Exercise versus Metformin to Improve Pregnancy Outcomes among Overweight Pregnant Women: A Systematic Review and Network Meta-Analysis
Abstract
1. Introduction
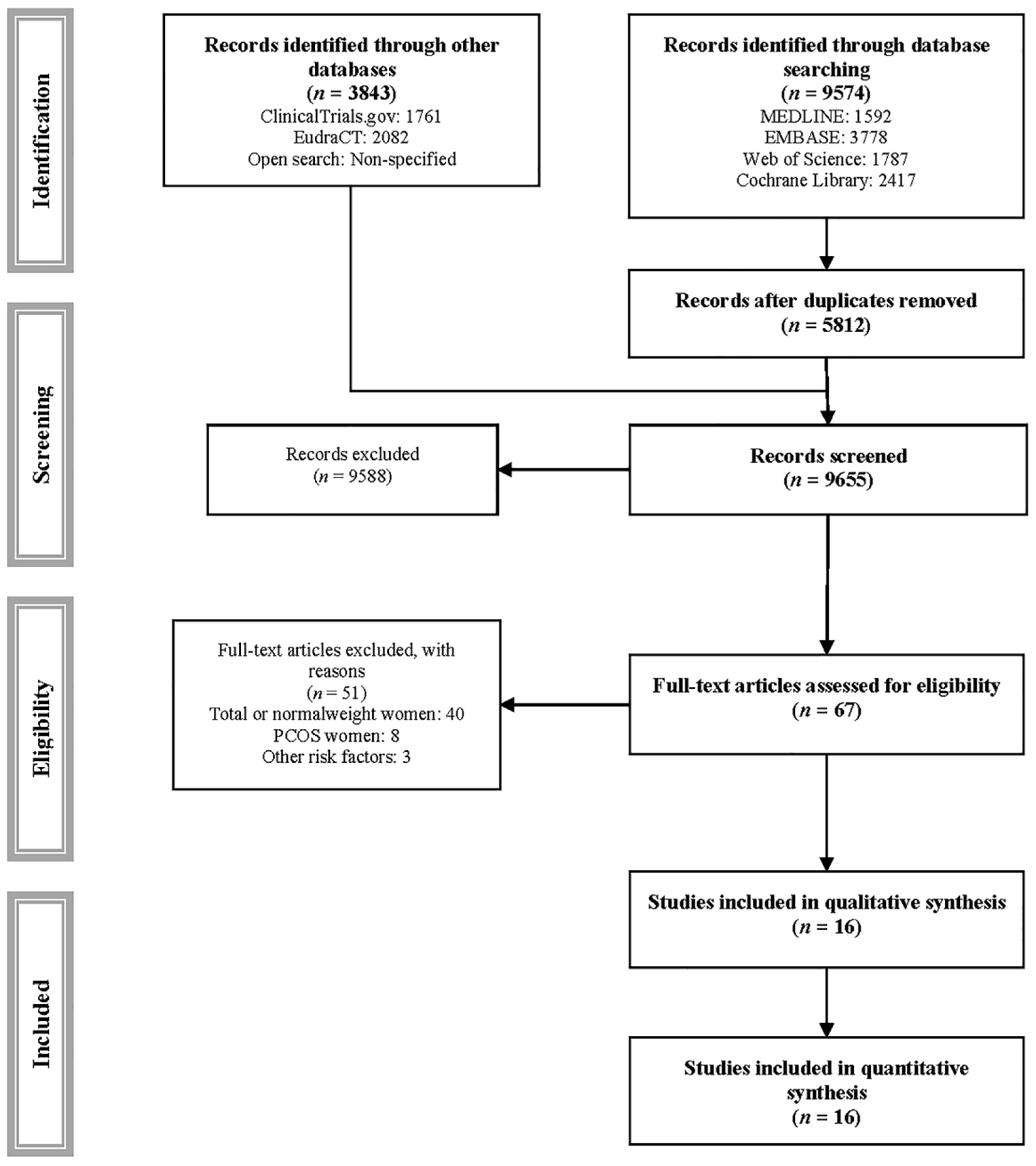
2. Materials and Methods
2.1. Search Strategy
2.2. Elegibility
2.3. Data Extraction
2.4. Categorization of Available Evidence
2.5. Risk of Bias Assessment
2.6. Grading of Quality of Evidence
2.7. Data Synthesis
2.8. Modifications to the Initial Protocol
3. Results
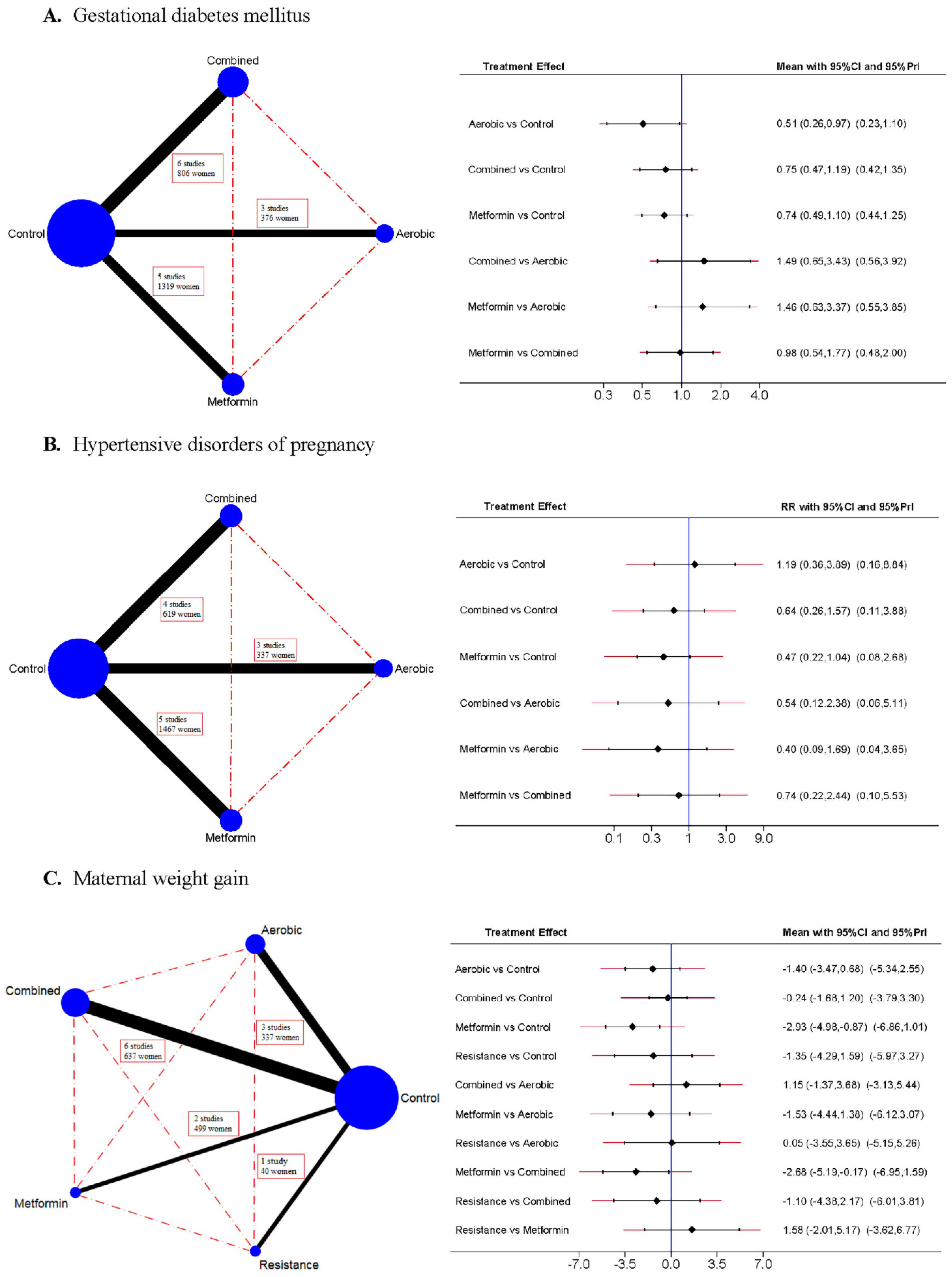
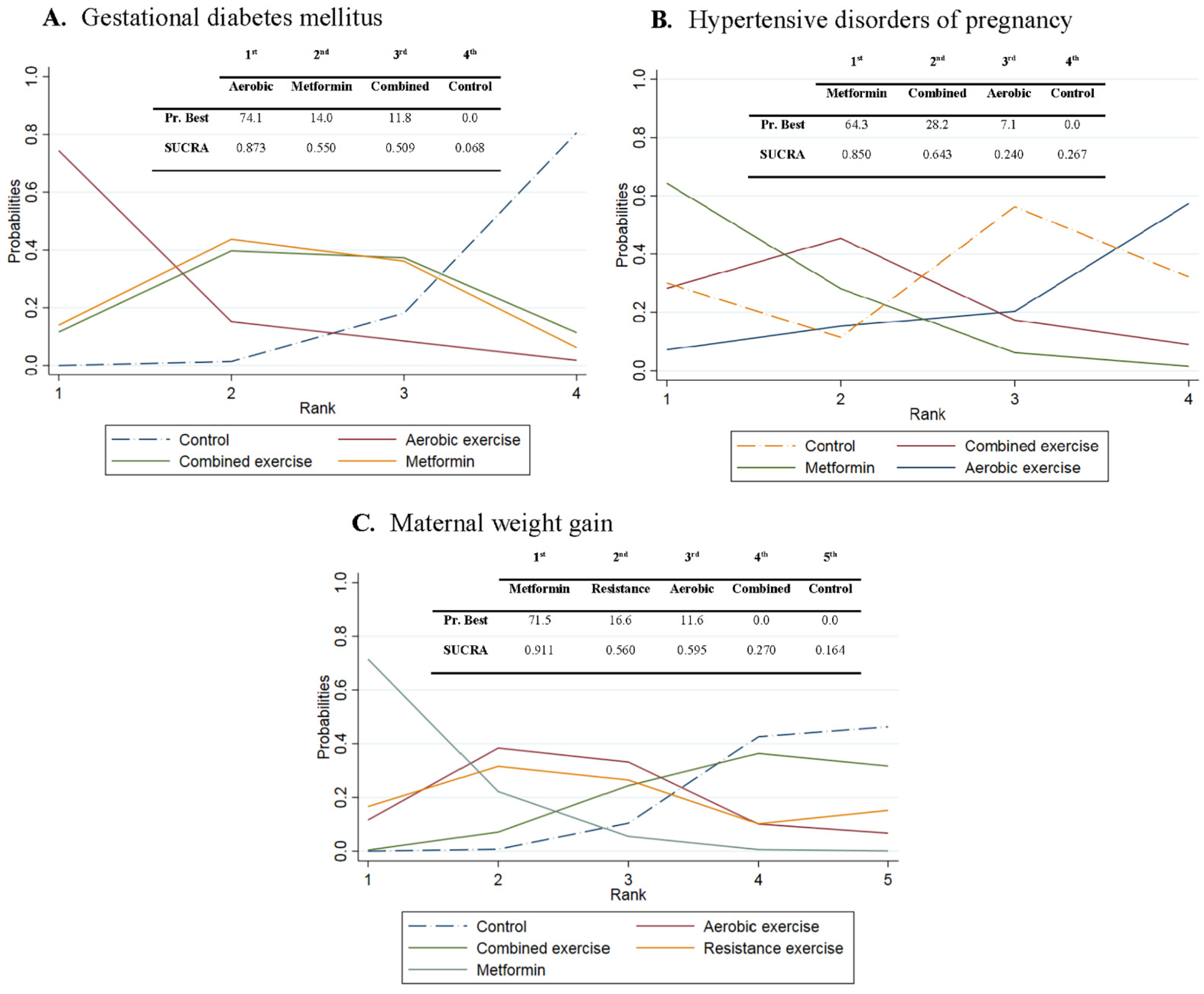
3.1. Gestational Diabetes Mellitus
3.2. Hypertensive Disorders of Pregnancy
3.3. Maternal Weight Gain
3.4. Risk of Bias
3.5. Grades of Recommendation, Assessment, Development, and Evaluation
3.6. Transitivity
3.7. Probabilities
3.8. Subgroup Analysis among Pregnant Women with Obesity
3.9. Sensitivity and Metarregresion Analyses
3.10. Heterogeneity and Publication Bias
4. Discussion
4.1. Main Findings
4.2. Interpretation
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Medline, EMBASE, Web of Science, Cochrane Library
Appendix A.2. NCT Trials
Appendix A.3. EudraCT
Appendix A.4. Other Databases
References
- Flegal, K.M.; Carroll, D.; Kit, B.K.; Ogden, C.L. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA J. Am. Med. Assoc. 2012, 307, 491–497. [Google Scholar] [CrossRef]
- Marchi, J.; Berg, M.; Dencker, A.; Olander, E.K.; Begley, C. Risks associated with obesity in pregnancy, for the mother and baby: A systematic review of reviews. Obes. Rev. 2015, 16, 621–638. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care 2015, 38, S8–S16. [Google Scholar] [CrossRef]
- Guariguata, L.; Linnenkamp, U.; Beagley, J.; Whiting, D.R.; Cho, N.H. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Res. Clin. Pract. 2014, 103, 176–185. [Google Scholar] [CrossRef]
- Metzger, B.E. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Wendland, E.M.; Torloni, M.R.; Falavigna, M.; Trujillo, J.; Dode, M.A.; Campos, M.A.; Duncan, B.B.; Schmidt, M.I. Gestational diabetes and pregnancy outcomes—A systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth 2012, 12. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, L.; Casas, J.P.; Hingorani, A.D.; Williams, D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 2009, 373, 1773–1779. [Google Scholar] [CrossRef]
- Chodick, G.; Elchalal, U.; Sella, T.; Heymann, A.D.; Porath, A.; Kokia, E.; Shalev, V. The risk of overt diabetes mellitus among women with gestational diabetes: A population-based study. Diabet. Med. 2010, 27, 779–785. [Google Scholar] [CrossRef]
