Functional and Cosmetic Outcome after Reconstruction of Isolated, Unilateral Orbital Floor Fractures (Blow-Out Fractures) with and without the Support of 3D-Printed Orbital Anatomical Models
Abstract
:1. Introduction
2. Experimental Section
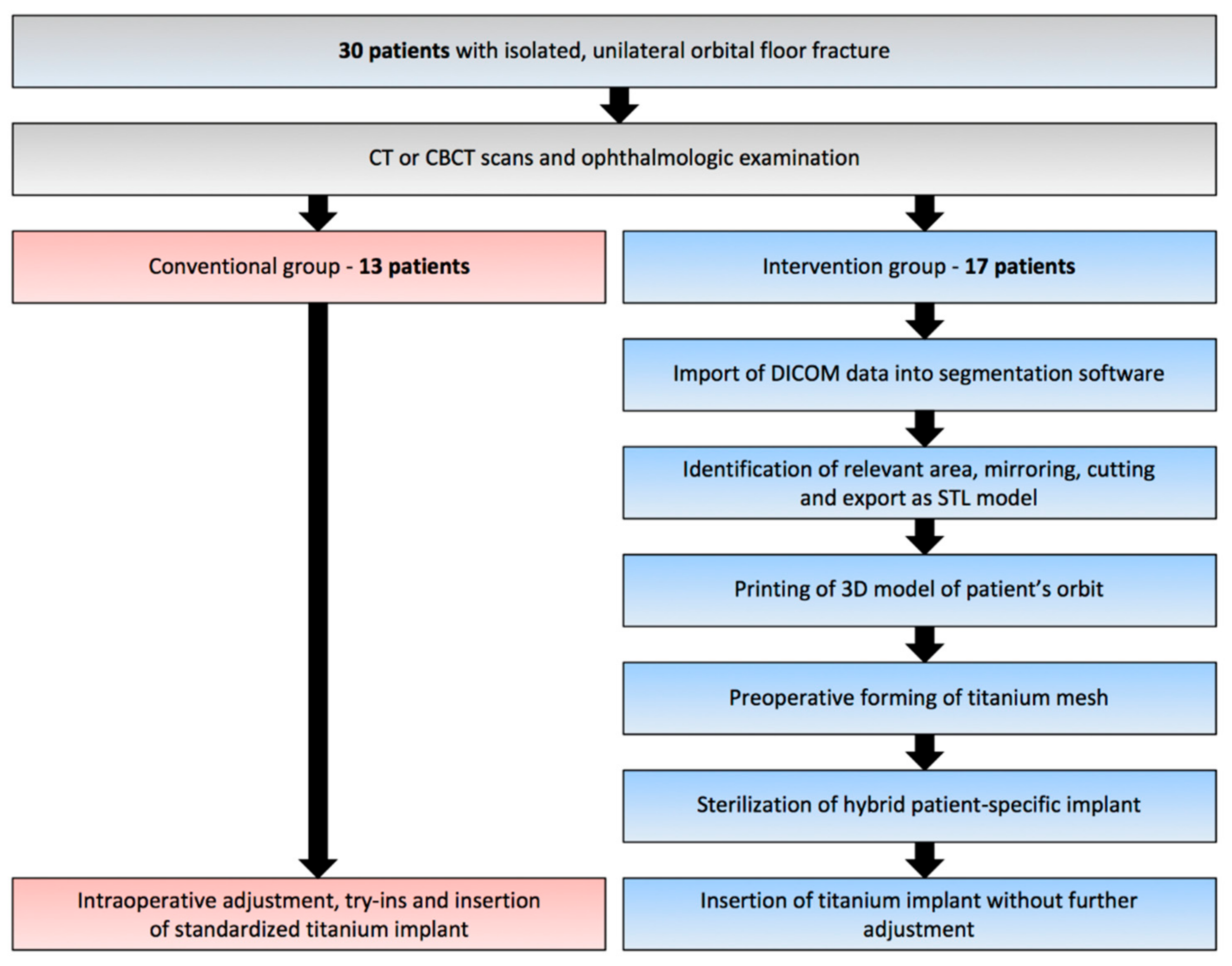
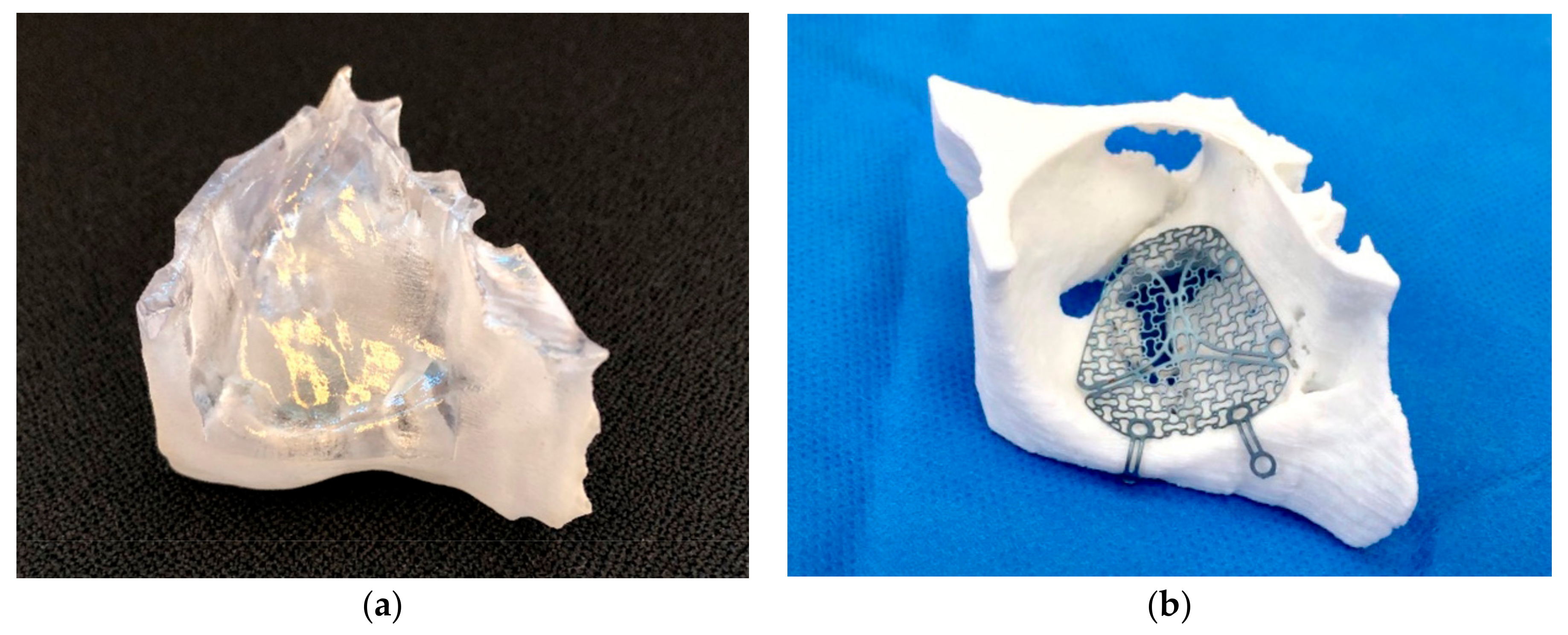
2.1. Virtual Planning and Manufacturing of the 3D Model
2.2. Surgical Procedure
2.3. Ophthalmic Examination
2.4. Statistical Analysis
3. Results
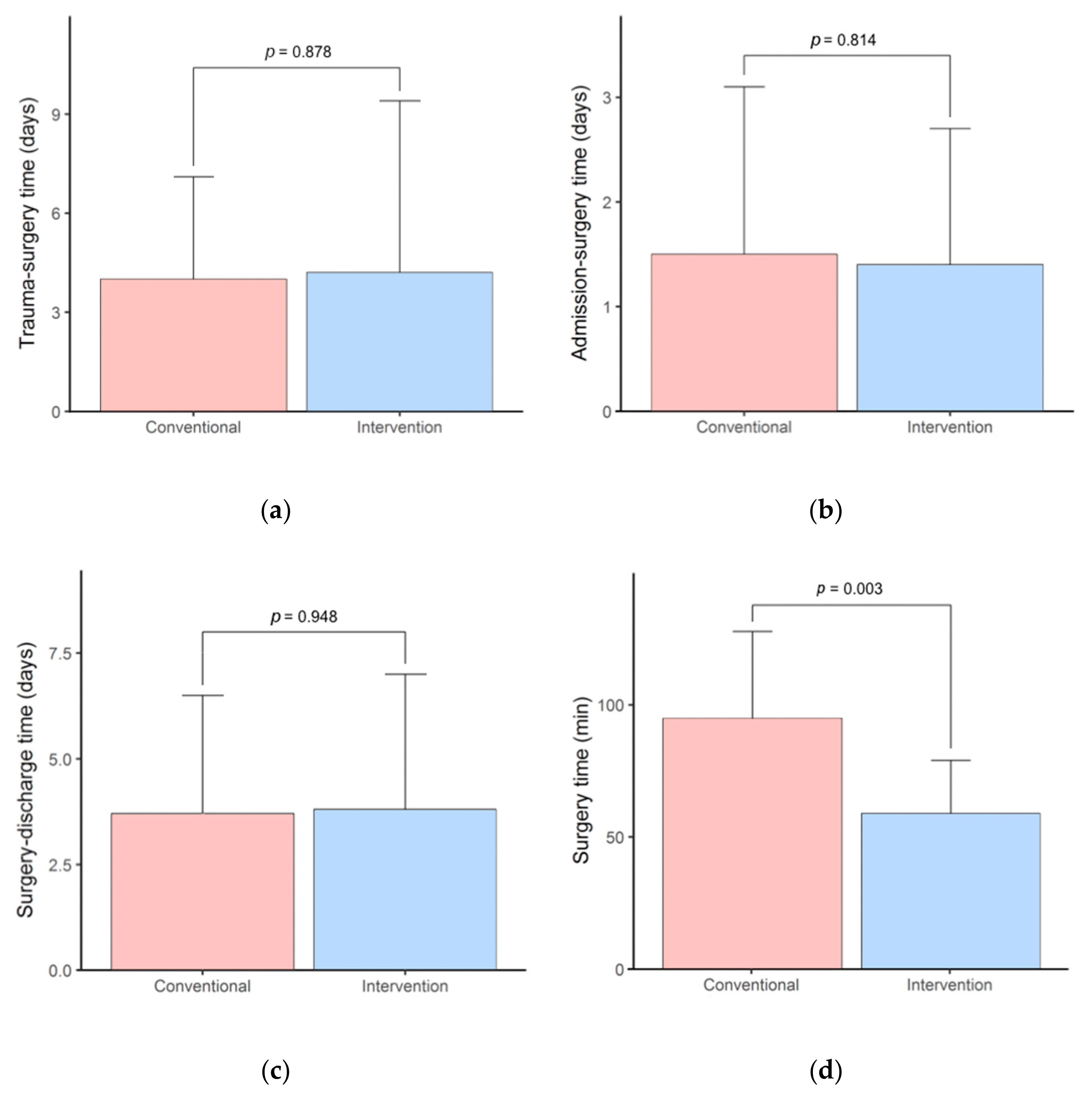
3.1. Surgery Time and Hospital Stay
3.2. Ophthalmic Examination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAD/DAM | Computer-aided design/computer-aided manufacturing |
| 3D | Three-dimensional |
| ROI | Region of interest |
| DICOM | Digital Imaging and Communications in Medicine |
| MAM | Medical Additive Manufacturing |
| MIMICS | Materialise Interactive Medical Image Control System |
| STL | Standard Tessellation Language |
| PLA | Polylactic acid |
| SD | Standard deviation |
| PDS | Polydioxanone |
References
- Ellis, E.; El-Attar, A.; Moos, K.F. An Analysis of 2067 cases of zygomatico-orbital fracture. J. Oral Maxillofac. Surg. 1985, 43, 417–428. [Google Scholar] [CrossRef]
- Shin, J.W.; Lim, J.S.; Yoo, G.; Byeon, J.H. An Analysis of Pure Blowout Fractures and Associated Ocular Symptoms. J. Craniofacial Surg. 2013, 24, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Hammer, B.; Prein, J. Correction of post-traumatic orbital deformities: Operative techniques and review of 26 patients. J. Cranio-Maxillofac. Surg. 1995, 23, 81–90. [Google Scholar] [CrossRef]
- Manson, P.N.; Iiff, N. Management of blow-out fractures of the orbital floor II. Early repair for selected injuries. Surv. Ophthalmol. 1991, 35, 280–292. [Google Scholar] [CrossRef]
- Kunz, C.; Sigron, G.R.; Jaquiéry, C. Functional outcome after non-surgical management of orbital fractures—The bias of decision-making according to size of defect: Critical review of 48 patients. Br. J. Oral Maxillofac. Surg. 2013, 51, 486–492. [Google Scholar] [CrossRef]
