Protocol and Evaluation of 3D-Planned Microsurgical and Dental Implant Reconstruction of Maxillary Cleft Critical Size Defects in Adolescents and Young Adults
Abstract
1. Introduction
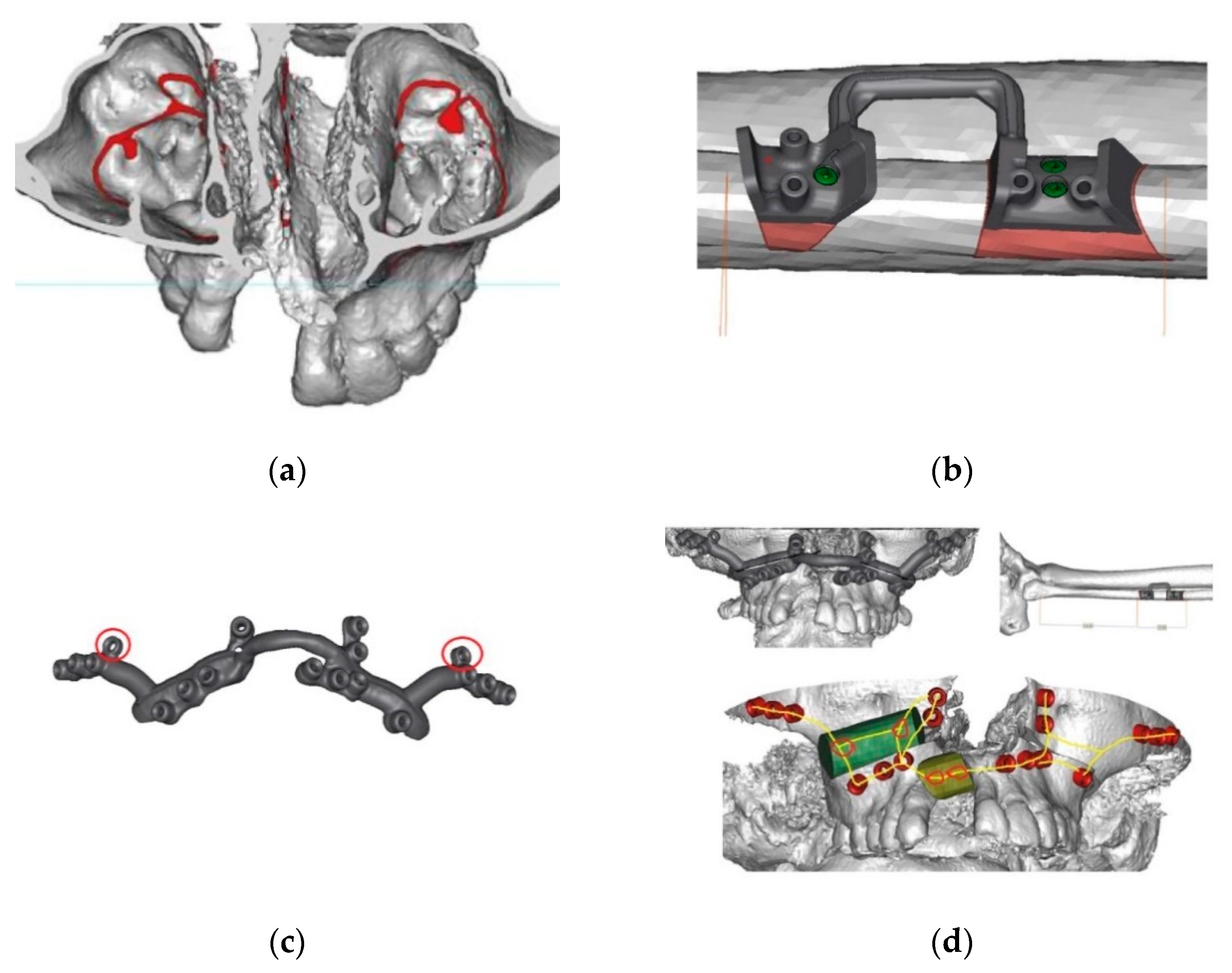
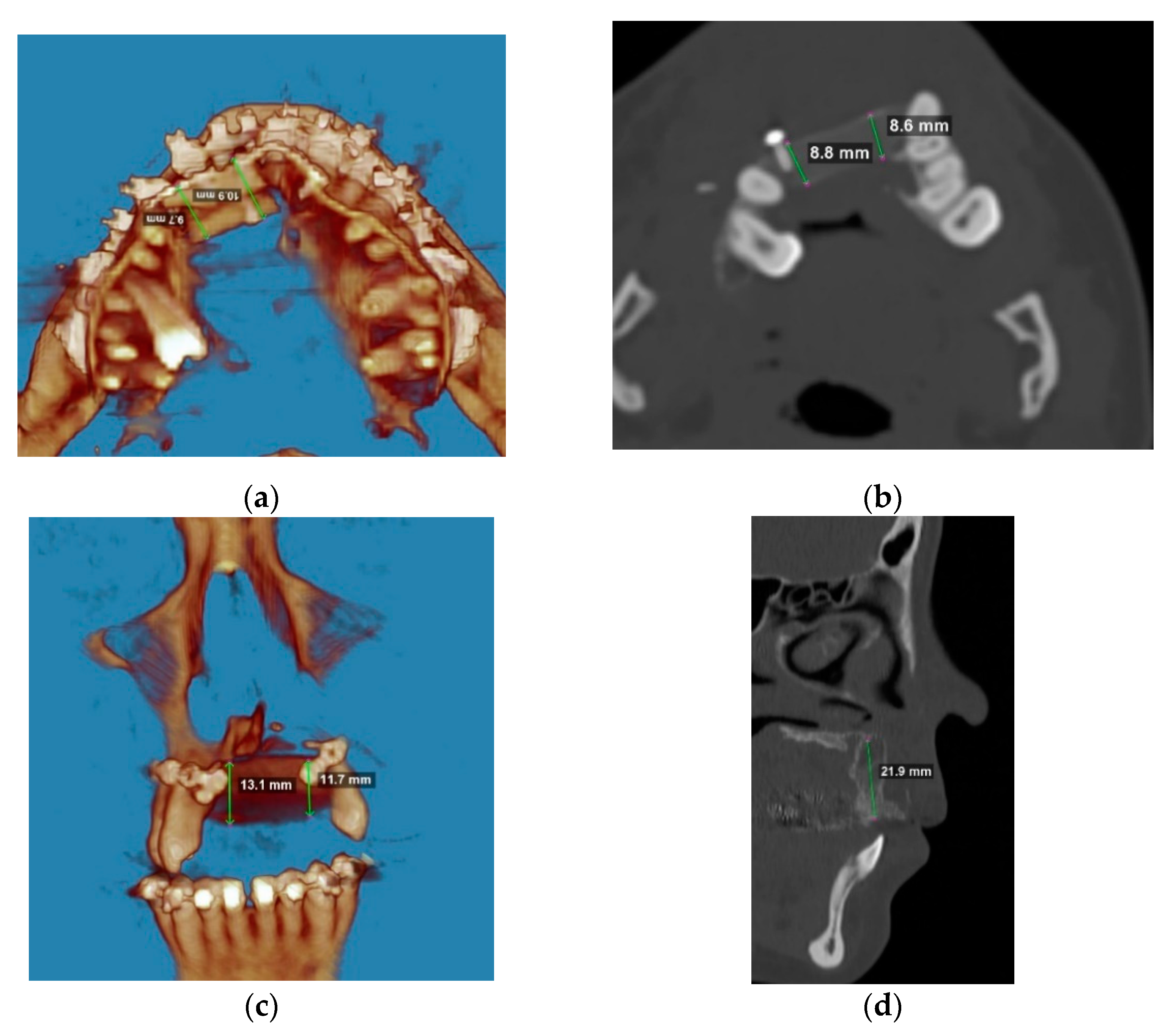
2. Materials and Methods
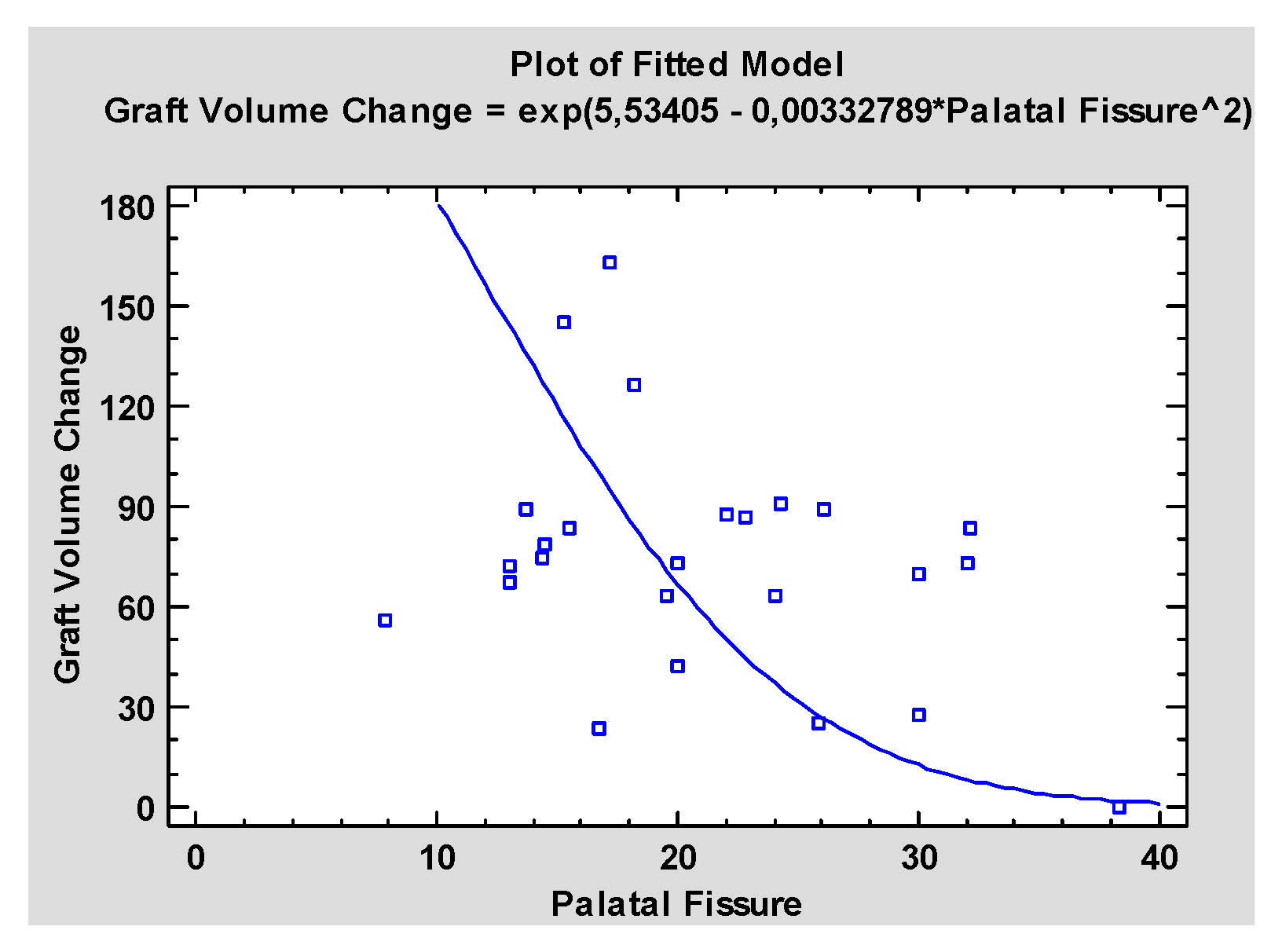
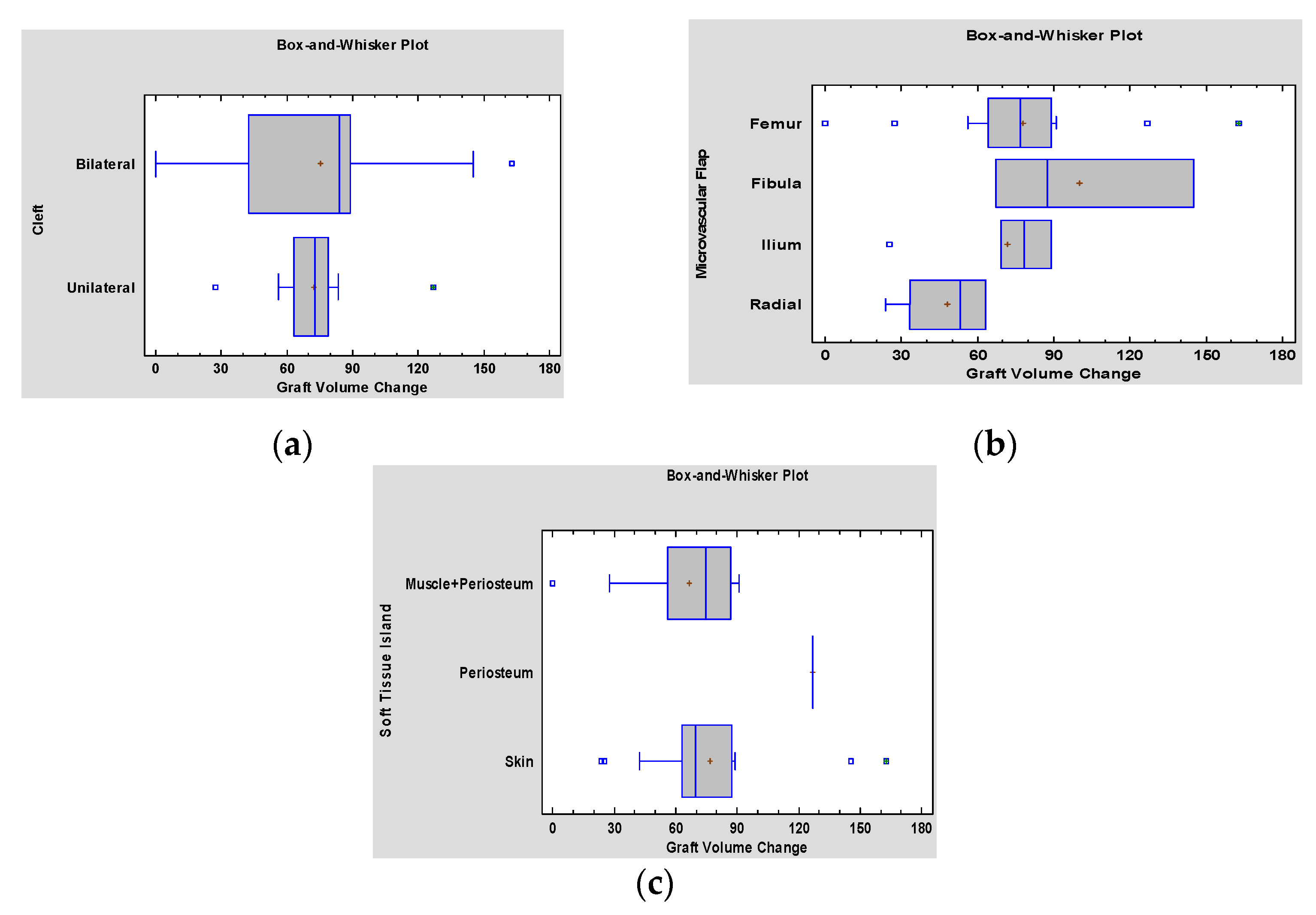
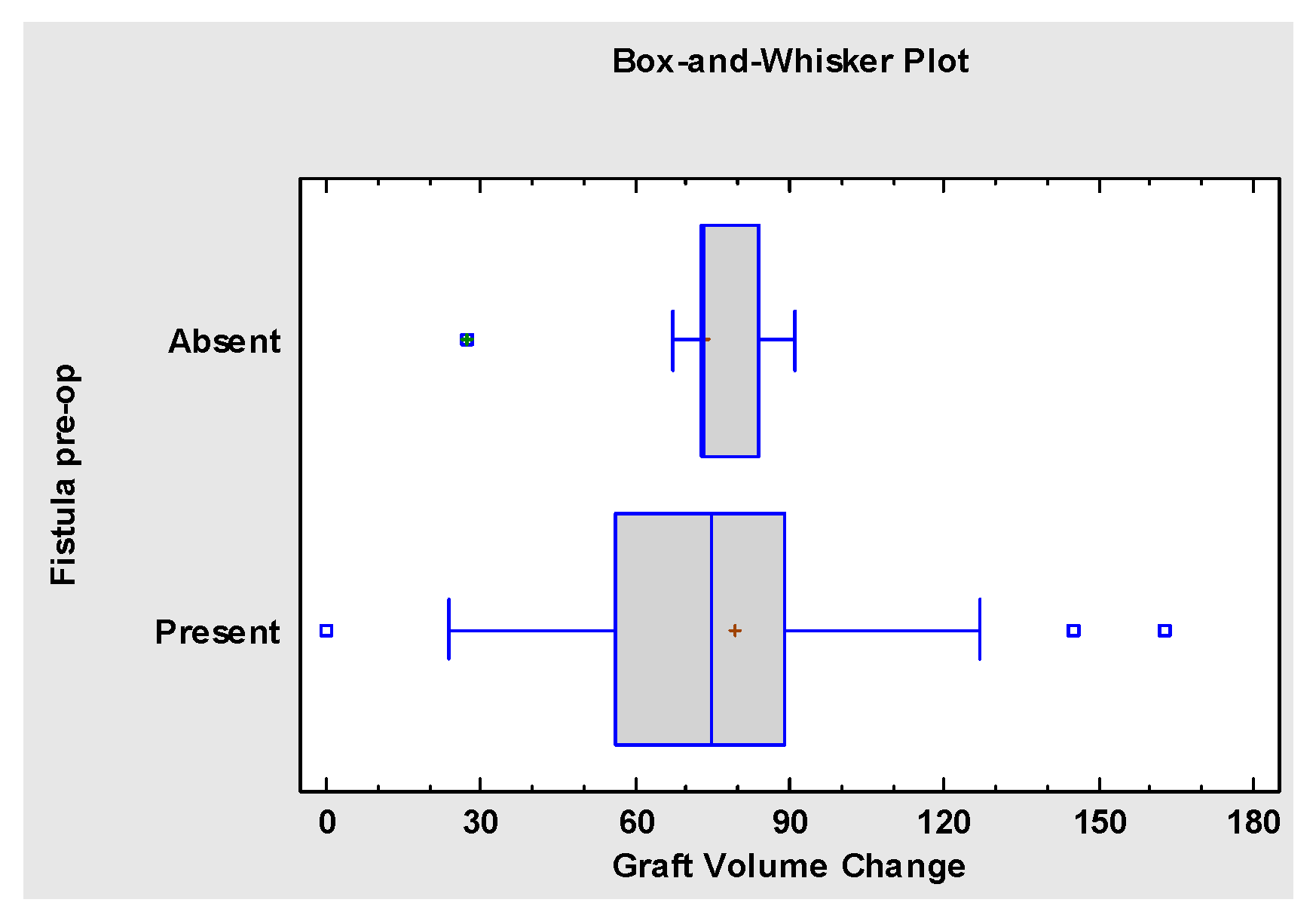
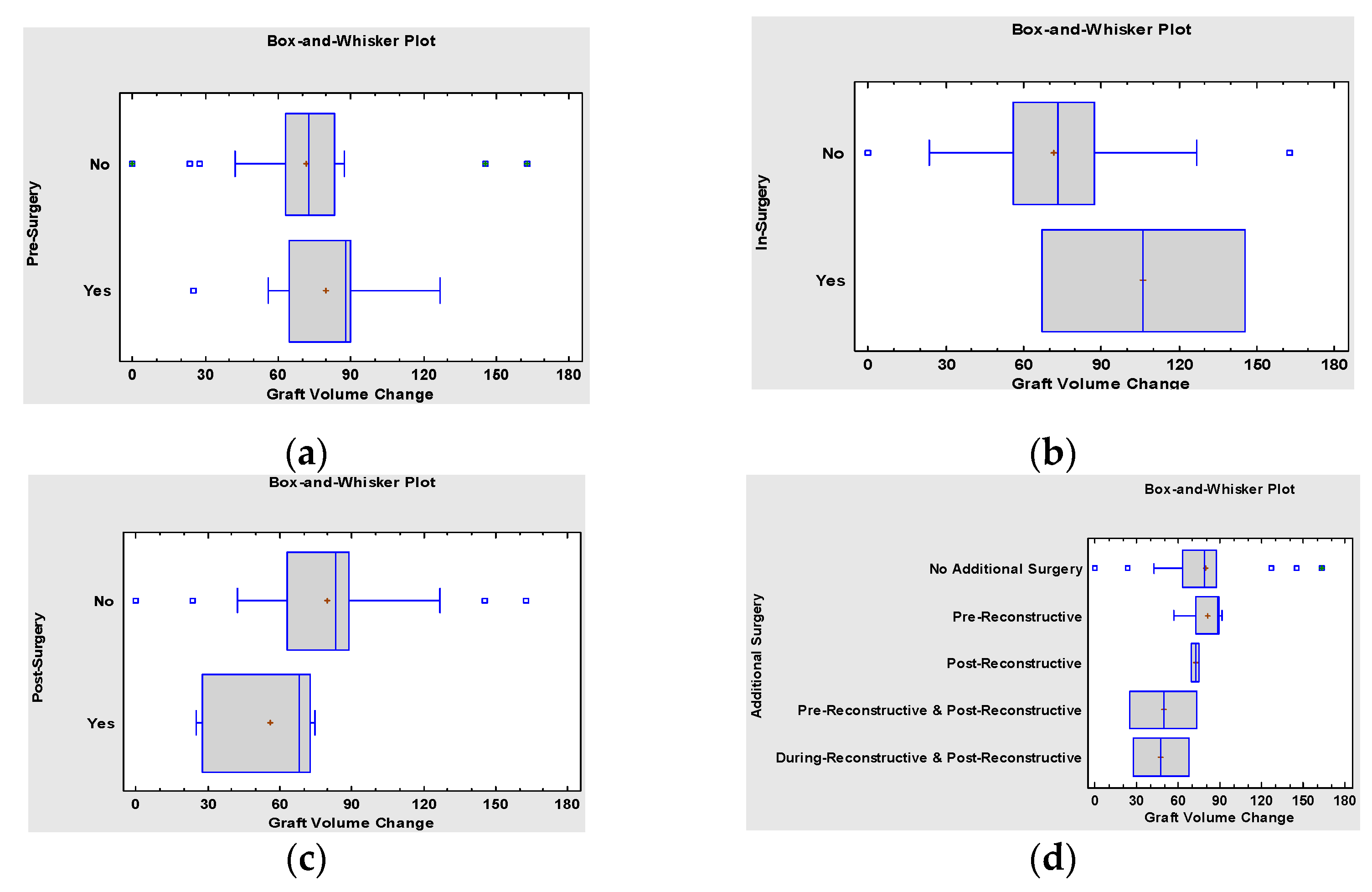
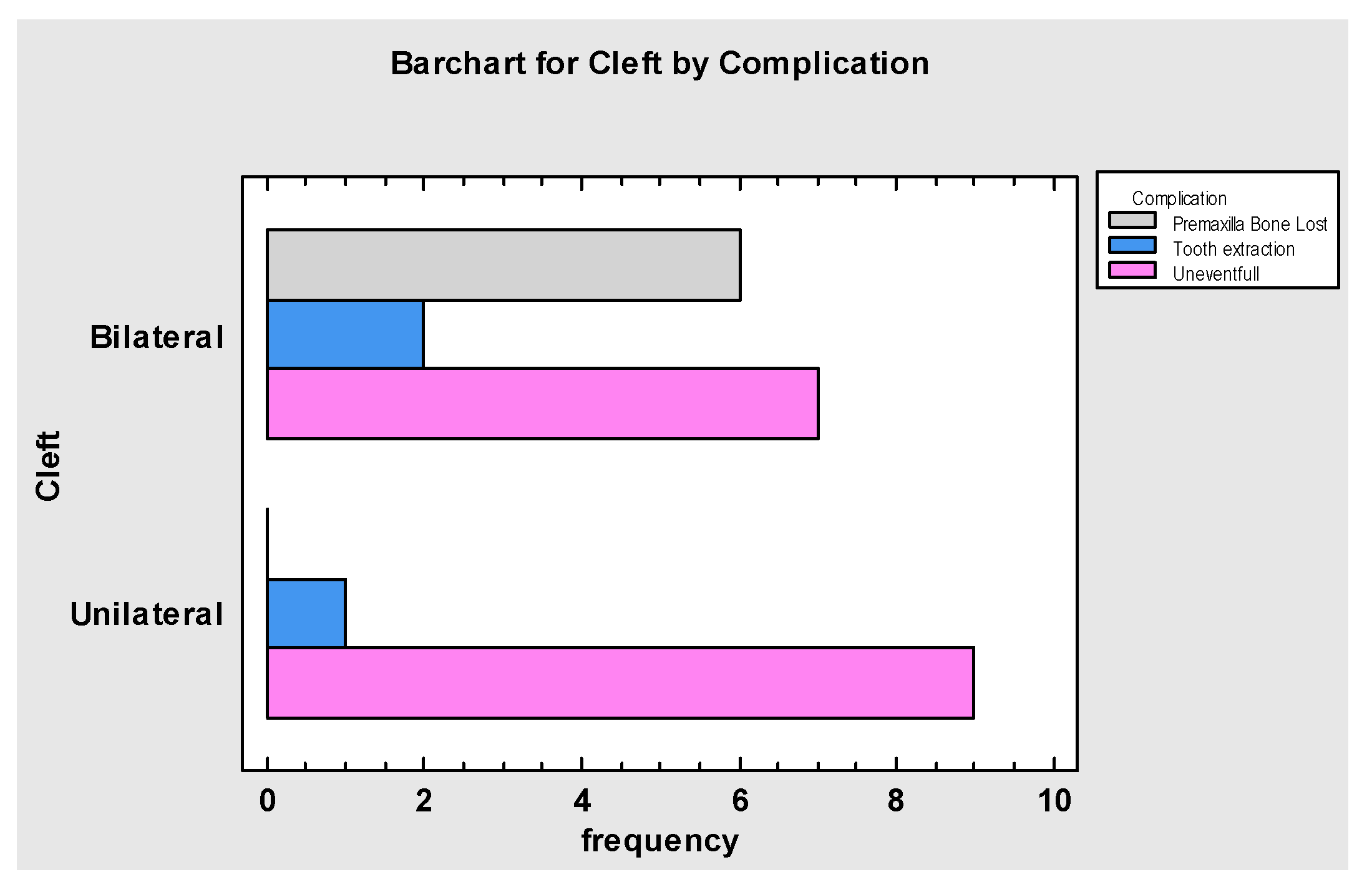
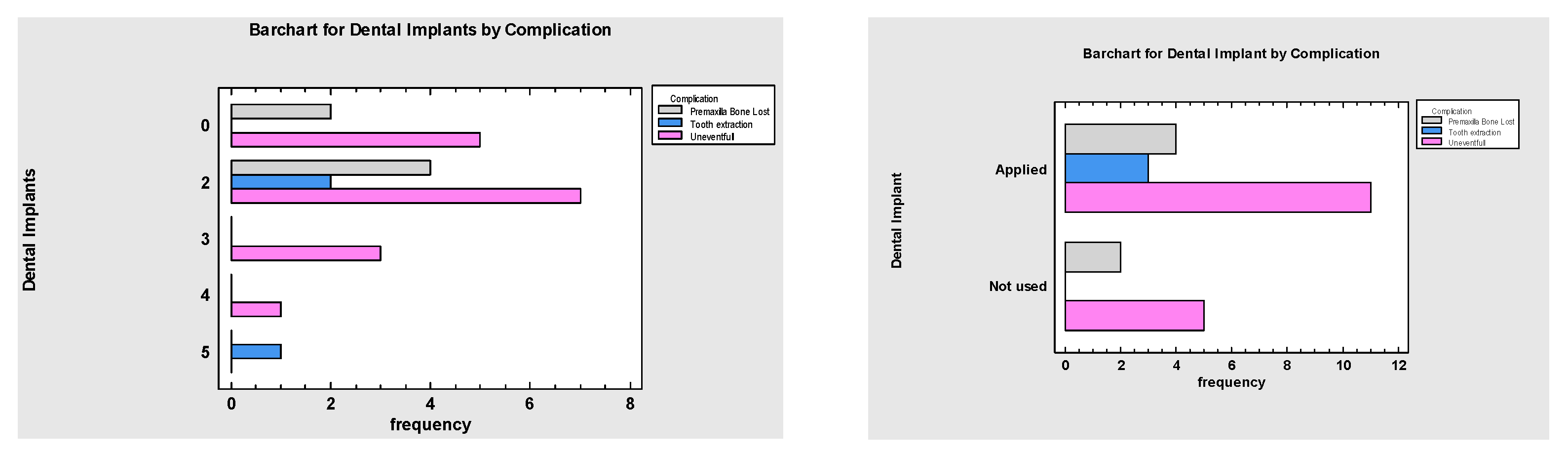
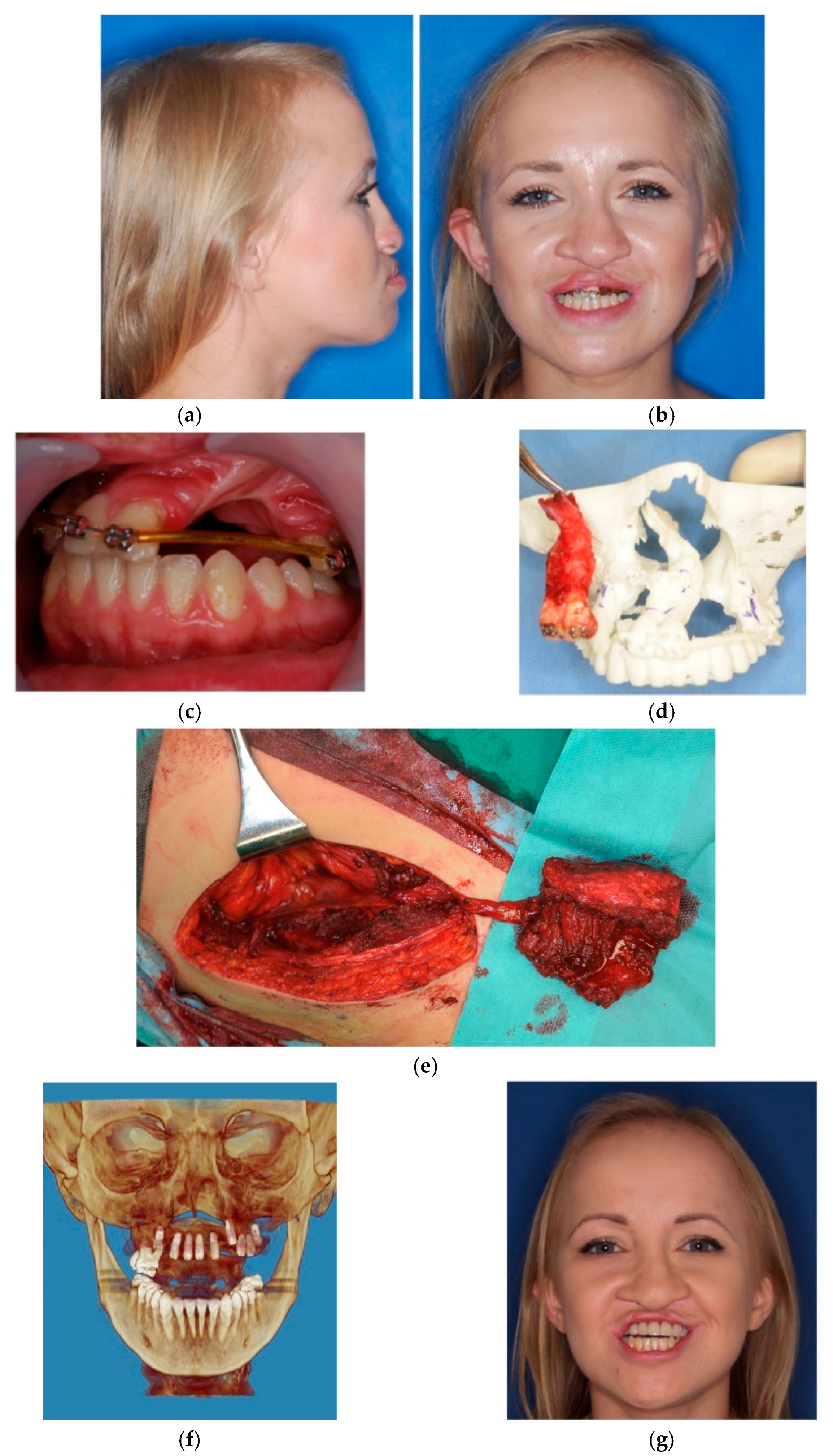
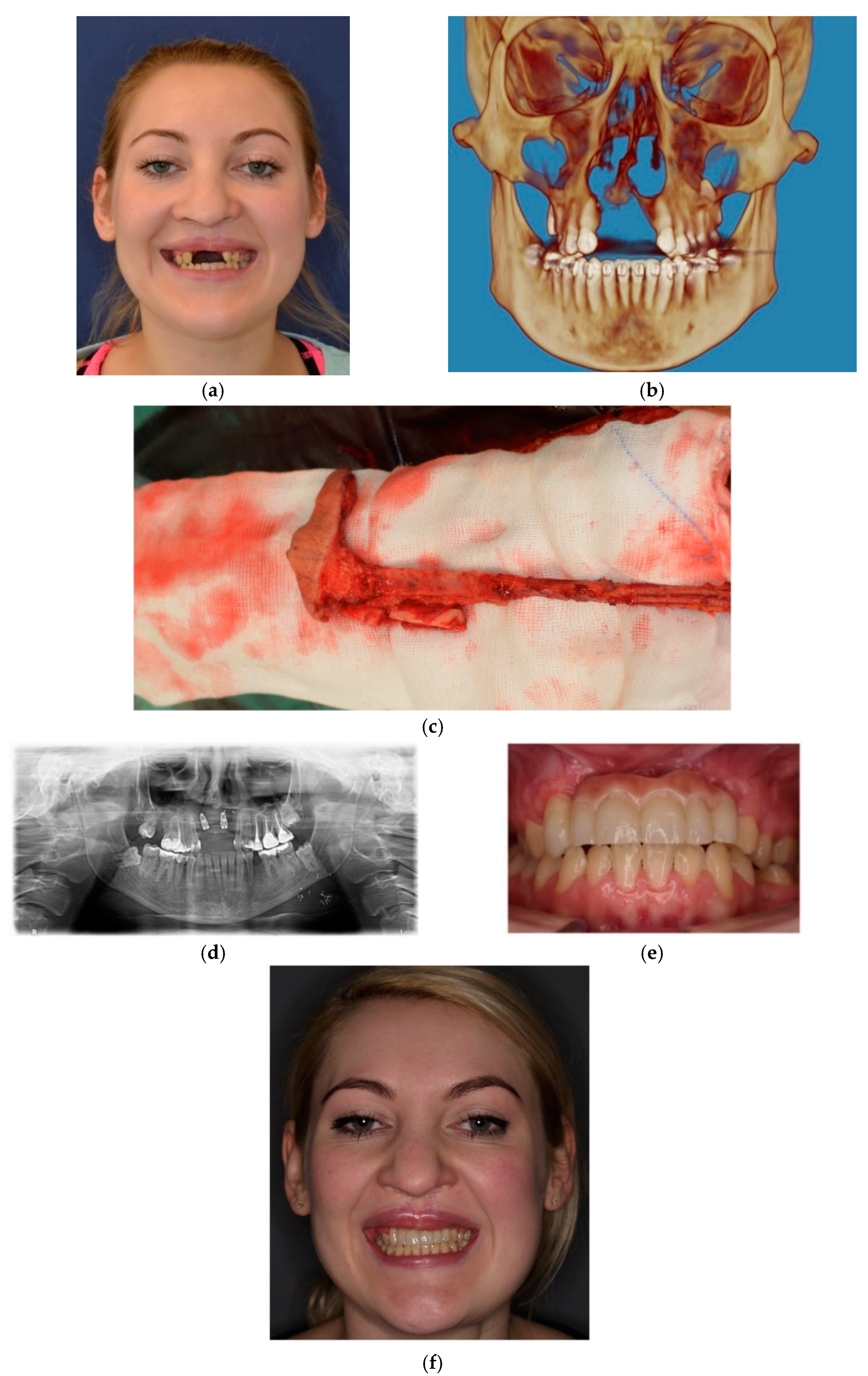
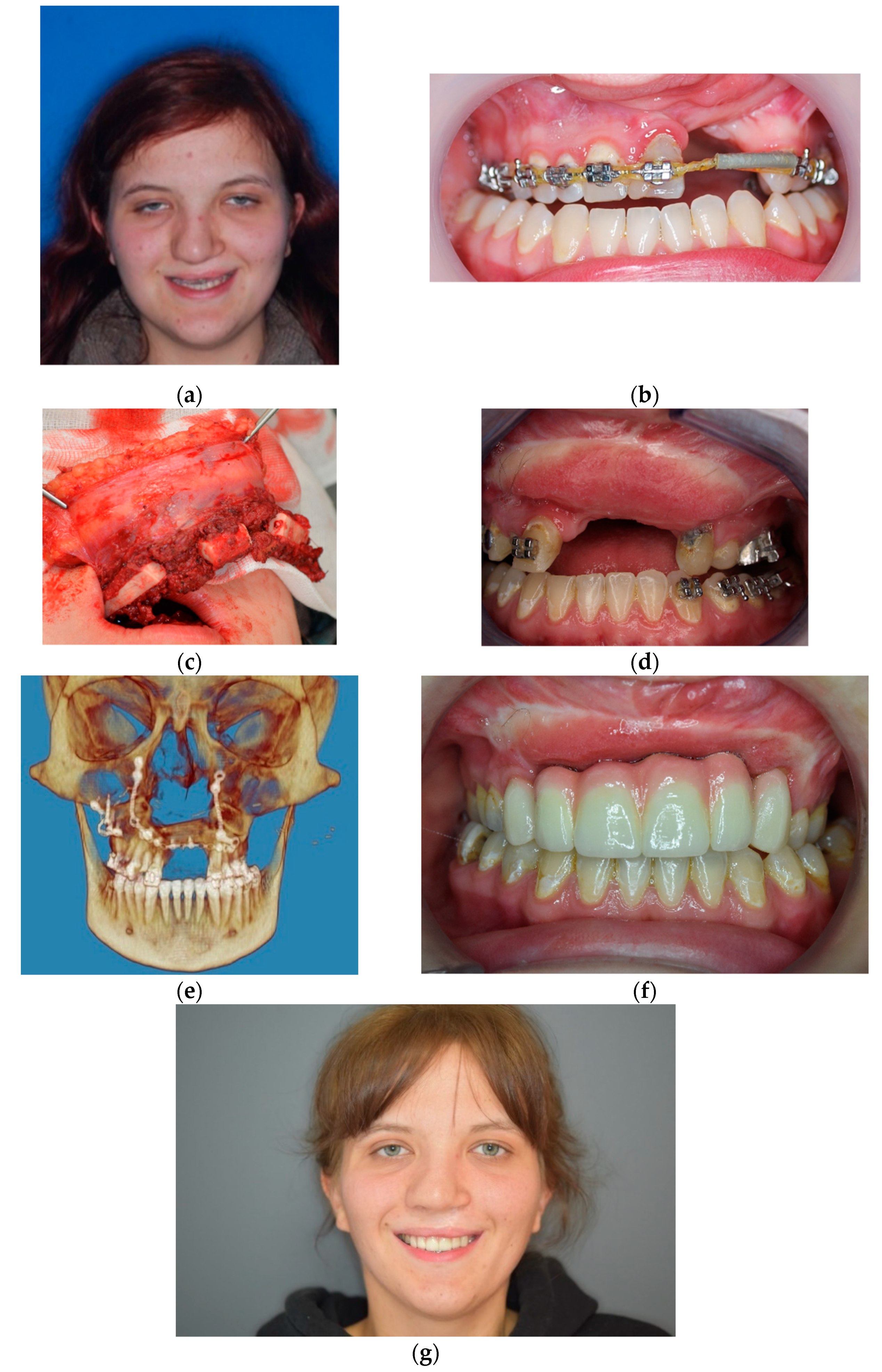
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Agrawal, K. Cleft palate repair and variations. Indian J. Plast. Surg. 2009, 42, S102–S109. [Google Scholar] [CrossRef] [PubMed]
- Cassi, D.; Di Blasio, A.; Gandolfinini, M.; Magnifico, M.; Pellegrino, F.; Piancino, M.G. Dentoalveolar Effects of Early Orthodontic Treatment in Patients With Cleft Lip and Palate. J. Craniofacial Surg. 2017, 28, 2021–2026. [Google Scholar] [CrossRef] [PubMed]
- Coots, B.K. Alveolar Bone Grafting: Past, Present, and New Horizons. Semin. Plast. Surg. 2012, 26, 178–183. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mahajan, R.; Ghildiyal, H.; Khasgiwala, A.; Muthukrishnan, G.; Kahlon, S. Evaluation of Secondary and Late Secondary Alveolar Bone Grafting on 66 Unilateral Cleft Lip and Palate Patients. Plast. Surg. 2017, 25, 194–199. [Google Scholar] [CrossRef]
- Duskova, M.; Kotova, M.; Sedlackova, K.; Leamerova, E.; Horak, J. Bone Reconstruction of the Maxillary Alveolus for Subsequent Insertion of a Dental Implant in Patients with Cleft Lip and Palate. J. Craniofacial Surg. 2007, 18, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Sandor, G.; Carmichael, R.P.; Brkovic, B.M. Dental implants placed into alveolar clefts reconstructed with tongue flaps and bone grafts. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 109, e1–e7. [Google Scholar] [CrossRef]