- Coustan, D.R. Gestational diabetes mellitus. Clin. Chem. 2013, 59, 1310–1321. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, K.M.; Lillycrop, K.A.; Burdge, G.C.; Gluckman, P.D.; Hanson, M.A. Epigenetic Mechanisms and the Mismatch Concept of the Developmental Origins of Health and Disease. Pediatr. Res. 2007, 61, 5R–10R. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.A.L.; Wolfe, L.A.; Mottola, M.F.; MacKinnon, C.; Arsenault, M.Y.; Bartellas, E.; Cargill, Y.; Gleason, T.; Iglesias, S.; Klein, M.; et al. Exercise in pregnancy and the postpartum period. J. Obstet. Gynaecol. Can. 2003, 25, 516–522. [Google Scholar] [CrossRef]
- Davenport, M.H.; Ruchat, S.M.; Poitras, V.J.; Jaramillo Garcia, A.; Gray, C.E.; Barrowman, N.; Skow, R.J.; Meah, V.L.; Riske, L.; Sobierajski, F.; et al. Prenatal exercise for the prevention of gestational diabetes mellitus and hypertensive disorders of pregnancy: A systematic review and meta-analysis. Br. J. Sports Med. 2018, 52, 1367–1375. [Google Scholar] [CrossRef]
- Sanabria-Martínez, G.; García-Hermoso, A.; Poyatos-León, R.; Álvarez-Bueno, C.; Sánchez-López, M.; Martínez-Vizcaíno, V. Effectiveness of physical activity interventions on preventing gestational diabetes mellitus and excessive maternal weight gain: A meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 1167–1174. [Google Scholar] [CrossRef]
- Wilkerson, R.G.; Ogunbodede, A.C. Hypertensive Disorders of Pregnancy. Emerg. Med. Clin. N. Am. 2019, 37, 301–316. [Google Scholar] [CrossRef]
- ACOG. Physical Activity and Exercise during Pregnancy and the Postpartum Period: ACOG Committee Opinion Summary, Number 804. Obstet. Gynecol. 2020, 135, 991–993. [Google Scholar] [CrossRef]
- Barakat, R.; Perales, M. Resistance exercise in pregnancy and outcome. Clin. Obstet. Gynecol. 2016, 59, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.A.L.; Wolfe, L.A.; Mottola, M.F.; MacKinnon, C. No. 129-Exercise in Pregnancy and the Postpartum Period. J. Obstet. Gynaecol. Can. 2018, 40, e58–e65. [Google Scholar] [CrossRef] [PubMed]
- Chiefari, E.; Arcidiacono, B.; Foti, D.; Brunetti, A. Gestational diabetes mellitus: An updated overview. J. Endocrinol. Investig. 2017, 40, 899–909. [Google Scholar] [CrossRef]
- Skeffington, K.L.; Higgins, J.S.; Mahmoud, A.D.; Evans, A.M.; Sferruzzi-Perri, A.N.; Fowden, A.L.; Yung, H.W.; Burton, G.J.; Giussani, D.A.; Moore, L.G. Hypoxia, AMPK activation and uterine artery vasoreactivity. J. Physiol. 2016, 594, 1357–1369. [Google Scholar] [CrossRef]
- Brownfoot, F.C.; Hastie, R.; Hannan, N.J.; Cannon, P.; Tuohey, L.; Parry, L.J.; Senadheera, S.; Illanes, S.E.; Kaitu’U-Lino, T.J.; Tong, S. Metformin as a prevention and treatment for preeclampsia: Effects on soluble fms-like tyrosine kinase 1 and soluble endoglin secretion and endothelial dysfunction. Am. J. Obstet. Gynecol. 2016, 214, 356.e1–356.e15. [Google Scholar] [CrossRef]