- Burnstine, M.A. Clinical recommendations for repair of isolated orbital floor fractures: An evidence-based analysis. Ophthalmology 2002, 109, 1207–1210. [Google Scholar] [CrossRef]
- Potter, J.K.; Ellis, E. Biomaterials for reconstruction of the internal orbit. J. Oral Maxillofac. Surg. 2004, 62, 1280–1297. [Google Scholar] [CrossRef]
- Ellis, E.; Tan, Y. Assessment of internal orbital reconstructions for pure blowout fractures: Cranial bone grafts versus titanium mesh. J. Oral Maxillofac. Surg. 2003, 61, 442–453. [Google Scholar] [CrossRef]
- Ellis, E.; Messo, E. Use of nonresorbable alloplastic implants for internal orbital reconstruction. J. Oral Maxillofac. Surg. 2004, 62, 873–881. [Google Scholar] [CrossRef]
- Sugar, A.W.; Kurlakose, M.; Walshaw, N.D. Titanium mesh in orbital wall reconstruction. Int. J. Oral Maxillofac. Surg. 1992, 21, 140–144. [Google Scholar] [CrossRef]
- Metzger, M.C.; Schön, R.; Weyer, N.; Rafii, A.; Gellrich, N.-C.; Schmelzeisen, R.; Strong, B.E. Anatomical 3-dimensional Pre-bent Titanium Implant for Orbital Floor Fractures. Ophthalmology 2006, 113, 1863–1868. [Google Scholar] [CrossRef] [PubMed]
- Kärkkäinen, M.; Wilkman, T.; Mesimäki, K.; Snäll, J. Primary reconstruction of orbital fractures using patient-specific titanium milled implants: The Helsinki protocol. Br. J. Oral Maxillofac. Surg. 2018, 56, 791–796. [Google Scholar] [CrossRef] [Green Version]
- Msallem, B.; Sharma, N.; Cao, S.; Halbeisen, F.S.; Zeilhofer, H.-F.; Thieringer, F.M. Evaluation of the Dimensional Accuracy of 3D-Printed Anatomical Mandibular Models Using FFF, SLA, SLS, MJ, and BJ Printing Technology. J. Clin. Med. 2020, 9, 817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatz, C.R.; Msallem, B.; Aghlmandi, S.; Brantner, P.; Thieringer, F.M. Can an entry-level 3D printer create high-quality anatomical models? Accuracy assessment of mandibular models printed by a desktop 3D printer and a professional device. Int. J. Oral Maxillofac. Surg. 2020, 49, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Maglitto, F.; Orabona, G.D.; Committeri, U.; Salzano, G.; De Fazio, G.R.; Vaira, L.A.; Abbate, V.; Bonavolontà, P.; Piombino, P.; Califano, L. Virtual Surgical Planning and the “In-House” Rapid Prototyping Technique in Maxillofacial Surgery: The Current Situation and Future Perspectives. Appl. Sci. 2021, 11, 1009. [Google Scholar] [CrossRef]