- Takahashi, T.; Inai, T.; Kochi, S.; Fukuda, M.; Yamaguchi, T.; Matsui, K.; Echigo, S.; Watanabe, M. Long-term follow-up of dental implants placed in a grafted alveolar cleft: Evaluation of alveolar bone height. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 105, 297–302. [Google Scholar] [CrossRef]
- Nawfal, F.; Hicham, B.; Achraf, B.; Rachid, B.; F, N.; B, H. Repair of large palatal fistula using tongue flap. Afr. J. Paediatr. Surg. 2014, 11, 82. [Google Scholar] [CrossRef]
- Shash, H.; Al-Halabi, B.; Jozaghi, Y.; Aldekhayel, S.; Gilardino, M.S. A Review of Tissue Expansion-Assisted Techniques of Cleft Palate Repair. J. Craniofacial Surg. 2016, 27, 760–766. [Google Scholar] [CrossRef]
- Van Damme, P.A.; Freihofer, H.P.M. Palatal Mucoperiosteal Expansion as an Adjunct to Palatal Fistula Repair: Case Report and Review of the Literature. Cleft Palate-Craniofacial J. 1996, 33, 255–257. [Google Scholar] [CrossRef]
- Rachmiel, A. Treatment of Maxillary Cleft Palate: Distraction Osteogenesis Versus Orthognathic Surgery—Part One: Maxillary Distraction. J. Oral Maxillofac. Surg. 2007, 65, 753–757. [Google Scholar] [CrossRef]
- Cheung, L.; Chua, H. A meta-analysis of cleft maxillary osteotomy and distraction osteogenesis. Int. J. Oral Maxillofac. Surg. 2006, 35, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Zemann, W.; Kruse, A.L.; Lüebbers, H.T.; Jacobsen, C.; Metzler, P.; Obwegeser, J.A. Microvascular Tissue Transfer in Cleft Palate Patients. J. Craniofacial Surg. 2011, 22, 2006–2010. [Google Scholar] [CrossRef] [PubMed]
- Liou, E.J.W.; Chen, P.K.T.; Huang, C.S.; Chen, Y.R. Interdental Distraction Osteogenesis and Rapid Orthodontic Tooth Movement: A Novel Approach to Approximate a Wide Alveolar Cleft or Bony Defect. Plast. Reconstr. Surg. 2000, 105, 1262–1272. [Google Scholar] [CrossRef] [PubMed]
- Dolanmaz, D.; Karaman, A.I.; Durmus, E.; Malkoc, S. Management of alveolar clefts using dento-osseous transport distraction osteogenesis. Angle Orthod. 2003, 73, 723–729. [Google Scholar] [PubMed]