- Agatisa, P.K.; Ness, R.B.; Roberts, J.M.; Costantino, J.P.; Kuller, L.H.; McLaughlin, M.K. Impairment of endothelial function in women with a history of preeclampsia: An indicator of cardiovascular risk. Am. J. Physiol. Heart Circ. Physiol. 2004, 286. [Google Scholar] [CrossRef]
- Banek, C.T.; Bauer, A.J.; Needham, K.M.; Dreyer, H.C.; Gilbert, J.S. AICAR (5-aminoimidazole-4-carboxamide-3-ribonucleoside) administration ameliorates hypertension and angiogenic imbalance in a model of preeclampsia in the rat. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1159–H1165. [Google Scholar] [CrossRef] [PubMed]
- Dodd, J.M.; Grivell, R.M.; Deussen, A.R.; Hague, W.M. Metformin for women who are overweight or obese during pregnancy for improving maternal and infant outcomes. Cochrane Database Syst. Rev. 2018, 2018, CD010564. [Google Scholar] [CrossRef] [PubMed]
- Chatzakis, C.; Goulis, D.G.; Mareti, E.; Eleftheriades, M.; Zavlanos, A.; Dinas, K.; Sotiriadis, A. Prevention of gestational diabetes mellitus in overweight or obese pregnant women: A network meta-analysis. Diabetes Res. Clin. Pract. 2019, 158. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6. [Google Scholar] [CrossRef]
- Hutton, B.; Catalá-López, F.; Moher, D. La extensión de la declaración PRISMA para revisiones sistemáticas que incorporan metaanálisis en red: PRISMA-NMA. Med. Clin. 2016, 147, 262–266. [Google Scholar] [CrossRef]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series; John Wiley and Sons: Hoboken, NJ, USA, 2008; ISBN 9780470699515. [Google Scholar]
- Pascual-Morena, C.; Martínez-Vizcaíno, V.; Álvarez-Bueno, C.; Pozuelo-Carrascosa, D.P.; Notario-Pacheco, B.; Saz-Lara, A.; Fernández-Rodriguez, R.; Cavero-Redondo, I. Exercise vs. metformin for gestational diabetes mellitus: Protocol for a network meta-analysis. Medicine 2019, 98, e16038. [Google Scholar] [CrossRef]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1902, 100, 126–131. [Google Scholar] [CrossRef]
- Riebe, D.; Ehrman, J.; Liguori, G.; Magal, M.; Medicine, A.C.S. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Kluwer, Wolters: Philadelphia, PA, USA, 2018; ISBN 9781496339065. [Google Scholar]
- Mcnair, D.; Lorr, M.; Droppleman, L.F. Manual for the Profile of Mood States; Educational and Industrial Testing Services: San Diego, CA, USA, 1971; 27p. [Google Scholar]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, S.; Campbell, M.K.; Campbell, M.J.; Drahota-Towns, A.; Giraudeau, B.; Higgins, J.P.T.; Reeves, B.C.; Siegfried, N. Revised Cochrane risk of bias tool for randomized trials (RoB 2.0): Additional considerations for cluster-randomized trials. Portsm. Res. Portal 2016. Available online: https://www.semanticscholar.org/paper/Revised-Cochrane-risk-of-bias-tool-for-randomized-Eldridge-Campbell/d0b0ef43d768e6d3a574cc84d75e91722a2f7d4e#citing-papers (accessed on 6 August 2021).