- Schön, R.; Metzger, M.C.; Zizelmann, C.; Weyer, N.; Schmelzeisen, R. Individually preformed titanium mesh implants for a true-to-original repair of orbital fractures. Int. J. Oral Maxillofac. Surg. 2006, 35, 990–995. [Google Scholar] [CrossRef]
- Strong, E.B.; Fuller, S.C.; Wiley, D.F.; Zumbansen, J.; Wilson, M.D.; Metzger, M.C. Preformed vs Intraoperative Bending of Titanium Mesh for Orbital Reconstruction. Otolaryngol. Head Neck Surg. 2013, 149, 60–66. [Google Scholar] [CrossRef]
- Kormi, E.; Männistö, V.; Lusila, N.; Naukkarinen, H.; Suojanen, J. Accuracy of Patient-Specific Meshes as a Reconstruction of Orbital Floor Blow-Out Fractures. J. Craniofacial Surg. 2021, 32, e116–e119. [Google Scholar] [CrossRef]
- Falkhausen, R.; Mitsimponas, K.; Adler, W.; Brand, M.; von Wilmowsky, C. Clinical outcome of patients with orbital fractures treated with patient specific CAD/CAM ceramic implants—A retrospective study. J. Cranio-Maxillofac. Surg. 2021, 49, 468–479. [Google Scholar] [CrossRef]
- Sigron, G.R.; Rüedi, N.; Chammartin, F.; Meyer, S.; Msallem, B.; Kunz, C.; Thieringer, F.M. Three-Dimensional Analysis of Isolated Orbital Floor Fractures Pre- and Post-Reconstruction with Standard Titanium Meshes and “Hybrid” Patient-Specific Implants. J. Clin. Med. 2020, 9, 1579. [Google Scholar] [CrossRef]
- Dubois, L.; Steenen, S.A.; Gooris, P.J.J.; Bos, R.R.M.; Becking, A.G. Controversies in orbital reconstruction—III. Biomaterials for orbital reconstruction: A review with clinical recommendations. Int. J. Oral Maxillofac. Surg. 2016, 45, 41–50. [Google Scholar] [CrossRef]
- Berg, B.-I.; Flury, E.; Thieringer, F.M.; Augello, M.; Savic, M.; Schötzau, A.; Kunz, C.; Goldblum, D. Retrobulbar haematoma in the era of anticoagulants. Injury 2019, 50, 1641–1648. [Google Scholar] [CrossRef] [PubMed]