- Dowgierd, K.; Pokrowiecki, R.; Borowiec, M.; Kozakiewicz, M.; Smyczek, D.; Krakowczyk, Ł. A Protocol for the Use of a Combined Microvascular Free Flap with Custom-Made 3D-Printed Total Temporomandibular Joint (TMJ) Prosthesis for Mandible Reconstruction in Children. Appl. Sci. 2021, 11, 2176. [Google Scholar] [CrossRef]
- Pradel, W.; Senf, D.; Mai, R.; Ludicke, G.; Eckelt, U.; Lauer, G. One-stage palate repair improves speech outcome and early maxillary growth in patients with cleft lip and palate. J. Physiol. Pharmacol. 2009, 60, 37–41. [Google Scholar]
- Belser, U.C.; Schmid, B.; Higginbottom, F.; Buser, D. Outcome analysis of implant restorations located in the anterior maxilla: A review of the recent literature. Int. J. Oral Maxillofac. Implant. 2004, 19, 30–42. [Google Scholar]
- Borba, A.M.; Borges, A.H.; da Silva, C.S.V.; Brozoski, M.A.; Naclério-Homem, M.D.G.; Miloro, M. Predictors of complication for alveolar cleft bone graft. Br. J. Oral Maxillofac. Surg. 2014, 52, 174–178. [Google Scholar] [CrossRef]
- Alexandra, M.P.E.; Andre, B.; Priscila, L.C.; Patricia, N.T. Alveolar Bone Graft: Clinical Profile and Risk Factors for Complications in Oral Cleft Patients. Cleft Palate-Craniofacial J. 2017, 54, 530–534. [Google Scholar] [CrossRef]
- Dowgierd, K.; Krakowczyk, Ł. The use of microsurgical reconstruction in treatment of craniofacial defects in paediatric patients. Int. J. Oral Maxillofac. Surg. 2019, 48, 136–137. [Google Scholar] [CrossRef]
- Shahzad, F. Pediatric Mandible Reconstruction: Controversies and Considerations. Plast. Reconstr. Surg. Glob. Open 2020, 8, e3285. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.; Jackson, I.T. Microvascular surgery as an adjunct to craniomaxillofacial reconstruction. Br. J. Plast. Surg. 1989, 42, 146–154. [Google Scholar] [CrossRef]