- Neumann, I.; Pantoja, T.; Peñaloza, B.; Cifuentes, L.; Rada, G. El sistema GRADE: Un cambio en la forma de evaluar la calidad de la evidencia y la fuerza de recomendaciones. Rev. Med. Chil. 2014, 142, 630–635. [Google Scholar] [CrossRef]
- Salanti, G.; Del Giovane, C.; Chaimani, A.; Caldwell, D.M.; Higgins, J.P.T. Evaluating the Quality of Evidence from a Network Meta-Analysis. PLoS ONE 2014, 9, e99682. [Google Scholar] [CrossRef]
- Chaimani, A.; Higgins, J.P.T.; Mavridis, D.; Spyridonos, P.; Salanti, G. Graphical Tools for Network Meta-Analysis in STATA. PLoS ONE 2013, 8, e76654. [Google Scholar] [CrossRef]
- Salanti, G.; Ades, A.E.; Ioannidis, J.P.A. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. J. Clin. Epidemiol. 2011, 64, 163–171. [Google Scholar] [CrossRef]
- Veroniki, A.A.; Vasiliadis, H.S.; Higgins, J.P.; Salanti, G. Evaluation of inconsistency in networks of interventions. Int. J. Epidemiol. 2013, 42, 332–345. [Google Scholar] [CrossRef]
- Der Simonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Spiegelhalter, D.J.; Abrams, K.R.; Myles, J.P. Bayesian Approaches to Clinical Trials and Health-Care Evaluation; John Wiley & Sons, Ltd.: Chichester, UK, 2003; ISBN 9780470092606. [Google Scholar] [CrossRef]
- Stettler, C.; Allemann, S.; Wandel, S.; Kastrati, A.; Morice, M.C.; Schömig, A.; Pfisterer, M.E.; Stone, G.W.; Leon, M.B.; de Lezo, J.S.; et al. Drug eluting and bare metal stents in people with and without diabetes: Collaborative network meta-analysis. BMJ 2008, 337. [Google Scholar] [CrossRef]
- Cipriani, A.; Higgins, J.P.T.; Geddes, J.R.; Salanti, G. Conceptual and technical challenges in network meta-analysis. Ann. Int. Med. 2013, 159, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test measures of funnel plot asymmetry. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.L.; Campbell, C.G.; Foster, R.C.; Peterson, A.D.; Lanningham-Foster, L. A pilot walking program promotes moderate-intensity physical activity during pregnancy. Med. Sci. Sports Exerc. 2014, 46, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, S.N.; Jiang, Y.; Derraik, J.G.B.; McCowan, L.M.E.; Parry, G.K.; Biggs, J.B.; Craigie, S.; Gusso, S.; Peres, G.; Rodrigues, R.O.; et al. Effects of antenatal exercise in overweight and obese pregnant women on maternal and perinatal outcomes: A randomised controlled trial. BJOG 2016, 123, 588–597. [Google Scholar] [CrossRef]
- Wang, C.; Wei, Y.; Zhang, X.; Zhang, Y.; Xu, Q.; Sun, Y.; Su, S.; Zhang, L.; Liu, C.; Feng, Y.; et al. A randomized clinical trial of exercise during pregnancy to prevent gestational diabetes mellitus and improve pregnancy outcome in overweight and obese pregnant women. Am. J. Obstet. Gynecol. 2017, 216, 340–351. [Google Scholar] [CrossRef]
- Barakat, R.; Pelaez, M.; Cordero, Y.; Perales, M.; Lopez, C.; Coteron, J.; Mottola, M.F. Exercise during pregnancy protects against hypertension and macrosomia: Randomized clinical trial. Am. J. Obstet. Gynecol. 2016, 214, 649.e1–649.e8. [Google Scholar] [CrossRef] [PubMed]
- Bisson, M.; Almeras, N.; Dufresne, S.S.; Robitaille, J.; Rheaume, C.; Bujold, E.; Frenette, J.; Tremblay, A.; Marc, I. A 12-Week Exercise Program for Pregnant Women with Obesity to Improve Physical Activity Levels: An Open Randomised Preliminary Study. PLoS ONE 2015, 10, e0137742. [Google Scholar] [CrossRef]
- Daly, N.; Farren, M.; McKeating, A.; O’Kelly, R.; Stapleton, M.; Turner, M.J. A Medically Supervised Pregnancy Exercise Intervention in Obese Women: A Randomized Controlled Trial. Obstet. Gynecol. 2017, 130, 1001–1010. [Google Scholar] [CrossRef]
- Garnæs, K.K.; Mørkved, S.; Salvesen, Ø.; Moholdt, T. Exercise Training and Weight Gain in Obese Pregnant Women: A Randomized Controlled Trial (ETIP Trial). PLoS Med. 2016, 13, e1002079. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, S.L.; Surita, F.G.; Parpinelli, M.A.; Siani, S.; Pinto e Silva, J.L. The effect of an antenatal physical exercise programme on maternal/perinatal outcomes and quality of life in overweight and obese pregnant women: A randomised clinical trial. BJOG 2011, 118, 1455–1463. [Google Scholar] [CrossRef]
- Oostdam, N.; Van Poppel, M.N.M.; Wouters, M.G.A.J.; Eekhoff, E.M.W.; Bekedam, D.J.; Kuchenbecker, W.K.H.; Quartero, H.W.P.; Heres, M.H.B.; Van Mechelen, W. No effect of the FitFor2 exercise programme on blood glucose, insulin sensitivity, and birthweight in pregnant women who were overweight and at risk for gestational diabetes: Results of a randomised controlled trial. BJOG 2012, 119, 1098–1107. [Google Scholar] [CrossRef]
- Ruiz, J.R.; Perales, M.; Pelaez, M.; Lopez, C.; Lucia, A.; Barakat, R. Supervised exercise-based intervention to prevent excessive gestational weight gain: A randomized controlled trial. Mayo Clin. Proc. 2013, 88, 1388–1397. [Google Scholar] [CrossRef]
- Barakat, R.; Lucia, A.; Ruiz, J.R. Resistance exercise training during pregnancy and newborn’s birth size: A randomised controlled trial. Int. J. Obes. 2009, 33, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Fattah, E. Can metformin limit weight gain in the obese with pregnancy? Int. J. Reprod. Contracept. Obstet. Gynecol. 2016, 5, 818–825. [Google Scholar] [CrossRef][Green Version]
- Brink, H.S.; Alkemade, M.; van der Lely, A.J.; van der Linden, J. Metformin in women at high risk of gestational diabetes mellitus. Diabetes Metab. 2018, 44, 300–302. [Google Scholar] [CrossRef]
- Chiswick, C.; Reynolds, R.M.; Denison, F.; Drake, A.J.; Forbes, S.; Newby, D.E.; Walker, B.R.; Quenby, S.; Wray, S.; Weeks, A.; et al. Effect of metformin on maternal and fetal outcomes in obese pregnant women (EMPOWaR): A randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2015, 3, 778–786. [Google Scholar] [CrossRef]
- do Nascimento, I.B.; Sales, W.B.; Dienstmann, G.; de Souza, M.L.R.; Fleig, R.; Silva, J.C. Metformin for prevention of cesarean delivery and large-for-gestational-age newborns in non-diabetic obese pregnant women: A randomized clinical trial. Arch. Endocrinol. Metab. 2020, 64, 290–297. [Google Scholar] [CrossRef]