- Zimmerer, R.M.; Ellis, E.; Aniceto, G.S.; Schramm, A.; Wagner, M.E.H.; Grant, M.P.; Cornelius, C.-P.; Strong, E.B.; Rana, M.; Chye, L.T. A prospective multicenter study to compare the precision of posttraumatic internal orbital reconstruction with standard preformed and individualized orbital implants. J. Cranio-Maxillofac. Surg. 2016, 44, 1485–1497. [Google Scholar] [CrossRef] [PubMed]
- Kozakiewicz, M.; Elgalal, M.; Piotr, L.; Broniarczyk-Loba, A.; Stefanczyk, L. Treatment with individual orbital wall implants in humans—1-Year ophthalmologic evaluation. J. Cranio-Maxillofac. Surg. 2011, 39, 30–36. [Google Scholar] [CrossRef]
- Jaquiéry, C.; Aeppli, C.; Cornelius, P.; Palmowsky, A.; Kunz, C.; Hammer, B. Reconstruction of orbital wall defects: Critical review of 72 patients. Int. J. Oral Maxillofac. Surg. 2007, 36, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Erickson, D.M.; Chance, D.; Schmitt, S.; Mathts, J. An opinion survey of reported benefits from the use of stereolithographic models. J. Oral Maxillofac. Surg. 1999, 57, 1040–1043. [Google Scholar] [CrossRef] [Green Version]
- Jansen, J.; Schreurs, R.; Dubois, L.; Maal, T.J.J.; Gooris, P.J.J.; Becking, A.G. The advantages of advanced computer-assisted diagnostics and three-dimensional preoperative planning on implant position in orbital reconstruction. J. Cranio-Maxillofac. Surg. 2018, 46, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Cao, S.; Msallem, B.; Kunz, C.; Brantner, P.; Honigmann, P.; Thieringer, F.M. Effects of Steam Sterilization on 3D Printed Biocompatible Resin Materials for Surgical Guides—An Accuracy Assessment Study. J. Clin. Med. 2020, 9, 1506. [Google Scholar] [CrossRef]
- Kozakiewicz, M.; Elgalal, M.; Loba, P.; Komuński, P.; Arkuszewski, P.; Broniarczyk-Loba, A.; Stefańczyk, L. Clinical application of 3D pre-bent titanium implants for orbital floor fractures. J. Cranio-Maxillofac. Surg. 2009, 37, 229–234. [Google Scholar] [CrossRef]
- Mustafa, S.F.; Evans, P.L.; Bocca, A.; Patton, D.W.; Sugar, A.W.; Baxter, P.W. Customized titanium reconstruction of post-traumatic orbital wall defects: A review of 22 cases. Int. J. Oral Maxillofac. Surg. 2011, 40, 1357–1362. [Google Scholar] [CrossRef]