- Futran, N.D. Retrospective case series of primary and secondary microvascular free tissue transfer reconstruction of midfacial defects. J. Prosthet. Dent. 2001, 86, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Ninkovic, M.; Hubli, E.H.; Schwabegger, A.; Anderl, H. Free Flap Closure of Recurrent Palatal Fistula in the Cleft Lip and Palate Patient. J. Craniofacial Surg. 1997, 8, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Monasterio, F.O.; Santamaría, E.; Morales, D.; Morales, C.; Yudovich, M.; Ramos, F.S. Reconstruction of the Premaxilla. J. Craniofacial Surg. 2009, 20, 1768–1770. [Google Scholar] [CrossRef]
- Holmes, J.D.; Aponte-Wesson, R. Dental Implants After Reconstruction with Free Tissue Transfer. Oral Maxillofac. Surg. Clin. N. Am. 2010, 22, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Urken, M.L.; Buchbinder, D.; Costantino, P.D.; Sinha, U.; Okay, D.; Lawson, W.; Biller, H.F. Oromandibular Reconstruction Using Microvascular Composite Flaps. Arch. Otolaryngol. Head Neck Surg. 1998, 124, 46–55. [Google Scholar] [CrossRef]
- Batchelor, A.; Palmer, J. A novel method of closing a palatal fistula: The free fascial flap. Br. J. Plast. Surg. 1990, 43, 359–361. [Google Scholar] [CrossRef]
- Chen, H.-C.; Ganos, D.L.; Coessens, B.C.; Kyutoku, S.; Noordhoff, M.S. Free Forearm Flap for Closure of Difficult Oronasal Fistulas in Cleft Palate Patients. Plast. Reconstr. Surg. 1992, 90, 757–762. [Google Scholar] [CrossRef]
- Barabás, J.; Szabó, G. Closure of cleft palate in adult patients, using a forearm flap with microvascular radial artery II. Fogorvosi Szle. 1993, 86, 71–76. [Google Scholar]
- MacLeod, A.; Morrison, W.; McCann, J.; Thistlethwaite, S.; VanderKolk, C.; Ryan, A. The free radial forearm flap with and without bone for closure of large palatal fistulae. Br. J. Plast. Surg. 1987, 40, 391–395. [Google Scholar] [CrossRef]
- Millesi, W.; Rath, T.; Millesi-Schobel, G.; Glaser, C. Reconstruction of the floor of the mouth with a fascial radial forearm flap, prelaminated with autologous mucosa. Int. J. Oral Maxillofac. Surg. 1998, 27, 106–110. [Google Scholar] [CrossRef]
- Kim, G.G.; Halvorson, E.G.; Hang, A.X.; Pederson, W.C.; De Santis, G.; Hackman, T.G. Prelamination of Radial Forearm Free Flap with Buccal Mucosa. Otolaryngol. Neck Surg. 2012, 148, 341–343. [Google Scholar] [CrossRef]