- Syngelaki, A.; Nicolaides, K.H.; Balani, J.; Hyer, S.; Akolekar, R.; Kotecha, R.; Pastides, A.; Shehata, H. Metformin versus Placebo in Obese Pregnant Women without Diabetes Mellitus. N. Engl. J. Med. 2016, 374, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Du, M.C.; Ouyang, Y.Q.; Nie, X.F.; Huang, Y.; Redding, S.R. Effects of physical exercise during pregnancy on maternal and infant outcomes in overweight and obese pregnant women: A meta-analysis. Birth 2019, 46, 211–221. [Google Scholar] [CrossRef]
- Nasiri-Amiri, F.; Sepidarkish, M.; Shirvani, M.A.; Habibipour, P.; Tabari, N.S.M. The effect of exercise on the prevention of gestational diabetes in obese and overweight pregnant women: A systematic review and meta-Analysis. Diabetol. Metab. Syndr. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Consitt, L.A.; Dudley, C.; Saxena, G. Impact of endurance and resistance training on skeletal muscle glucose metabolism in older adults. Nutrients 2019, 11, 2636. [Google Scholar] [CrossRef] [PubMed]
- Moghetti, P.; Bacchi, E.; Brangani, C.; Donà, S.; Negri, C. Metabolic Effects of Exercise. Front. Horm. Res. 2016, 47, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Alves, C.R.R.; Stanford, K.I.; Middelbeek, R.J.W.; Nigro, P.; Ryan, R.E.; Xue, R.; Sakaguchi, M.; Lynes, M.D.; So, K.; et al. TGF-β2 is an exercise-induced adipokine that regulates glucose and fatty acid metabolism. Nat. Metab. 2019, 1, 291–303. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Atkin, S.L.; Simental-Mendía, L.E.; Sahebkar, A. Molecular mechanisms by which aerobic exercise induces insulin sensitivity. J. Cell. Physiol. 2019, 234, 12385–12392. [Google Scholar] [CrossRef]
- Pourranjbar, M.; Arabnejad, N.; Naderipour, K.; Rafie, F. Effects of Aerobic Exercises on Serum Levels of Myonectin and Insulin Resistance in Obese and Overweight Women. J. Med. Life 2018, 11, 381–386. [Google Scholar] [CrossRef]
- Nascimento, I.B.D.; Dienstmann, G.; De Souza, M.L.R.; Fleig, R.; Hoffmann, C.B.P.C.; Silva, J.C. Evaluation of Preeclampsia Results after Use of Metformin in Gestation: Systematic Review and Meta-analysis. Rev. Bras. Ginecol. Obstet. 2018, 40, 713–721. [Google Scholar] [CrossRef]
- Kalafat, E.; Sukur, Y.E.; Abdi, A.; Thilaganathan, B.; Khalil, A. Metformin for prevention of hypertensive disorders of pregnancy in women with gestational diabetes or obesity: Systematic review and meta-analysis of randomized trials. Ultrasound Obstet. Gynecol. 2018, 52, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Elmaraezy, A.; Abushouk, A.I.; Emara, A.; Elshahat, O.; Ahmed, H.; Mostafa, M.I. Effect of metformin on maternal and neonatal outcomes in pregnant obese non-diabetic women: A meta-analysis. Int. J. Reprod. Biomed. 2017, 15, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Erez, O.; Huttemann, M.; Maymon, E.; Panaitescu, B.; Conde-Agudelo, A.; Pacora, P.; Yoon, B.H.; Grossman, L.I. Metformin, the aspirin of the 21st century: Its role in gestational diabetes mellitus, prevention of preeclampsia and cancer, and the promotion of longevity. Am. J. Obstet. Gynecol. 2017, 217, 282–302. [Google Scholar] [CrossRef] [PubMed]
- Soobryan, N.; Murugesan, S.; Pandiyan, A.; Moodley, J.; Mackraj, I. Angiogenic Dysregulation in Pregnancy-Related Hypertension—A Role for Metformin. Reprod. Sci. 2018, 25, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, V.; Brunelli, R.; Vena, F.; Di Mascio, D.; Marchetti, C.; Boccherini, C.; Piccioni, M.G.; Benedetti Panici, P.; Giancotti, A. Metformin reduces maternal weight gain in obese pregnant women: A systematic review and meta-analysis of two randomized controlled trials. Diabetes Metab. Res. Rev. 2019, 35. [Google Scholar] [CrossRef]
- Nicodemus, N.A., Jr. Prevention of Excessive Gestational Weight Gain and Postpartum Weight Retention. Curr. Obes. Rep. 2018, 7, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Yerevanian, A.; Soukas, A.A. Metformin: Mechanisms in Human Obesity and Weight Loss. Curr. Obes. Rep. 2019, 8, 156–164. [Google Scholar] [CrossRef]
- Malin, S.K.; Kashyap, S.R. Effects of metformin on weight loss: Potential mechanisms. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 323–329. [Google Scholar] [CrossRef]
- Broberg, L.; Ersbøll, A.S.; Backhausen, M.G.; Damm, P.; Tabor, A.; Hegaard, H.K. Compliance with national recommendations for exercise during early pregnancy in a Danish cohort. BMC Pregnancy Childbirth 2015, 15, 317. [Google Scholar] [CrossRef]
- Amezcua-Prieto, C.; Lardelli-Claret, P.; Olmedo-Requena, R.; Mozas-Moreno, J.; Bueno-Cavanillas, A.; Jiménez-Moleón, J.J. Compliance with leisure-time physical activity recommendations in pregnant women. Acta Obstet. Gynecol. Scand. 2011, 90, 245–252. [Google Scholar] [CrossRef]
- Martin-Arias, A.; Brik, M.; Vargas-Terrones, M.; Barakat, R.; Santacruz, B. Predictive factors of compliance with a program of supervised exercise during pregnancy. Acta Obstet. Gynecol. Scand. 2019, 98, 807–808. [Google Scholar] [CrossRef]
- Brislane, Á.; Jones, H.; Holder, S.M.; Low, D.A.; Hopkins, N.D. The Effect of Exercise during Pregnancy on Maternal and Offspring Vascular Outcomes: A Pilot Study. Reprod. Sci. 2021, 28, 510–523. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, S.R.; Cecatti, J.G.; Pereira, R.I.; Baciuk, E.P.; Bernardo, A.L.; Silveira, C. Water aerobics II: Maternal body composition and perinatal outcomes after a program for low risk pregnant women. Reprod. Health 2009, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Clapp, J.F., 3rd; Kim, H.; Burciu, B.; Lopez, B. Beginning regular exercise in early pregnancy: Effect on fetoplacental growth. Am. J. Obstet. Gynecol. 2000, 183, 1484–1488. [Google Scholar] [CrossRef]
- de Oliveria Melo, A.S.; Silva, J.L.P.; Tavares, J.S.; Barros, V.O.; Leite, D.F.B.; Amorim, M.M.R. Effect of a physical exercise program during pregnancy on uteroplacental and fetal blood flow and fetal growth: A randomized controlled trial. Obstet. Gynecol. 2012, 120, 302–310. [Google Scholar] [CrossRef]
