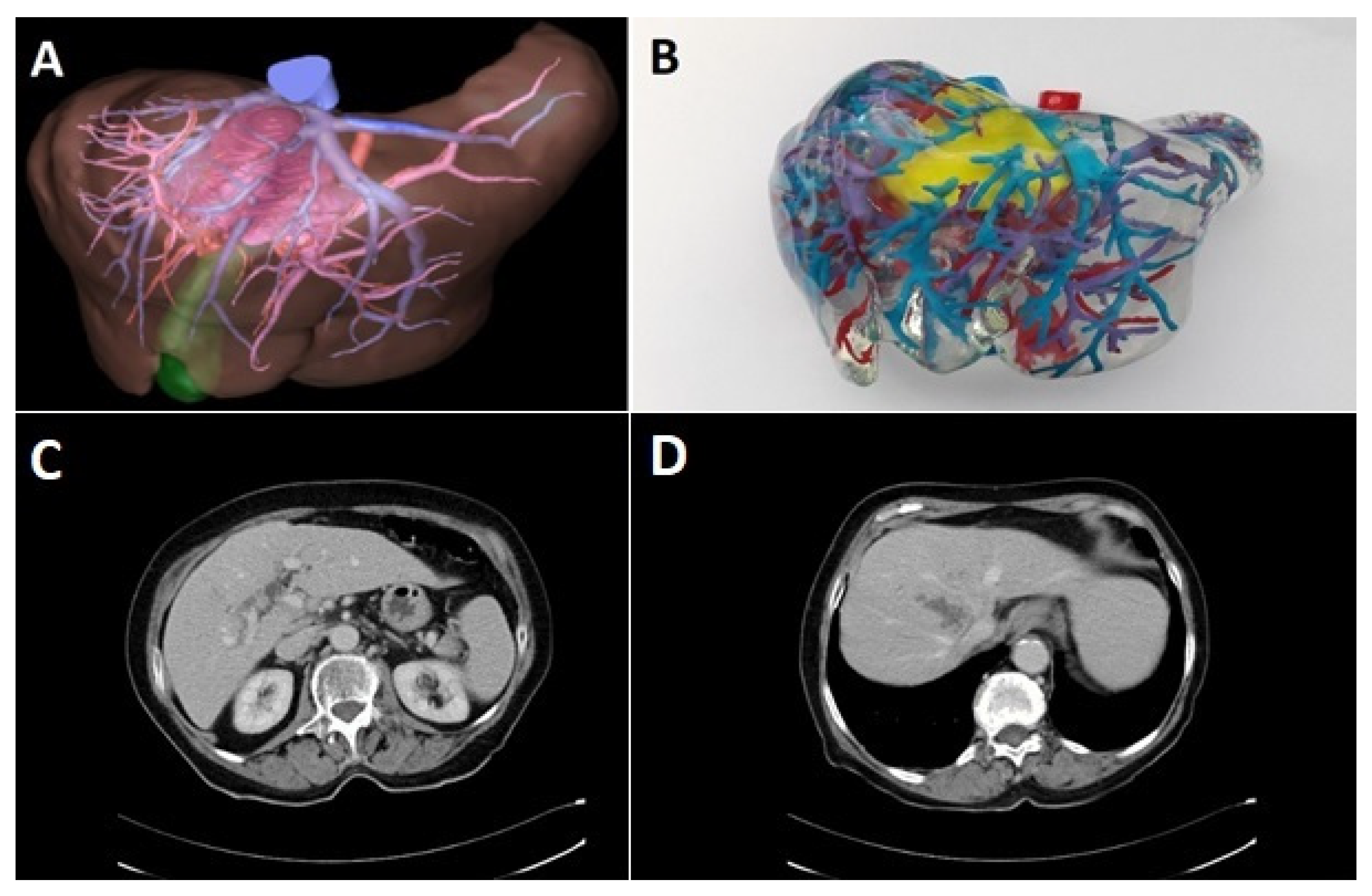
Preoperative diagnostic work-up included ultrasound of the abdomen and computed tomography (CT) of the abdomen and chest. Upper and lower gastrointestinal endoscopy was performed to exclude extrahepatic primary tumor in cases where the diagnosis of an iCCA was not made by biopsy. In selected cases, three-dimensional CT-scan of the liver including volumetry, virtual tumor resection, and computer-assisted risk analysis was performed prior to the resection. These were performed either by an external provider (MeVis Distant Services, MeVis AG, Bremen, Germany) or with a local reconstruction software (Synapse 3D, Fujifilm AG, Tokyo, Japan) by a trained surgical resident [
8]. Since 2017, 3D-prints of the liver were performed on special request of the surgeon with non-flexible polyurethane rubber [
9]. Especially in cases with anticipated complex vascular reconstructions, 3D-prints were ordered for preoperative planning (
Figure 1).