- Kelly, C.P.; Moreira-Gonzalez, A.; Ali, M.A.; Topf, J.; Persiani, R.J.; Jackson, I.T. Vascular Iliac Crest With Inner Table of the Ilium as an Option in Maxillary Reconstruction. J. Craniofacial Surg. 2004, 15, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Mücke, T.; Hölzle, F.; Loeffelbein, D.J.; Ljubic, A.; Kesting, M.; Wolff, K.-D.; Mitchell, D.A. Maxillary reconstruction using microvascular free flaps. Oral Surgery Oral Med. Oral Pathol. Oral Radiol. Endodontology 2011, 111, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Kademani, D.; Salinas, T.; Moran, S.L. Medial Femoral Periosteal Microvascular Free Flap: A New Method for Maxillary Reconstruction. J. Oral Maxillofac. Surg. 2009, 67, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Gaggl, A.; Bürger, H.; Chiari, F.M. The Microvascular Osteocutaneous Femur Transplant for Covering Combined Alveolar Ridge and Floor of the Mouth Defects: Preliminary Report. J. Reconstr. Microsurg. 2008, 24, 169–175. [Google Scholar] [CrossRef]
- Werle, A.H.; Tsue, T.T.; Toby, E.B.; Girod, D.A. Osteocutaneous radial forearm free flap: Its use without significant donor site morbidity. Otolaryngol. Neck Surg. 2000, 123, 711–717. [Google Scholar] [CrossRef]
- Landes, C.; Korzinskas, T.; Dehner, J.-F.; Santo, G.; Ghanaati, S.; Sader, R. One-stage microvascular mandible reconstruction and alloplastic TMJ prosthesis. J. Cranio-Maxillofacial Surg. 2014, 42, 28–34. [Google Scholar] [CrossRef]
- Ni, Y.; Lu, P.; Yang, Z.; Wang, W.; Dai, W.; Qi, Z.-Z.; Duan, W.; Xu, Z.-F.; Sun, C.-F.; Liu, F. The application of fibular free flap with flexor hallucis longus in maxilla or mandible extensive defect: A comparison study with conventional flap. World J. Surg. Oncol. 2018, 16, 149. [Google Scholar] [CrossRef] [PubMed]
- Weitz, J.S.; Kreutzer, K.; Bauer, F.; Wolff, K.-D.; Nobis, C.; Kesting, M.R. Sandwich flaps as a feasible solution for the management of huge mandibular composite tissue defects. J. Cranio-Maxillofacial Surg. 2015, 43, 1769–1775. [Google Scholar] [CrossRef]