- Ghodsi, Z.; Asltoghiri, M. Effects of aerobic exercise training on maternal and neonatal outcome: A randomized controlled trial on pregnant women in Iran. J. Pak. Med. Assoc. 2014, 64, 1053–1056. [Google Scholar] [PubMed]
- Guelfi, K.J.; Ong, M.J.; Crisp, N.A.; Fournier, P.A.; Wallman, K.E.; Grove, J.R.; Doherty, D.A.; Newnham, J.P. Regular Exercise to Prevent the Recurrence of Gestational Diabetes Mellitus: A Randomized Controlled Trial. Obstet. Gynecol. 2016, 128, 819–827. [Google Scholar] [CrossRef]
- Hopkins, S.A.; Baldi, J.C.; Cutfield, W.S.; McCowan, L.; Hofman, P.L. Effects of exercise training on maternal hormonal changes in pregnancy. Clin. Endocrinol. 2011, 74, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Kasawara, K.T.; Burgos, C.S.G.; do Nascimento, S.L.; Ferreira, N.O.; Surita, F.G.; Pinto, E.; Silva, J.L. Maternal and perinatal outcomes of exercise in pregnant women with chronic hypertension and/or previous preeclampsia: A randomized controlled trial. ISRN Obstet. Gynecol. 2013, 2013, 857047. [Google Scholar] [CrossRef] [PubMed]
- Khoram, S.; Loripoor, M.; Pirhadi, M.; Beigi, M. The effect of walking on pregnancy blood pressure disorders in women susceptible to pregnancy hypertension: A randomized clinical trial. J. Educ. Health Promot. 2019, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Kihlstrand, M.; Stenman, B.; Nilsson, S.; Axelsson, O. Water-gymnastics reduced the intensity of back/low back pain in pregnant women. Acta Obstet. Gynecol. Scand. 1999, 78, 180–185. [Google Scholar] [PubMed]
- Ko, C.W.; Napolitano, P.G.; Lee, S.P.; Schulte, S.D.; Ciol, M.A.; Beresford, S.A.A. Physical activity, maternal metabolic measures, and the incidence of gallbladder sludge or stones during pregnancy: A randomized trial. Am. J. Perinatol. 2014, 31, 39–48. [Google Scholar] [CrossRef]
- Labonte-Lemoyne, E.; Curnier, D.; Ellemberg, D. Exercise during pregnancy enhances cerebral maturation in the newborn: A randomized controlled trial. J. Clin. Exp. Neuropsychol. 2017, 39, 347–354. [Google Scholar] [CrossRef]
- Marquez-Sterling, S.; Perry, A.C.; Kaplan, T.A.; Halberstein, R.A.; Signorile, J.F. Physical and psychological changes with vigorous exercise in sedentary primigravidae. Med. Sci. Sports Exerc. 2000, 32, 58–62. [Google Scholar] [CrossRef]
- McDonald, S.M.; Newton, E.; Strickland, D.; Isler, C.; Haven, K.; Kelley, G.; Chasan-Taber, L.; Kuehn, D.; May, L.E. Influence of Prenatal Aerobic Exercise on Fetal Morphometry. Matern. Child Health J. 2020, 24, 1367–1375. [Google Scholar] [CrossRef]
- Navas, A.; Carrascosa, M.D.C.; Artigues, C.; Ortas, S.; Portells, E.; Soler, A.; Yañez, A.M.; Bennasar-Veny, M.; Leiva, A. Effectiveness of Moderate-Intensity Aerobic Water Exercise during Pregnancy on Quality of Life and Postpartum Depression: A Multi-Center, Randomized Controlled Trial. J. Clin. Med. 2021, 10, 2432. [Google Scholar] [CrossRef]
- Sedaghati, P.; Ziaee, V.; Ardjmand, A. The effect of an ergometric training program on pregnants’ weight gain and low back pain. Gazz. Med. Ital. 2007, 166, 209–213. [Google Scholar]
- Taniguchi, C.; Sato, C. Home-based walking during pregnancy affects mood and birth outcomes among sedentary women: A randomized controlled trial. Int. J. Nurs. Pract. 2016, 22, 420–426. [Google Scholar] [CrossRef]
- Tomić, V.; Sporiš, G.; Tomić, J.; Milanović, Z.; Zigmundovac-Klaić, D.; Pantelić, S. The effect of maternal exercise during pregnancy on abnormal fetal growth. Croat. Med. J. 2013, 54, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.-C.; Chen, C.H. Effects of non-supervised aerobic exercise on sleep quality and maternal-fetal attachment in pregnant women: A randomized controlled trial. Complement. Ther. Med. 2021, 57, 102671. [Google Scholar] [CrossRef] [PubMed]
- Rakhshani, A.; Nagarathna, R.; Mhaskar, R.; Mhaskar, A.; Thomas, A.; Gunasheela, S. The effects of yoga in prevention of pregnancy complications in high-risk pregnancies: A randomized controlled trial. Prev. Med. 2012, 55, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Sonmezer, E.; Özköslü, M.A.; Yosmaoğlu, H.B. The effects of clinical pilates exercises on functional disability, pain, quality of life and lumbopelvic stabilization in pregnant women with low back pain: A randomized controlled study. J. Back Musculoskelet. Rehabil. 2021, 34, 69–76. [Google Scholar] [CrossRef]
- Sun, Y.-C.; Hung, Y.-C.; Chang, Y.; Kuo, S.-C. Effects of a prenatal yoga programme on the discomforts of pregnancy and maternal childbirth self-efficacy in Taiwan. Midwifery 2010, 26, e31–e36. [Google Scholar] [CrossRef]
- Bacchi, M.; Mottola, M.F.; Perales, M.; Refoyo, I.; Barakat, R. Aquatic Activities during Pregnancy Prevent Excessive Maternal Weight Gain and Preserve Birth Weight: A Randomized Clinical Trial. Am. J. Health Promot. 2018, 32, 729–735. [Google Scholar] [CrossRef]
- Backhausen, M.G.; Tabor, A.; Albert, H.; Rosthøj, S.; Damm, P.; Hegaard, H.K. The effects of an unsupervised water exercise program on low back pain and sick leave among healthy pregnant women—A randomised controlled trial. PLoS ONE 2017, 12, e0182114. [Google Scholar] [CrossRef]
- Barakat, R.; Pelaez, M.; Montejo, R.; Luaces, M.; Zakynthinaki, M. Exercise during pregnancy improves maternal health perception: A randomized controlled trial. Am. J. Obstet. Gynecol. 2011, 204, 402.e1–402.e7. [Google Scholar] [CrossRef]
- Barakat, R.; Pelaez, M.; Lopez, C.; Montejo, R.; Coteron, J. Exercise during pregnancy reduces the rate of cesarean and instrumental deliveries: Results of a randomized controlled trial. J. Matern. Fetal. Neonatal Med. 2012, 25, 2372–2376. [Google Scholar] [CrossRef]
- Barakat, R.; Pelaez, M.; Lopez, C.; Lucia, A.; Ruiz, J.R. Exercise during pregnancy and gestational diabetes-related adverse effects: A randomised controlled trial. Br. J. Sports Med. 2013, 47, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Barakat, R.; Vargas, M.; Brik, M.; Fernandez, I.; Gil, J.; Coteron, J.; Santacruz, B. Does Exercise During Pregnancy Affect Placental Weight? A Randomized Clinical Trial. Eval. Health Prof. 2018, 41, 400–414. [Google Scholar] [CrossRef] [PubMed]