- Kim, Y.C.; Jeong, W.S.; Park, T.-K.; Choi, J.W.; Koh, K.S.; Oh, T.S. The accuracy of patient specific implant prebented with 3D-printed rapid prototype model for orbital wall reconstruction. J. Cranio-Maxillofac. Surg. 2017, 45, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Chen, H.; Sun, Y.-J.; Wang, B.-F.; Che, L.; Liu, S.-Y.; Li, G.-Y. Clinical effects of 3-D printing-assisted personalized reconstructive surgery for blowout orbital fractures. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 2051–2057. [Google Scholar] [CrossRef] [PubMed]
- Holmes, S.; Schlittler, F.L. Going beyond the limitations of the non-patient-specific implant in titanium reconstruction of the orbit. Br. J. Oral Maxillofac. Surg. 2021. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, R.; Malińska, M.; Kozakiewicz, M. Classical versus custom orbital wall reconstruction: Selected factors regarding surgery and hospitalization. J. Cranio-Maxillofac. Surg. 2017, 45, 710–715. [Google Scholar] [CrossRef]
- Lim, C.G.T.; Campbell, D.I.; Clucas, D.M. Rapid Prototyping Technology in Orbital Floor Reconstruction: Application in Three Patients. Craniomaxillofac. Trauma Reconstr. 2014, 7, 143–146. [Google Scholar] [CrossRef] [Green Version]
- Msallem, D.M.B.; Beiglboeck, F.; Honigmann, P.; Jaquiéry, D.M.C.; Thieringer, F. Craniofacial Reconstruction by a Cost-Efficient Template-Based Process Using 3D Printing. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1582. [Google Scholar] [CrossRef] [Green Version]
- Legocki, A.T.; Duffy-Peter, A.; Scott, A.R. Benefits and Limitations of Entry-Level 3-Dimensional Printing of Maxillofacial Skeletal Models. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 389–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zweifel, D.F.; Simon, C.; Hoarau, R.; Pasche, P.; Broome, M. Are Virtual Planning and Guided Surgery for Head and Neck Reconstruction Economically Viable? J. Oral Maxillofac. Surg. 2015, 73, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Macario, A. What does one minute of operating room time cost? J. Clin. Anesth. 2010, 22, 233–236. [Google Scholar] [CrossRef] [PubMed]
| Variable | Overall | Conventional Group | Intervention Group |
