Transcatheter versus Isolated Surgical Aortic Valve Replacement in Young High-Risk Patients: A Propensity Score-Matched Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. TAVR Cohort
2.2. iSAVR Cohort
2.3. Study Design and Endpoint Definitions
2.4. Statistical Analysis
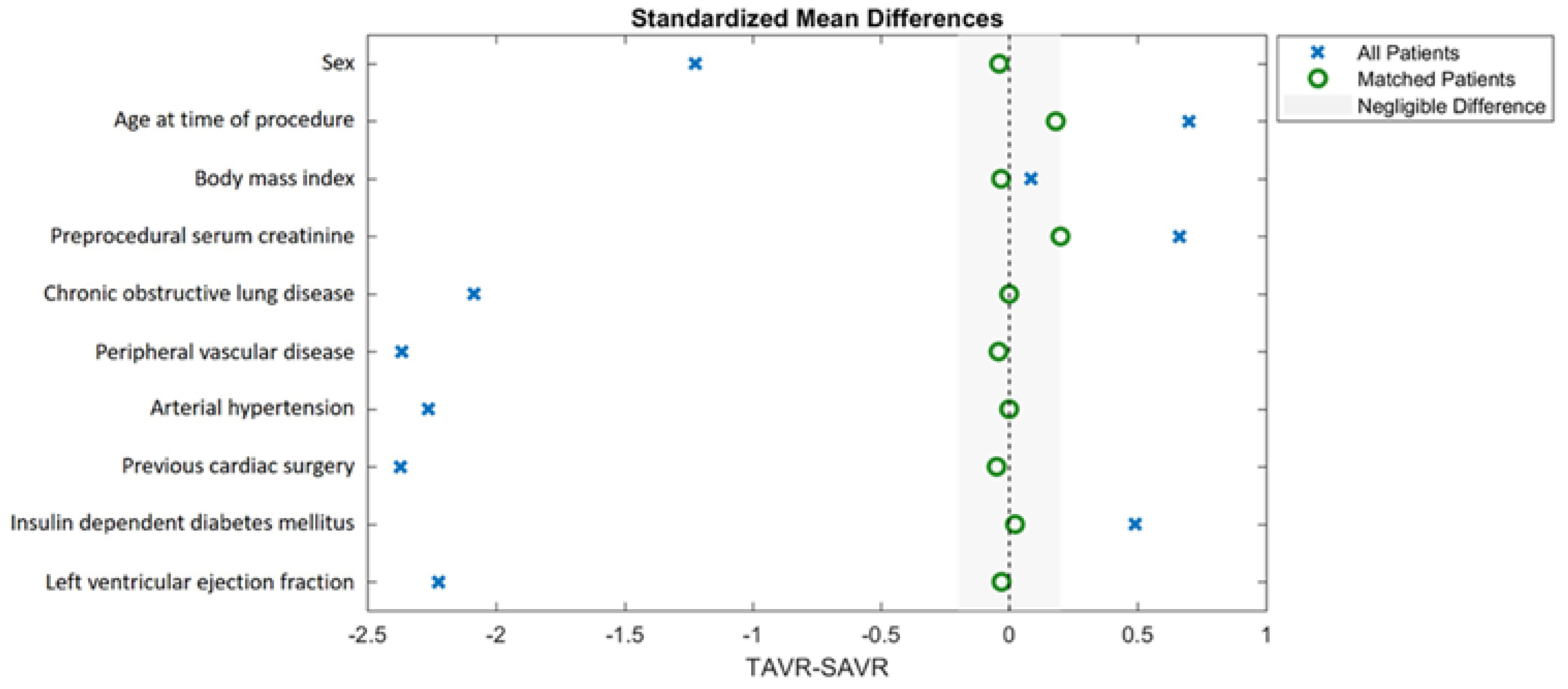
2.5. Propensity Score Matching
3. Results
3.1. Baseline Characteristics
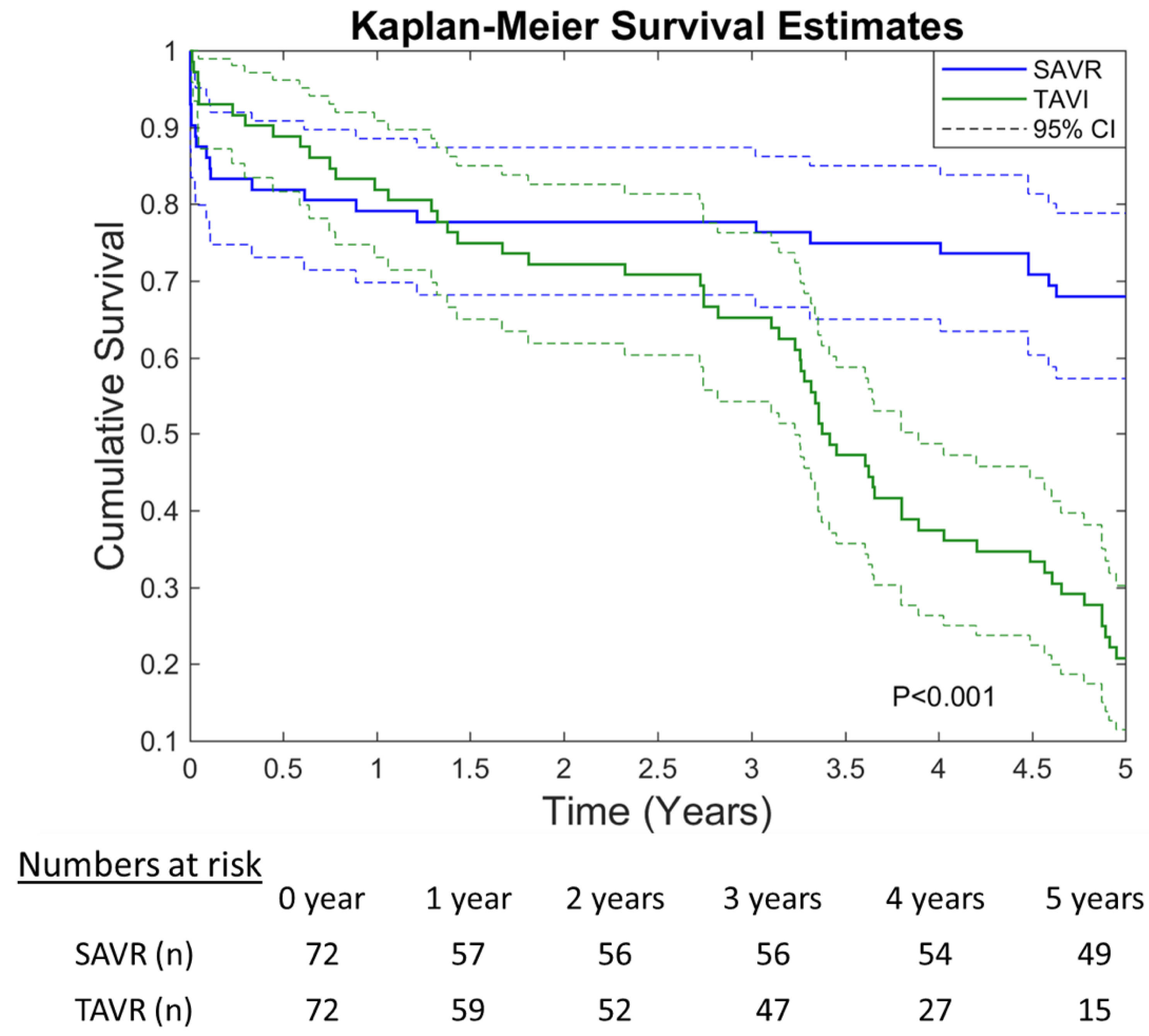
3.2. Survival and Safety Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Backer, O.; Sondergaard, L. Challenges When Expanding Transcatheter Aortic Valve Implantation to Younger Patients. Front. Cardiovasc. Med. 2018, 5, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Backer, O.; Luk, N.H.; Olsen, N.T.; Olsen, P.S.; Sondergaard, L. Choice of Treatment for Aortic Valve Stenosis in the Era of Transcatheter Aortic Valve Replacement in Eastern Denmark (2005 to 2015). JACC Cardiovasc. Interv. 2016, 9, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Popma, J.J.; Adams, D.H.; Reardon, M.J.; Yakubov, S.J.; Kleiman, N.S.; Heimansohn, D.; Hermiller, J., Jr.; Hughes, G.C.; Harrison, J.K.; Coselli, J.; et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J. Am. Coll. Cardiol. 2014, 63, 1972–1981. [Google Scholar] [CrossRef] [Green Version]
- Luscher, T.F. TAVI: From an experimental procedure to standard of care. Eur. Heart J. 2018, 39, 2605–2608. [Google Scholar] [CrossRef] [Green Version]
- De Sciscio, P.; Brubert, J.; De Sciscio, M.; Serrani, M.; Stasiak, J.; Moggridge, G.D. Quantifying the Shift Toward Transcatheter Aortic Valve Replacement in Low-Risk Patients: A Meta-Analysis. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e003287. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Lung, B.; Lancellotti, P.; Lansac, E.; Muñoz, D.R.; et al. 2017 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Rev. Esp. Cardiol. (Engl. Ed.) 2018, 71, 110. [Google Scholar]