- Barakat, R.; Refoyo, I.; Coteron, J.; Franco, E. Exercise during pregnancy has a preventative effect on excessive maternal weight gain and gestational diabetes. A randomized controlled trial. Braz. J. Phys. Ther. 2019, 23, 148–155. [Google Scholar] [CrossRef]
- Brik, M.; Fernandez-Buhigas, I.; Martin-Arias, A.; Vargas-Terrones, M.; Barakat, R.; Santacruz, B. Does exercise during pregnancy impact on maternal weight gain and fetal cardiac function? A randomized controlled trial. Ultrasound Obstet. Gynecol. 2019, 53, 583–589. [Google Scholar] [CrossRef]
- Broberg, L.; Tabor, A.; Rosthøj, S.; Backhausen, M.; Frokjaer, V.G.; Damm, P.; Hegaard, H.K. Effect of supervised group exercise on psychological well-being among pregnant women with or at high risk of depression (the EWE Study): A randomized controlled trial. Acta Obstet. Gynecol. Scand. 2021, 100, 129–138. [Google Scholar] [CrossRef]
- Cordero, Y.; Peláez, M.; De Miguel, M.; Perales, M.; Barakat Carballo, R. Can moderate physical exercise during pregnancy act as a factor in preventing gestational diabetes? [Puede el ejercicio físico moderado durante el embarazo actuar como un factor de prevención de la diabetes gestacional?]. RICYDE Rev. Int. Cienc. Deport. 2012, 8, 3–19. [Google Scholar] [CrossRef]
- Cordero, Y.; Mottola, M.F.; Vargas, J.; Blanco, M.; Barakat, R. Exercise Is Associated with a Reduction in Gestational Diabetes Mellitus. Med. Sci. Sports Exerc. 2015, 47, 1328–1333. [Google Scholar] [CrossRef]
- da Silva, S.G.; Hallal, P.C.; Domingues, M.R.; Bertoldi, A.D.A.D.; da Silveira, M.F.; Bassani, D.; da Silva, I.C.M.I.; da Silva, B.G.C.; de Coll, C.V.N.; Evenson, K. A randomized controlled trial of exercise during pregnancy on maternal and neonatal outcomes: Results from the PAMELA study. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 175. [Google Scholar] [CrossRef]
- Fernandez-Buhigas, I.; Brik, M.; Martin-Arias, A.; Vargas-Terrones, M.; Varillas, D.; Barakat, R.; Santacruz, B. Maternal physiological changes at rest induced by exercise during pregnancy: A randomized controlled trial. Physiol. Behav. 2020, 112863. [Google Scholar] [CrossRef]
- Haakstad, L.A.H.; Bo, K. Effect of regular exercise on prevention of excessive weight gain in pregnancy: A randomised controlled trial. Eur. J. Contracept. Reprod. Health Care 2011, 16, 116–125. [Google Scholar] [CrossRef]
- Pelaez, M.; Gonzalez-Cerron, S.; Montejo, R.; Barakat, R. Protective Effect of Exercise in Pregnant Women Including Those Who Exceed Weight Gain Recommendations: A Randomized Controlled Trial. Mayo Clin. Proc. 2019, 94, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Perales, M.; Calabria, I.; Lopez, C.; Franco, E.; Coteron, J.; Barakat, R. Regular Exercise Throughout Pregnancy Is Associated With a Shorter First Stage of Labor. Am. J. Health Promot. 2016, 30, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Price, B.B.; Amini, S.B.; Kappeler, K. Exercise in pregnancy: Effect on fitness and obstetric outcomes-a randomized trial. Med. Sci. Sports Exerc. 2012, 44, 2263–2269. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Vélez, R.; Lobelo, F.; Aguilar-de Plata, A.C.; Izquierdo, M.; García-Hermoso, A. Exercise during pregnancy on maternal lipids: A secondary analysis of randomized controlled trial. BMC Pregnancy Childbirth 2017, 17, 396. [Google Scholar] [CrossRef]
- Rodríguez-Blanque, R.; Sánchez-García, J.C.; Sánchez-López, A.M.; Mur-Villar, N.; Fernández-Castillo, R.; Aguilar-Cordero, M.J. Influence of physical exercise during pregnancy on newborn weight: A randomized clinical trial [Influencia del ejercicio físico durante el embarazo sobre el peso del recién nacido: Un ensayo clínico aleatorizado]. Nutr. Hosp. 2017, 34, 834–840. [Google Scholar] [CrossRef]
- Stafne, S.N.; Salvesen, K.Å.; Romundstad, P.R.; Eggebø, T.M.; Carlsen, S.M.; Mørkved, S. Regular exercise during pregnancy to prevent gestational diabetes: A randomized controlled trial. Obstet. Gynecol. 2012, 119, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Abd El Hameed, A.A.; Shreif, H.E.; Mowafy, H.E. The role of continuing metformin therapy during pregnancy in the reduction of gestational diabetes and improving pregnancy outcomes in women with polycystic ovary syndrome. Middle East Fertil. Soc. J. 2011, 16, 204–208. [Google Scholar] [CrossRef]
- Begum, M.R.; Khanam, N.N.; Quadir, E.; Ferdous, J.; Begum, M.S.; Khan, F.; Begum, A. Prevention of gestational diabetes mellitus by continuing metformin therapy throughout pregnancy in women with polycystic ovary syndrome. J. Obstet. Gynaecol. Res. 2009, 35, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Glueck, C.J.; Wang, P.; Kobayashi, S.; Phillips, H.; Sieve-Smith, L. Metformin therapy throughout pregnancy reduces the development of gestational diabetes in women with polycystic ovary syndrome. Fertil. Steril. 2002, 77, 520–525. [Google Scholar] [CrossRef]
- Jamal, A.; Milani, F.; Al-Yasin, A. Evaluation of the effect of metformin and aspirin on utero placental circulation of pregnant women with PCOS. Iran. J. Reprod. Med. 2012, 10, 265–270. [Google Scholar]
- Khattab, S.; Mohsen, I.A.; Aboul Foutouh, I.; Ashmawi, H.S.; Mohsen, M.N.; Van Wely, M.; Van Der Veen, F.; Youssef, M.A. Can metformin reduce the incidence of gestational diabetes mellitus in pregnant women with polycystic ovary syndrome? Prospective cohort study. Gynecol. Endocrinol. 2011, 27, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Lovvik, T.S.; Carlsen, S.M.; Salvesen, O.; Steffensen, B.; Bixo, M.; Gomez-Real, F.; Lonnebotn, M.; Hestvold, K.V.; Zabielska, R.; Hirschberg, A.L.; et al. Use of metformin to treat pregnant women with polycystic ovary syndrome (PregMet2): A randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 256–266. [Google Scholar] [CrossRef]
- Valdés, E.; Sepúlveda-Martínez, A.; Candia, P.; Abusada, N.; Orellana, R.; Manukian, B.B.B.; Cuellar, E. Metformin as a prophylactic treatment of gestational diabetes in pregnant patients with pregestational insulin resistance: A randomized study. J. Obstet. Gynaecol. Res. 2018, 44, 81–86. [Google Scholar] [CrossRef]