- Schmelzeisen, R.; Schliephake, H. Interdisciplinary microvascular reconstruction of maxillary, midfacial and skull base defects. J. Cranio-Maxillofacial Surg. 1998, 26, 1–10. [Google Scholar] [CrossRef]
- Turk, A.E.; Chang, J.; Soroudi, E.A.; Hui, K.; Lineaweaver, W.C. Free Flap Closure in Complex Congenital and Acquired Defects of the Palate. Ann. Plast. Surg. 2000, 45, 274–279. [Google Scholar] [CrossRef]



| Gender | Age [Year] | Diagnosis | Malignancy | Mandibulectomy Range | TMJ Replacement | Reconstruction Type | Follow-up [Years] | Additional Surgery | Final Treatment |
|---|---|---|---|---|---|---|---|---|---|
| Male | 8 | Fibroma Ossificans | Benign | Ramus only | Unilateral | ICFF | 5 | None | Ortodontic |
| Male | 8 | Fibrous Displasia | Benign | Ramus only | Unilateral | ICFF | 5 | None | Ortodontic |
| Female | 10 | Sarcoma | Cancer | Ramus & Body | Unilateral | FFF | 4 | None | Ortodontic & Dental Implants |
| Male | 11 | Sarcoma | Cancer | Ramus & Body | Unilateral | FFF | 6 | None | Ortodontic |
| Female | 12 | Severe Deformation | Benign | Ramus & Body | Bilateral | FFF | 4 | Ortognathic | Ortodontic |
| Male | 13 | Fibrous Displasia | Benign | Ramus & Body | Bilateral | FFF | 2 | None | Ortodontic |
| Male | 13 | Severe Deformation | Benign | Ramus & Body | Unilateral | FFF | 5 | Ortognathic | Ortodontic & Dental Implants |
| Male | 13 | Sarcoma | Cancer | Ramus & Body | Unilateral | FFF | 5 | None | Ortodontic |
| Male | 13 | Sarcoma | Cancer | Ramus & Body | Unilateral | ICFF | 5 | Reconstructive | Ortodontic & Dental Implants |
| Male | 14 | Amelobastoma | Benign | Ramus & Body | Unilateral | FFF | 4 | None | Ortodontic & Dental Implants |
| Male | 15 | Fibrous Displasia | Benign | Ramus & Body | Unilateral | FFF | 2 | None | Ortodontic |
| Female | 15 | Amelobastoma | Benign | Ramus & Body | Unilateral | FFF | 5 | Reconstructive | Ortodontic & Dental Implants |