- Gleason, T.G.; Reardon, M.J.; Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Lee, J.S.; Kleiman, N.S.; Chetcuti, S.; Hermiller, J.B.; Heiser, J.; et al. 5-Year Outcomes of Self-Expanding Transcatheter Versus Surgical Aortic Valve Replacement in High-Risk Patients. J. Am. Coll. Cardiol. 2018, 72, 2687–2696. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Guyton, R.A., 3rd; O’Gara, P.T.; Ruiz, C.E.; Skubas, N.J.; Sorajja, P.; et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2438–2488. [Google Scholar] [CrossRef] [Green Version]
- Webb, J.G.; Doshi, D.; Mack, M.J.; Makkar, R.; Smith, C.R.; Pichard, A.D.; Kodali, S.; Kapadia, S.; Miller, D.C.; Babaliaros, V.; et al. A Randomized Evaluation of the SAPIEN XT Transcatheter Heart Valve System in Patients With Aortic Stenosis Who Are Not Candidates for Surgery. JACC Cardiovasc. Interv. 2015, 8, 1797–1806. [Google Scholar] [CrossRef] [Green Version]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Del Trigo, M.; Munoz-Garcia, A.J.; Wijeysundera, H.C.; Nombela-Franco, L.; Cheema, A.N.; Gutierrez, E.; Serra, V.; Kefer, J.; Amat-Santos, I.J.; Benitez, L.M.; et al. Incidence, Timing, and Predictors of Valve Hemodynamic Deterioration After Transcatheter Aortic Valve Replacement: Multicenter Registry. J. Am. Coll. Cardiol. 2016, 67, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Cerrato, E.; Nombela-Franco, L.; Nazif, T.M.; Eltchaninoff, H.; Sondergaard, L.; Ribeiro, H.B.; Barbanti, M.; Nietlispach, F.; De Jaegere, P.; Agostoni, P.; et al. Evaluation of current practices in transcatheter aortic valve implantation: The WRITTEN (WoRldwIde TAVI ExperieNce) survey. Int. J. Cardiol. 2017, 228, 640–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bekeredjian, R.; Szabo, G.; Balaban, U.; Bleiziffer, S.; Bauer, T.; Ensminger, S.; Frerker, C.; Herrmann, E.; Beyersdorf, F.; Hamm, C.; et al. Patients at low surgical risk as defined by the Society of Thoracic Surgeons Score undergoing isolated interventional