|---|---|---|---|
| Number of patients, n (%) | 30 (100) | 13 (43.3) | 17 (56.7) |
| Age (years) | |||
| Mean (SD) | 51.2 (20.4) | 49.6 (19.1) | 52.4 (21.8) |
| Range | 20–91 | 21–79 | 20–91 |
| Sex, n (%) | |||
| Female | 15 (50) | 4 (30.8) | 11 (64.7) |
| Male | 15 (50) | 9 (69.2) | 6 (35.3) |
| Cause of injury, n (%) | |||
| Fall | 16 (53.3) | 7 (53.8) | 9 (52.9) |
| Assault | 9 (30.0) | 5 (38.5) | 4 (23.5) |
| Sports accident | 3 (10.0) | 1 (7.7) | 2 (11.8) |
| Vehicle accident | 1 (3.3) | 0 (0.0) | 1 (5.9) |
| Work accident | 1 (3.3) | 0 (0.0) | 1 (5.9) |
| Site of injury, n (%) | |||
| right | 11 (36.7) | 2 (15.4) | 9 (52.9) |
| left | 19 (63.3) | 11 (84.6) | 8 (47.1) |
| Follow-up (SD) (days) | 196 (233) | 191.2 (279) | 200 (200) |
| Conventional Group | Intervention Group | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre-Operative | Post-Operative | Pre-Operative | Post-Operative | |||||
| Position of globe | ||||||||
| Normal | 10 | (76.9) | 12 | (92.3) | 12 | (70.6) | 16 | (94.1) |
| Enopthalmos | 2 | (15.4) | 0 | (0.0) | 4 | (23.5) | 0 | (0.0) |
| Exophthalmos | 1 | (7.7) | 1 | (7.7) | 1 | (5.9) | 1 | (5.9) |
| Total | 13 | (100) | 13 | (100) | 17 | (100) | 17 | (100) |
| Diplopia | ||||||||
| None | 5 | (41.7) | 8 | (66.6) | 5 | (29.4) | 13 | (76.4) |
| Upgaze | 3 | (25.0) | 2 | (16.7) | 3 | (17.7) | 2 | (11.8) |
| Upgaze and other directions | 4 | (33.3) | 2 | (16.7) | 9 | (52.9) | 2 | (11.8) |
| Total | 12 | (100) | 12 | (100) | 17 | (100) | 17 | (100) |
| Motility impairment | ||||||||
| None | 8 | (61.5) | 11 | (84.6) | 8 | (47.0) | 14 | (82.3) |
| Elevation | 3 | (23.1) | 1 | (7.7) | 6 | (35.3) | 1 | (5.9) |
| Elevation and other directions | 2 | (15.4) | 1 | (7.7) | 3 | (17.7) | 2 | (11.8) |
| Total | 13 | (100) | 13 | (100) | 17 | (100) | 17 | (100) |
| Herniation of muscle | ||||||||
| None | 10 | (76.9) | 11 | (64.7) | ||||
| M. rectus inferior | 3 | (23.1) | 6 | (35.3) | ||||
| Total | 13 | (100) | 17 | (100) | ||||
| Herniation of fat tissue | ||||||||
| None | 3 | (23.1) | 5 | (29.4) | ||||
| Yes | 10 | (76.9) | 12 | (70.6) | ||||