All surgical explorations and resections were performed by a team of experienced surgeons with special expertise in hepato-biliary surgery, and retrospectively classified according to the “New World” terminology [
10]. Postoperatively, for at least two years, we conducted follow-up every three months; later on, the interval was increased to 6 months, if reasonable. Preferably, CT imaging was obtained at least every 6 months alternating with ultrasound examinations. For patients who were not able to undergo follow-up at our center, further information was retrieved from treating physicians.
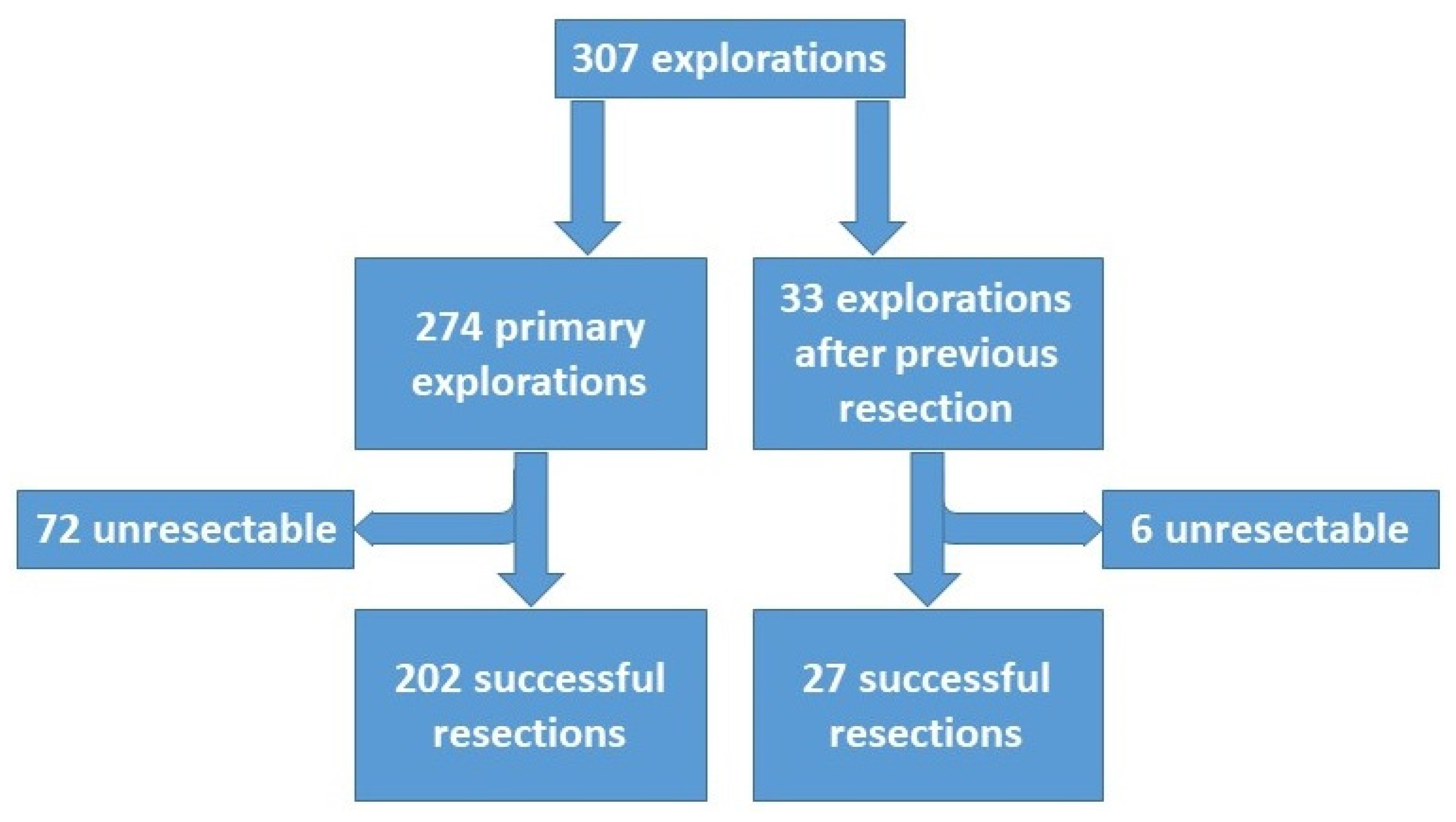
The data collection was completed in February 2021. The data of the patients undergoing liver resection (
n = 223) were further analyzed with regard to (1) preoperative treatment of tumor, (2) operative details, (3) perioperative morbidity and mortality, (4) pathologic findings, (5) outcome measured by tumor recurrence, treatment of recurrence and survival, and (6) prognostic factors for overall and disease-free survival. Surgical complications were assessed according to the Dindo–Clavien classification [
11]. The TNM classification was performed according to the 8th edition of the classification of the Union for International Cancer Control (UICC) [
12] (
Table 1).