- Vanky, E.; Salvesen, K.A.; Heimstad, R.; Fougner, K.J.; Romundstad, P.; Carlsen, S.M. Metformin reduces pregnancy complications without affecting androgen levels in pregnant polycystic ovary syndrome women: Results of a randomized study. Hum. Reprod. 2004, 19, 1734–1740. [Google Scholar] [CrossRef] [PubMed]
- Vanky, E.; Stridsklev, S.; Heimstad, R.; Romundstad, P.; Skogøy, K.; Kleggetveit, O.; Hjelle, S.; Von Brandis, P.; Eikeland, T.; Flo, K.; et al. Metformin Versus placebo from first trimester to delivery in polycystic ovary syndrome: A randomized, controlled multicenter study. J. Clin. Endocrinol. Metab. 2010, 95, E448–E455. [Google Scholar] [CrossRef]
| Reference | Design | Country | Intervention | Target Women | Sample | Age | Outcome | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | I | C | I | C | GDM | HDP | MWG | |||||
| Kong K et al. (2014)-1 [44] | RCT | United States | Aerobic exercise | Overweight | 19 | 9 | 9 | 26.2 ± 2.6 | 27.3 ± 3.6 | ✓ | ✓ | ✓ |
| Kong K et al. (2014)-2 [44] | RCT | United States | Aerobic exercise | Obese | 18 | 9 | 10 | 28.6 ± 5.3 | 25.7 ± 4.0 | ✓ | ✓ | ✓ |
| Seneviratne SN et al. (2016) [45] | RCT | New Zealand | Aerobic exercise | Obese | 75 | 38 | 37 | NA | NA | ✓ | ✓ | ✓ |
| Wang C et al. (2017) [46] | RCT | China | Aerobic exercise | Overweight | 300 | 150 | 150 | 32.1 ± 4.6 | 32.5 ± 4.9 | ✓ | ✓ | ✓ |
| Barakat R et al. (2016)-1 [47] | RCT | Spain | Combined exercise | Overweight | 164 | 90 | 78 | NA | NA | ✓ | ✓ | - |
| Barakat R et al. (2016)-2 [47] | RCT | Spain | Combined exercise | Obese | 54 | 25 | 29 | NA | NA | ✓ | ✓ | - |
| Bisson M et al. (2015) [48] | RCT | Canada | Combined exercise | Obese | 50 | 25 | 25 | 30.5 ± 3.7 | 31.0 ± 4.0 | ✓ | ✓ | ✓ |
| Daly N et al. (2017) [49] | RCT | Ireland | Combined exercise | Obese | 88 | 44 | 44 | 30.0 ± 5.1 | 29.4 ± 4.8 | ✓ | - | ✓ |
| Garnæs KK et al. (2016) [50] | RCT | Norway | Combined exercise | Obese | 91 | 46 | 45 | 31.3 ± 3.8 | 31.4 ± 4.7 | ✓ | ✓ | ✓ |
| Nascimento SL et al. (2011) [51] | RCT | Brazil | Combined exercise | Overweight and Obese | 82 | 40 | 42 | 29.7 ± 6.8 | 30.9 ± 5.9 | - | - | ✓ |
| Oostdam N et al. (2012) [52] | RCT | Netherlands | Combined exercise | Obese | 121 | 62 | 59 | 30.8 ± 5.2 | 30.1 ± 4.5 | ✓ | - | ✓ |
| Ruiz JR et al. (2013) [53] | RCT | Spain | Combined exercise | Overweight and Obese | 275 | 146 | 129 | NA | NA | ✓ | ✓ | ✓ |
| Barakat R et al. (2009)-1 [54] | RCT | Spain | Resistance exercise | Overweight | 28 | 14 | 14 | NA | NA | - | - | ✓ |
| Barakat R et al. (2009)-2 [54] | RCT | Spain | Resistance exercise | Obese | 12 | 9 | 3 | NA | NA | - | - | ✓ |
| Abd El Fattah EA et al. (2016) [55] | RCT | Egypt | Metformin | Obese | 200 | 100 | 100 | 26.9 ± 5.2 | 26.2 ± 5.5 | ✓ | ✓ | ✓ |
| Brink HS et al. (2018) [56] | RCT | Netherlands | Metformin | Obese | 49 | 24 | 25 | 29.3 ± 5.2 | 30.7 ± 5.2 | ✓ | ✓ | - |
| Chiswick C et al. (2015) [57] | RCT | UK | Metformin | Obese | 449 | 226 | 223 | 28.7 ± 5.8 | 28.9 ± 5.1 | ✓ | ✓ | ✓ |
| Nascimento IB et al. (2020) [58] | RCT | Brazil | Metformin | Obese | 378 | 189 | 189 | 28.6 ± 6.2 | 29.6 ± 6.1 | ✓ | ✓ | - |
| Syngelaki A et al. (2016) [59] | RCT | UK | Metformin | Obese | 450 | 225 | 225 | 32.9 | 30.8 | ✓ | ✓ | - |
| A. Gestational Diabetes Mellitus | |||||
|---|---|---|---|---|---|
| Control | Aerobic | Combined | Resistance | Metformin | |
| Control | 0.59 * (0.41, 0.85) | 0.91 (0.67, 1.22) | 0.78 (0.59, 1.02) | ||
| Aerobic | 0.51 * (0.26, 0.97) | - | - | ||
| Combined | 0.75 (0.47, 1.19) | 1.49 (0.65, 3.43) | - | ||
| Resistance | |||||
| Metformin | 0.74 (0.49, 1.10) | 1.46 (0.63, 3.37) | 0.98 (0.54, 1.77) | ||
| B. Hypertensive disorders of pregnancy | |||||
| Control | 0.95 (0.56, 1.62) | 0.65 (0.36, 1.14) | 0.48 (0.19, 1.22) | ||
| Aerobic | 1.19 (0.36, 3.89) | - | - | ||
| Combined | 0.64 (0.26, 1.57) | 0.54 (0.12, 2.38) | - | ||
| Resistance | |||||
| Metformin | 0.47 (0.22, 1.04) | 0.40 (0.09, 1.69) | 0.74 (0.22, 2.44) | ||
| C. Maternal weight gain | |||||
| Control | −1.91 * (−2.74, −1.07) | −0.31 (−1.06, 0.44) | −1.35 (−3.57, 0.88) | −2.82 (−7.26, 1.62) | |
| Aerobic | −1.40 (−3.47, 0.68) | - | - | - | |
| Combined | −0.24 (−1.68, 1.20) | 1.15 (−1.37, 3.68) | - | - | |
| Resistance | −1.35 (−4.29, 1.59) | 0.05 (−3.55, 3.65) | −1.10 (−4.38, 2.17) | - | |
| Metformin | −2.93 * (−4.98, −0.87) | −1.53 (−4.44, 1.38) | −2.68 * (−5.19, −0.17) | −1.58 (−5.17, 2.01) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascual-Morena, C.; Cavero-Redondo, I.; Álvarez-Bueno, C.; Lucerón-Lucas-Torres, M.; Sanabria-Martínez, G.; Poyatos-León, R.; Rodríguez-Martín, B.; Martínez-Vizcaíno, V. Exercise versus Metformin to Improve Pregnancy Outcomes among Overweight Pregnant Women: A Systematic Review and Network Meta-Analysis. J. Clin. Med. 2021, 10, 3490. https://doi.org/10.3390/jcm10163490
Pascual-Morena C, Cavero-Redondo I, Álvarez-Bueno C, Lucerón-Lucas-Torres M, Sanabria-Martínez G, Poyatos-León R, Rodríguez-Martín B, Martínez-Vizcaíno V. Exercise versus Metformin to Improve Pregnancy Outcomes among Overweight Pregnant Women: A Systematic Review and Network Meta-Analysis. Journal of Clinical Medicine. 2021; 10(16):3490. https://doi.org/10.3390/jcm10163490
Chicago/Turabian StylePascual-Morena, Carlos, Iván Cavero-Redondo, Celia Álvarez-Bueno, Maribel Lucerón-Lucas-Torres, Gema Sanabria-Martínez, Raquel Poyatos-León, Beatriz Rodríguez-Martín, and Vicente Martínez-Vizcaíno. 2021. "Exercise versus Metformin to Improve Pregnancy Outcomes among Overweight Pregnant Women: A Systematic Review and Network Meta-Analysis" Journal of Clinical Medicine 10, no. 16: 3490. https://doi.org/10.3390/jcm10163490
APA StylePascual-Morena, C., Cavero-Redondo, I., Álvarez-Bueno, C., Lucerón-Lucas-Torres, M., Sanabria-Martínez, G., Poyatos-León, R., Rodríguez-Martín, B., & Martínez-Vizcaíno, V. (2021). Exercise versus Metformin to Improve Pregnancy Outcomes among Overweight Pregnant Women: A Systematic Review and Network Meta-Analysis. Journal of Clinical Medicine, 10(16), 3490. https://doi.org/10.3390/jcm10163490