or surgical aortic valve implantation: In-hospital data and 1-year results from the German Aortic Valve Registry (GARY). Eur. Heart J. 2019, 40, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Mach, M.; Koschutnik, M.; Wilbring, M.; Winkler, B.; Reinweber, M.; Alexiou, K.; Kappert, U.; Adlbrecht, C.; Delle-Karth, G.; Grabenwöger, M.; et al. Impact of COPD on Outcome in Patients Undergoing Transfemoral versus Transapical TAVI. Thorac. Cardiovasc. Surg. 2019, 67, 251–256. [Google Scholar] [CrossRef]
- Kappetein, A.P.; Head, S.J.; Genereux, P.; Piazza, N.; van Mieghem, N.M.; Blackstone, E.H.; Brott, T.G.; Cohen, D.J.; Cutlip, D.E.; Van Es, G.-A.; et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document. Eur. Heart J. 2012, 33, 2403–2418. [Google Scholar] [CrossRef]
- McMurry, T.L.; Hu, Y.; Blackstone, E.H.; Kozower, B.D. Propensity scores: Methods, considerations, and applications in the Journal of Thoracic and Cardiovascular Surgery. J. Thorac. Cardiovasc. Surg. 2015, 150, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Klein, J.P.; Moeschberger, M.L. Survival Analysis: Techniques for Censored and Truncated Data, 2nd ed.; Springer: New York, NY, USA, 1997. [Google Scholar]
- Smith, C.R.; Leon, M.B.; Mack, M.J.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N. Engl. J. Med. 2011, 364, 2187–2198. [Google Scholar] [CrossRef] [Green Version]
- Mack, M.J.; Leon, M.B.; Smith, C.R.; Miller, D.C.; Moses, J.W.; Tuzcu, E.M.; Webb, J.G.; Douglas, P.S.; Anderson, W.N.; Blackstone, E.H.; et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): A randomised controlled trial. Lancet 2015, 385, 2477–2484. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef]