All patients signed an informed consent that allowed the data and follow-up to be collected anonymously and potentially used for scientific analysis. Abiding by the regulations of the federal state law (state hospital law §36 & §37) and according to the independent ethics committee of Rhineland-Palatinate, no ethical approval was necessary for this study.
Statistical Analyses
SPSS 23 (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY, USA: IBM Corp.) was used to perform statistics. Categorical data was analyzed using the Chi2 test in cross tabulation. Survival analyses were conducted with the Kaplan Meier model and for comparison of factors influencing survival the log rank test was utilized. A
p-value of <0.05 was considered significant. All analyses were an intention to treat, and no patients were excluded. Recurrence-free survival was defined according to Punt and colleagues [
13].
4. Discussion
Surgical treatment of iCCA is still one of the main challenges in hepatobiliary surgery. Most often, iCCAs are diagnosed late in the course of the disease when tumors are locally advanced or even in a metastatic stage. In our series, the vast majority of tumors required major hepatectomy (in almost 80%) and additional operative procedures such as complex vascular and biliary reconstructions in nearly 50% of our cases, exceeding in this regard other reports by far. Thus, the presented series is one of the largest in the Western world, not just because of the numbers but also with regard to complexity of procedures. Further, as a single center series pursuing the same aggressive surgical strategy over the entire inclusion period, the comparability of data is also relatively well in contrast to often inhomogeneous multicentric data.
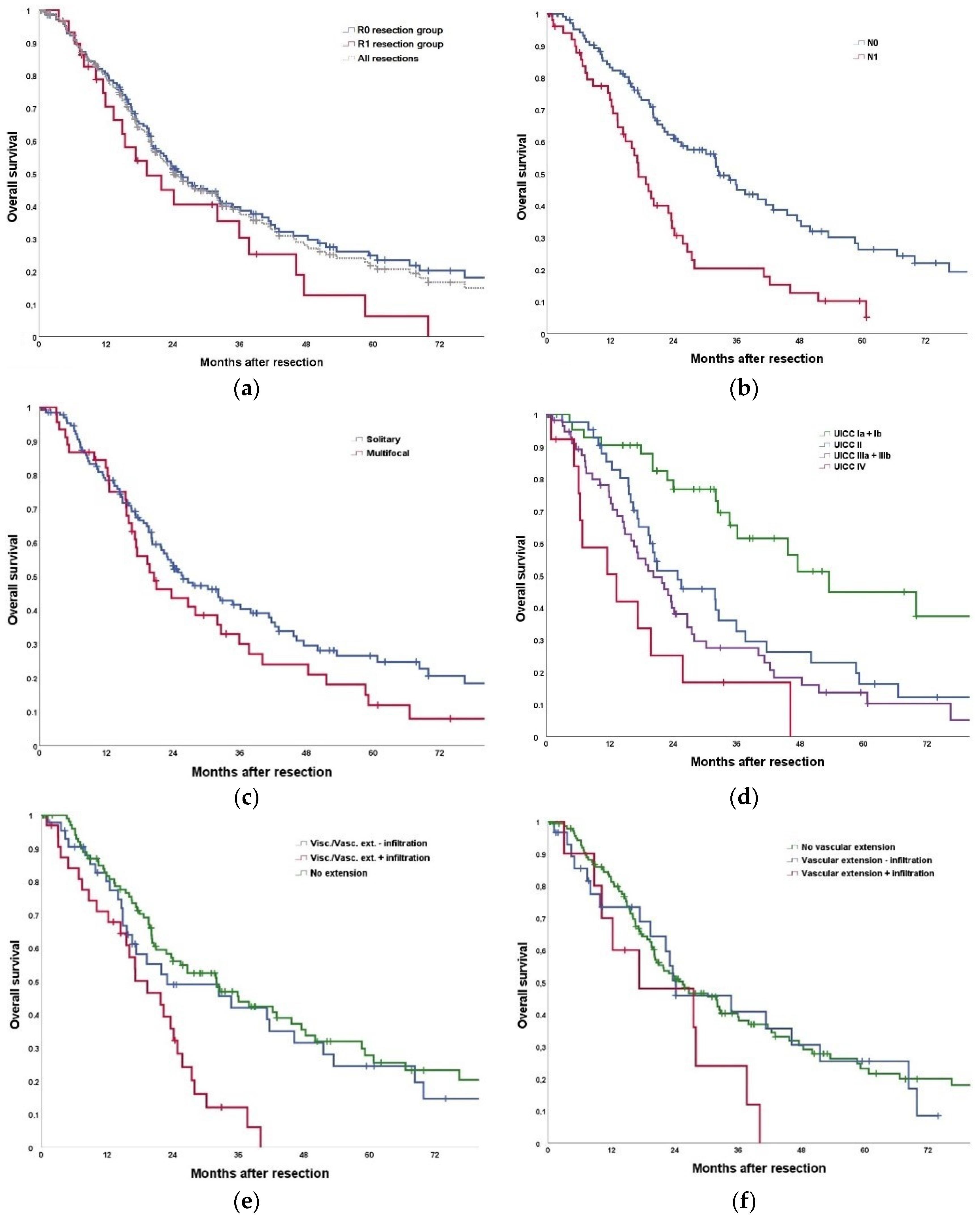
The reported survival after hepatectomy for iCCA ranges between 31% and 59% at 3 years and 21% to 45% at 5 years, depending on the selection criteria for surgery (
Table 8). The herein presented results with a 3- and 5-year-survival of 35% and 22% are at the lower range of the reported data, but in our series the extent of liver resection and additional procedures such as vascular reconstructions exceeded those of most other reports by far, indicating more advanced tumors and more difficult resections [
19,
20]. Looking exclusively at the subgroup undergoing hepatectomy without operative extension, the results with 46% and 28% survival at 3- and 5-years are in accordance with most published data. These results are comparable to the results of surgical therapy in many other gastrointestinal malignancies, endorsing that therapeutic nihilism is not justified in iCCA. However, the presented data also clearly indicate that even with an aggressive surgical approach the chance of cure is still small in iCCA.
In our series, there was resection/reconstruction of major hepatic vessels or the inferior vena cava in 22% of the cases. Notably, pathology confirmed tumor infiltration of major vessels in only 29% of the suspected cases. As infiltration is difficult to assess by preoperative or intraoperative imaging, our approach is to resect and reconstruct vessels whenever infiltration is suspected. This aggressive approach seems to be justified as this can be done with acceptable low morbidity and mortality.