| Female | 17 | Amelobastoma | Benign | Ramus & Body | Unilateral | ICFF | 5 | Ortognathic | Ortodontic & Dental Implants |
| Male | 14 | Central Cell Giant Granuloma | Benign | Ramus & Body | Unilateral | FFF | 4 | None | Ortodontic & Dental Implants |
| Gender | Age [Years] | Mandibulectomy Range | TMJ Replacement | Reconstruction Type | MIOpre [mm] | MIOpost [mm] | SNBpre [deg.] | SNBpost [deg.] | Ramus OP [mm] | Ramus OP’ [mm] | Body OP [mm] | Body OP’ [mm] | Ramus C [mm] | Ramus C’ [mm] | Body C [mm] | Body C’ [mm] | Assymetry [mm] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | 8 | Ramus only | Unilateral | f_Iliac_F | 5 | 30 | 69.2 | 72.5 | 19.3 | 20.7 | 47.3 | 50 | 52 | 54 | 47.2 | 51 | 6 |
| Male | 8 | Ramus only | Unilateral | f_Iliac_F | 10 | 35 | 77 | 74 | 28.7 | 28.7 | 68.4 | 68.4 | 49 | 51 | 56 | 57 | 0 |
| Female | 10 | Ramus & Body | Unilateral | f_Fibula_F | 0.5 | 30 | 77.2 | 74.7 | 41 | 43.3 | 89 | 91 | 58 | 65 | 68 | 70 | 0 |
| Male | 11 | Ramus & Body | Unilateral | f_Fibula_F | 5 | 35 | 78.5 | 79 | 43 | 43.5 | 63.8 | 64 | 50 | 53 | 53 | 56 | 0 |
| Female | 12 | Ramus & Body | Bilateral | f_Fibula_F | 25 | 35 | 56 | 82.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 0 |
| Male | 13 | Ramus & Body | Bilateral | f_Fibula_F | 5 | 35 | 74 | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 3 |
| Male | 13 | Ramus & Body | Unilateral | f_Fibula_F | 0.5 | 35 | 69.1 | 75.5 | 45.8 | 51.8 | 50.5 | 58.6 | 52.7 | 53 | 54.8 | 61 | 5 |
| Male | 13 | Ramus & Body | Unilateral | f_Fibula_F | 15 | 35 | 77.2 | 75.4 | 29.9 | 31 | 35.4 | 37.5 | 56 | 57.5 | 70 | 77.4 | 5 |
| Male | 13 | Ramus & Body | Unilateral | f_Iliac_F | 40 | 40 | 65 | 73.1 | 34 | 42.2 | 41 | 42.9 | 51 | 56 | 67 | 70 | 0 |
| Male | 14 | Ramus & Body | Unilateral | f_Fibula_F | 10 | 40 | 64 | 76 | 37 | 39 | 98 | 99 | 47.7 | 56 | 62.1 | 72 | 4 |
| Male | 15 | Ramus & Body | Unilateral | f_Fibula_F | 10 | 35 | 72 | 74.4 | 41.6 | 43 | 27.5 | 27.5 | 45 | 56 | 49 | 72 | 6 |
| Female | 15 | Ramus & Body | Unilateral | f_Fibula_F | 10 | 45 | 69.4 | 71 | 32.3 | 37.2 | 56.3 | 61.5 | 53 | 61 | 64 | 73 | 7 |