- Thyregod, H.G.; Steinbruchel, D.A.; Ihlemann, N.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; Chang, Y.; Franzen, O.W.; Engstrøm, T.; Clemmensen, P.; et al. Transcatheter Versus Surgical Aortic Valve Replacement in Patients With Severe Aortic Valve Stenosis: 1-Year Results From the All-Comers NOTION Randomized Clinical Trial. J. Am. Coll. Cardiol. 2015, 65, 2184–2194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thyregod, H.G.H.; Ihlemann, N.; Jorgensen, T.H.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; Chang, Y.; Franzen, O.W.; Engstrøm, T.; Clemmensen, P.; et al. Five-Year Clinical and Echocardiographic Outcomes from the Nordic Aortic Valve Intervention (NOTION) Randomized Clinical Trial in Lower Surgical Risk Patients. Circulation 2019, 139, 2714–2723. [Google Scholar] [CrossRef] [PubMed]
- Beyersdorf, F.; Bauer, T.; Freemantle, N.; Walther, T.; Frerker, C.; Herrmann, E.; Bleiziffer, S.; Möllmann, H.; Landwehr, S.; Ensminger, S.; et al. Five-year outcome in 18 010 patients from the German Aortic Valve Registry. Eur. J. Cardiothorac. Surg. 2021, ezab216. [Google Scholar] [CrossRef] [PubMed]
- Maisano, F.; Taramasso, M.; Nietlispach, F. Prognostic influence of paravalvular leak following TAVI: Is aortic regurgitation an active incremental risk factor or just a mere indicator? Eur. Heart J. 2015, 36, 413–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burkhart, C.S.; Dell-Kuster, S.; Gamberini, M.; Moeckli, A.; Grapow, M.; Filipovic, M.; Seeberger, M.; Monsch, A.U.; Strebel, S.P.; Steiner, L.A. Modifiable and nonmodifiable risk factors for postoperative delirium after cardiac surgery with cardiopulmonary bypass. J. Cardiothorac. Vasc. Anesth. 2010, 24, 555–559. [Google Scholar] [CrossRef]
- Maniar, H.S.; Lindman, B.R.; Escallier, K.; Avidan, M.; Novak, E.; Melby, S.J.; Damiano, M.S.; Lasala, J.; Quader, N.; Rao, R.S.; et al. Delirium after surgical and transcatheter aortic valve replacement is associated with increased mortality. J. Thorac. Cardiovasc. Surg. 2016, 151, 815–823.e2. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, A.; Schofer, N.; Gossling, A.; Seiffert, M.; Schirmer, J.; Deuschl, F.; Schneeberger, Y.; Voigtländer, L.; Detter, C.; Schaefer, U.; et al. Transcatheter aortic valve implantation versus surgical aortic valve replacement in low-risk patients: A propensity score-matched analysis. Eur. J. Cardiothorac. Surg. 2019, 56, 1131–1139. [Google Scholar] [CrossRef] [Green Version]