The most aggressive surgical approach to iCCA is ALPPS [
35]. Our results after ALPPS for iCCA are consistent with data from a multicentric analysis by Li et al. [
36]. While there seems to be hardly any benefit of such an aggressive approach in multifocal tumors ALPPS seems to be justified in selected cases of solitary iCCA. The patient surviving 11.5 years is to the best of our knowledge the longest survivor worldwide after an ALPPS procedure at all.
Depending on the aggressiveness of the surgical approach and the quality of preoperative diagnostic and staging procedures reported resectability rates of iCCA show great variability ranging between 50% and 75% [
14,
23,
37,
38]. This is considerably lower than in most if not in all other hepatobiliary malignancies. Most often multinodular intrahepatic tumor spread or less often peritoneal seedings are the main causes of irresectability. In our series the resectability rate of almost 80% is high but this is at the expense of the need for often extensive resections with frequently complex resections and reconstruction of adjacent structures. We follow this aggressive approach as resection offers the only chance for cure.
It has already been shown that routine use of staging laparoscopy results in a reduction of about 20% of explorative laparotomies in biliary cancer [
39,
40]. Avoiding a large laparotomy can help to guide these patients towards an immediate palliative treatment. In addition, computer-assisted operation planning and 3D reconstruction of liver anatomy that may provide significant anatomical information. In recent years, we used 3D-prints of the liver for operation planning in selected cases [
9]. Although very instructive, it needs to be evaluated whether these techniques can also contribute to a better assessment of resectability, to a reduction of perioperative morbidity and mortality and finally to an improvement of the oncological outcome.
One aim of our analysis was to identify prognostic markers and risk factors for tumor recurrence after hepatic resection. Like other studies, we could confirm extended resection, visceral extension, vascular infiltration, visceral infiltration, tumor size, T-stage, N-stage, M-stage, Pn-stage, and UICC stage to be significantly associated with tumor recurrence and poor survival after R0 resection. In multivariate analysis, tumor size, T stage and N stage were significantly associated with a worse overall survival, while N stage, preoperative therapy, T stage, tumor size and M stage were associated with worse recurrence-free survival. Therefore, based on the presented data, we do not consider the presence of any of these prognostic factors (with the exception of UICC stage IV) as a contraindication to liver resection. In particular, we do not consider the potential need for extension of resections a contraindication, the more as even modern imaging modalities do not reliably predict macro- or micro-vascular infiltration.