| Female | 17 | Ramus & Body | Unilateral | f_Iliac_F | 35 | 45 | 68 | 74 | 30.3 | 30.5 | 30 | 29 | 48 | 58 | 49 | 61 | 0 |
| Male | 14 | Ramus & Body | Unilateral | f_Fibula_F | 10 | 40 | 71 | 74 | 35 | 38.6 | 31.1 | 47.2 | 55 | 59 | 60 | 67 | 4 |
| Type of Flap | Advantages | Disadvantages | Indications |
|---|---|---|---|
| Radial forearm flap (RFF) |
|
|
|
| Medial femoral condyle free flap (MFCFF) |
|
|
|
| Iliac crest free flap (ICF) |
|
|
|
| Fibula free flap (FFF) |
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dowgierd, K.; Pokrowiecki, R.; Borowiec, M.; Sokolowska, Z.; Dowgierd, M.; Wos, J.; Kozakiewicz, M.; Krakowczyk, Ł. Protocol and Evaluation of 3D-Planned Microsurgical and Dental Implant Reconstruction of Maxillary Cleft Critical Size Defects in Adolescents and Young Adults. J. Clin. Med. 2021, 10, 2267. https://doi.org/10.3390/jcm10112267
Dowgierd K, Pokrowiecki R, Borowiec M, Sokolowska Z, Dowgierd M, Wos J, Kozakiewicz M, Krakowczyk Ł. Protocol and Evaluation of 3D-Planned Microsurgical and Dental Implant Reconstruction of Maxillary Cleft Critical Size Defects in Adolescents and Young Adults. Journal of Clinical Medicine. 2021; 10(11):2267. https://doi.org/10.3390/jcm10112267
Chicago/Turabian StyleDowgierd, Krzysztof, Rafał Pokrowiecki, Maciej Borowiec, Zuzanna Sokolowska, Martyna Dowgierd, Jan Wos, Marcin Kozakiewicz, and Łukasz Krakowczyk. 2021. "Protocol and Evaluation of 3D-Planned Microsurgical and Dental Implant Reconstruction of Maxillary Cleft Critical Size Defects in Adolescents and Young Adults" Journal of Clinical Medicine 10, no. 11: 2267. https://doi.org/10.3390/jcm10112267
APA StyleDowgierd, K., Pokrowiecki, R., Borowiec, M., Sokolowska, Z., Dowgierd, M., Wos, J., Kozakiewicz, M., & Krakowczyk, Ł. (2021). Protocol and Evaluation of 3D-Planned Microsurgical and Dental Implant Reconstruction of Maxillary Cleft Critical Size Defects in Adolescents and Young Adults. Journal of Clinical Medicine, 10(11), 2267. https://doi.org/10.3390/jcm10112267