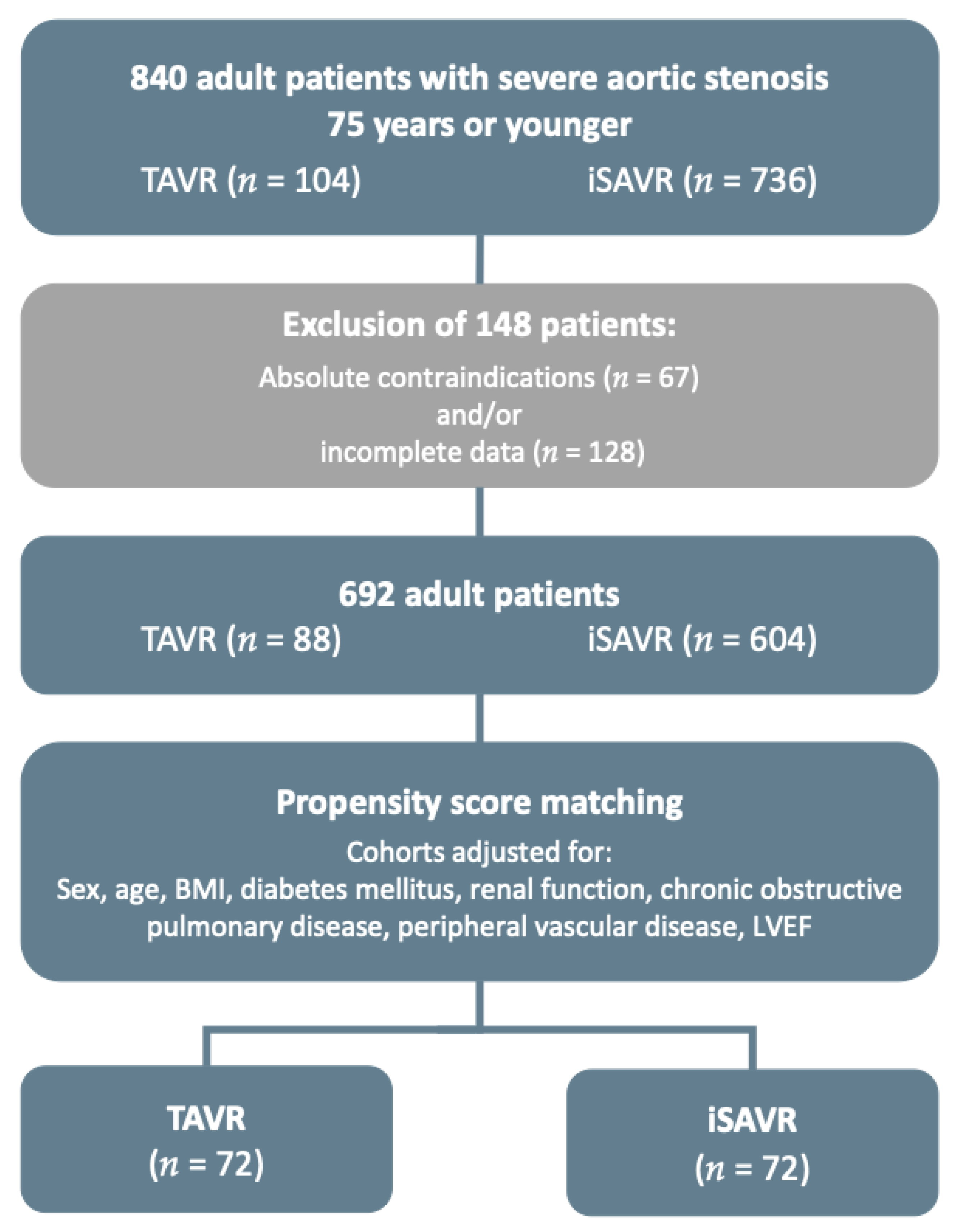
| TAVR < 75 Years n = 104 | |
|---|---|
| Prohibitive surgical risk, n (%) 1 | 8 (7.7) |
| Porcelain aorta, n (%) 1 | 9 (8.7) |
| High-risk reoperation, n (%) | 42 (40.4) |
| Respiratory impairment, n (%) | 41 (39.4) |
| Severely reduced LVEF, n (%) | 34 (32.7) |
| Severe renal insufficiency, n (%) | 32 (30.8) |
| Substance abuse, n (%) | 23 (22.1) |
| Adipositas per magna, n (%) | 16 (15.4) |
| Valve-in-Valve procedure, n (%) | 13 (12.5) |
| Neurological impairment, n (%) | 12 (11.5) |
| Hepatopathy, n (%) | 10 (9.6) |
| History of radiation to the chest, n (%) | 9 (8.7) |
| Severe mental disorder, n (%) | 9 (8.7) |
| Pulmonary hypertension, n (%) | 7 (6.7) |
| Frailty, n (%) | 3 (2.9) |
| Severe rhythm disorder, n (%) | 2 (1.9) |
| History of severe bleeding, n (%) | 1 (1.0) |
| Other, n (%) | 17 (16.3) |
| Patients with 2 or more reasons listed above | 74 (71.2) |
| Patients with 3 or more reasons listed above | 35 (33.7) |
| Unmatched-Population (n = 692) | PS-Matched-Population (n = 144) | |||||
|---|---|---|---|---|---|---|
| Overall n = 692 | iSAVR < 75 Years n = 604 | TAVR < 75 Years n = 88 | iSAVR < 75 Years n = 72 | TAVR < 75 Years n = 72 | p-Value | |
| Demographics | ||||||
| Age, mean (±SD) | 64.1 (9.5) | 63.4 (9.8) | 68.9 (5.2) | 67.6 (7) | 68.7 (5.5) | 0.190 |
| Female, n (%) | 287 (41.5) | 237 (39.2) | 50 (56.8) | 33 (45.8) | 39 (54.2) | 0.418 |
| Body mass index kg/m2, median (IQR) | 28.7 (5.5) | 28.6 (5.4) | 29.2 (6.5) | 29.3 (4.8) | 29.1 (6.7) | 0.854 |
| Risk profile | ||||||
| EuroSCORE II, median (IQR) | 2.7 (3.7) | 1.7 (2.2) | 5.9 (5.3) | 6.4 (4.3) | 4.3 (3.2) | 0.194 |
| Chronic Health Conditions and Risk Factors | ||||||