There is agreement that resection of stage IV iCCA should only be performed in selected cases in palliative intention where tumor associated symptoms cannot be controlled otherwise. However, there is still discussion about surgical therapy in the presence of lymph node involvement [
41]. Up to now, there is no evidence whether lymphadenectomy is of prognostic value only or also beneficial for survival [
42]. Our data with a median survival of 18 months and a 5-year-survival of 12% after R0 resections in the presence of lymph node metastases suggest a probable survival benefit of liver resection. However, in this patient group the need for effective perioperative therapy is highly evident.
The new UICC classification recommends the removal of at least 6 lymph nodes from the hilar region and along the lesser gastric curve in left-sided iCCA or retropancreatic in right-sided iCCA [
12]. Hopefully, this will help to further improve both treatment stratification of iCCA and, with ongoing progress in adjuvant chemotherapy, also oncological outcome.
Tumor recurrence is by far the most frequent cause of death after resection of iCCA [
43,
44,
45]. Our data reveal that the liver is the most frequent primary site of tumor recurrence with 50% of cases where the liver is the only initial site of recurrence. We performed repeated resection in 27 cases with a survival even longer than in the entire resection group [
16]. In a recent German multicenter study with 113 repeated resections, mainly by minor hepatectomies or segmentectomies, a 3- and 5-year disease-free survival of 36% and 28% was reported [
46]. These survival rates are even slightly better than after primary resection, suggesting that there might be a selection bias indicating a more favorable biology in those recurrent tumors remaining confined to the liver [
47].
Due to the often-advanced disease at the time of diagnosis, there is a substantial number of cases where an R1 resection cannot be avoided. In particular, in large or centrally located tumors, sometimes there is no more margin to be left or final histology shows microscopic tumor invasion of the resection margin which at operation had been assumed to be tumor-free. Similarly, although in the current series all resections were performed with curative intention, we achieved an R1-resection rate of 16%. In former years, R1 resection was supposed to provide little survival benefit rather than to face the patients to the risks of major hepatic surgery [
48,
49,
50,
51]. However, with the availability of effective chemotherapy, the value of R1 resections probably needs to be re-evaluated, in particular in combination with neoadjuvant and downsizing therapy strategies [
52].
Although significantly better than the data of non-resected tumors, the achieved long-term results even after extensive resections with complex reconstructions are poor. This indicates that there is a limit to the contribution made by radical resections alone. Hence, since the first results of the BILCAP trial (adjuvant capecitabine versus observation following R0/R1 resection of bile duct cancer) became available, adjuvant chemotherapy with capecitabine is currently the standard of care after resection of iCCA in our institution [
53].
In our series, we had 18 resections following neoadjuvant CTx for down-sizing. The survival data in these patients are at least comparable with those of patients undergoing upfront surgery without chemotherapy. Assuming that the tumors in the downsizing group were more advanced, these results suggest the effectiveness of CTx. Consistently, in a multivariate analysis, preoperative chemotherapy was one prognostic factor significantly associated with improved recurrence-free survival. Certainly, our series is too small to draw any valid conclusion, but our data are in line with some recent reports from the literature. In a French study neoadjuvant chemotherapy led to a secondary resectability in 39/74 (53%) patients with initially borderline resectable or irresectable iCCA [
54]. These patients had survival rates similar to the group with initially resectable iCCA. Similarly, in a recent propensity-matched analysis (203 versus 487 patients with iCCA) based on data from the National Cancer Database (NCDB, USA), neoadjuvant treatment was associated with a significantly higher R0 resection rate and better survival compared to adjuvant therapy (median OS: 40.3 vs 32.8 months;
p = 0.01) [
55]. This suggests the potential effectiveness of neoadjuvant (downsizing) treatment for iCCA and justifies further evaluation of this concept [
56,
57].
Further, cholangiocarcinoma has become a hallmark of modern precision medicine. With the development of next-generation sequencing (NGS) targeted therapies become increasingly available. In the palliative setting, first promising results have been reported after treatment with fibroblast growth factor receptor (FGFR) and isocitrate dehydrogenase (IDH) inhibitors [
58]. In future it is possible that these targeted therapies may also play a role in preoperative neoadjuvant or downsizing therapy [
59,
60].