| Total | 13 | (100) | 17 | (100) | ||||
| Sensory disturbances N. V2 | ||||||||
| None | 9 | (69.2) | 9 | (69.2) | 7 | (41.2) | 10 | (58.8) |
| Yes | 4 | (30.8) | 4 | (30.8) | 10 | (58.8) | 7 | (41.2) |
| Total | 13 | (100) | 13 | (100) | 17 | (100) | 17 | (100) |
| Variable | Conventional Group | Intervention Group | p-Value * |
|---|---|---|---|
| Post-operative findings, n (%) | |||
| No post-operative diplopia | 8 (38.1) | 13 (61.9) | 0.335 |
| No post-operative motility impairment | 11 (44.0) | 14 (56.0) | 0.439 |
| No post-operative sensory disturbance N. V2 | 9 (47.4) | 10 (52.6) | 0.473 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sigron, G.R.; Barba, M.; Chammartin, F.; Msallem, B.; Berg, B.-I.; Thieringer, F.M. Functional and Cosmetic Outcome after Reconstruction of Isolated, Unilateral Orbital Floor Fractures (Blow-Out Fractures) with and without the Support of 3D-Printed Orbital Anatomical Models. J. Clin. Med. 2021, 10, 3509. https://doi.org/10.3390/jcm10163509
Sigron GR, Barba M, Chammartin F, Msallem B, Berg B-I, Thieringer FM. Functional and Cosmetic Outcome after Reconstruction of Isolated, Unilateral Orbital Floor Fractures (Blow-Out Fractures) with and without the Support of 3D-Printed Orbital Anatomical Models. Journal of Clinical Medicine. 2021; 10(16):3509. https://doi.org/10.3390/jcm10163509
Chicago/Turabian StyleSigron, Guido R., Marina Barba, Frédérique Chammartin, Bilal Msallem, Britt-Isabelle Berg, and Florian M. Thieringer. 2021. "Functional and Cosmetic Outcome after Reconstruction of Isolated, Unilateral Orbital Floor Fractures (Blow-Out Fractures) with and without the Support of 3D-Printed Orbital Anatomical Models" Journal of Clinical Medicine 10, no. 16: 3509. https://doi.org/10.3390/jcm10163509
APA StyleSigron, G. R., Barba, M., Chammartin, F., Msallem, B., Berg, B.-I., & Thieringer, F. M. (2021). Functional and Cosmetic Outcome after Reconstruction of Isolated, Unilateral Orbital Floor Fractures (Blow-Out Fractures) with and without the Support of 3D-Printed Orbital Anatomical Models. Journal of Clinical Medicine, 10(16), 3509. https://doi.org/10.3390/jcm10163509