| Hypertension, n (%) | 538 (77.7) | 464 (92.2) | 74 (84.1) | 53 (73.6) | 62 (86.1) | 0.089 |
| Dyslipidaemia, n (%) | 433 (62.6) | 380 (82.3) | 53 (60.2) | 42 (36.2) | 41 (35.3) | 0.999 |
| Diabetes mellitus, n (%) | 200 (28.9) | 164 (27.2) | 36 (40.9) | 9 (12.5) | 8 (11.1) | 0.371 |
| Active smoker, n (%) | 126 (18.2) | 106 (17.5) | 20 (22.7) | 9 (12.5) | 18 (25.0) | 0.121 |
| Serum creatinine mg/dL, mean (±SD) | 1.1 (0.6) | 1.0 (0.4) | 1.5 (1.2) | 1.4 (0.7) | 1.3 (0.6) | 0.894 |
| Preoperative dialysis, n (%) | 6 (0.9) | 2 (0.3) | 4 (4.5) | 2 (2.8) | 1 (1.4) | 0.999 |
| Chronic obstructive pulmonary disease, n (%) | 217 (31.4) | 168 (24.3) | 49 (7.1) | 41 (56.9) | 37 (51.4) | 0.608 |
| Peripheral vascular disease, n (%) | 67 (9.7) | 43 (6.2) | 24 (27.3) | 14 (19.4) | 14 (19.4) | 0.999 |
| Cerebrovascular disease, n (%) | 111 (16.0) | 87 (14.4) | 24 (3.5) | 9 (12.5) | 19 (26.4) | 0.031 |
| Previous cerebrovascular accident, n (%) | 17 (2.5) | 7 (1.2) | 10 (11.4) | 4 (5.6) | 9 (12.5) | 0.227 |
| Atrial fibrillation, n (%) | 119 (17.2) | 101 (16.7) | 18 (20.5) | 14 (19.4) | 13 (18.1) | 0.999 |
| Previous myocardial infarction, n (%) | 54 (7.8) | 37 (6.1) | 17 (19.3) | 10 (13.9) | 13 (18.1) | 0.629 |
| New York Heart Association class III/IV, n (%) | 367 (53.1) | 288 (47.7) | 79 (90) | 51 (70.8) | 63 (87.5) | 0.072 |
| Preprocedural PCI, n (%) | 43 (6.2) | 26 (4.3) | 17 (19.3) | 4 (5.6) | 14 (19.4) | 0.021 |
| Previous pacemaker implantation, n (%) | 32 (4.6) | 17 (2.8) | 15 (17) | 5 (6.9) | 11 (15.3) | 0.210 |
| Previous cardiac surgery, n (%) | 64 (9.2) | 26 (4.3) | 38 (43.2) | 16 (22.2) | 26 (36.1) | 0.064 |
| Previous CABG, n (%) | 34 (4.9) | 11 (1.8) | 23 (26.1) | 9 (12.5) | 17 (23.6) | |
| Previous valve surgery, n (%) | 34 (4.9) | 16 (2.6) | 18 (20.5) | 10 (13.9) | 10 (13.9) | |
| aortic, n (%) | 25 (3.6) | 12 (2.0) | 13 (14.8) | 9 (12.5) | 10 (13.9) | |
| mitral, n (%) | 9 (1.5) | 4 (0.7) | 5 (5.7) | 1 (1.4) | 0 (0) | |
| tricuspid, n (%) | 3 (0.4) | 2 (0.3) | 1 (1.0) | 0 (0) | 0 (0) | |
| Previous other cardiac surgery, n (%) | 17 (2.5) | 2 (0.3) | 15 (17) | 0 (0) | 10 (13.9) | |
| Preoperative Echocardiographic Data | ||||||
| Mean pressure gradient, mean (±SD) | 48 (17.3) | 48.6 (17.6) | 46.3 (18.3) | 48.3 (17.9) | 46.7 (18.6) | 0.266 |
| Left ventricular ejection fraction %, mean (±IQR) 1 | 52.7 (9.9) | 53.4 (9.2) | 46.5 (11.9) | 51.7 (12.8) | 47.7 (11.3) | 0.061 |
| Unmatched-Population (n = 692) | PS-Matched-Population (n = 144) | |||||
|---|---|---|---|---|---|---|
| Overall n = 692 | iSAVR < 75 Years n = 604 | TAVR < 75 Years n = 88 | iSAVR < 75 Years n = 72 | TAVR < 75 Years n = 72 | p-Value | |
| Procedural Characteristics | ||||||
| Biological valve prosthesis, n (%) | 595 (86.0) | 507 (83.9) | 88 (100) | 62 (86.1) | 72 (100) | n/a ‡ |
| Balloon-expandable THV, n (%) | 56 (63.6) | 43 (59.7) | n/a ‡ | |||
| Prosthesis size in mm, mean (±SD) | 23.2 (3.2) | 22.8 (3.1) | 26.3 (2.2) | 22.7 (2.2) | 26.5 (2.1) | <0.001 |
| Full sternotomy, n (%) | 494 (81.2) | 66 (91.7) | n/a ‡ | |||
| Cross-clamp time, mean (±SD) | 58.3 (31) | 58.3 (31) | 0 (0) | 62.8 (21.5) | 0 (0) | n/a ‡ |
| Perfusion time, mean (±SD) | 87.8 (52.1) | 87.8 (52.1) | 0 (0) | 111.2 (40.2) | 0 (0) | n/a ‡ |
| Transfemoral access, n (%) | 42 (47.7) | 29 (40.3) | n/a ‡ | |||
| Predilatation, n (%) | 43 (48.9) | 38 (52.8) | n/a ‡ | |||
| Postdilatation, n (%) | 9 (10.2) | 5 (6.9) | n/a ‡ | |||
| Paravalvular leak > mild, n (%) | 1 (0.1) | 0 (0) | 1 (1.1) | 0 (0) | 1 (1.4) | 0.999 |
| Postoperative circulatory support, n (%) | 8 (1.2) | 7 (1.2) | 1 (1.1) | 4 (5.6) | 0 (0) | n/a ‡ |
| Extubated in the operating room, n (%) | 10 (1.4) | 0 (0) | 10 (12) | 0 (0) | 6 (8.3) | n/a ‡ |
| Total hours ventilated, median (±IQR) | 8 (8) | 8.0 (8) | 4 (7) | 12 (27) | 4 (7) | <0.001 |
| Re-intubated during hospital stay, n (%) | 22 (3.2) | 19 (3.1) | 3 (3.6) | 4 (5.6) | 3 (4.5) | 0.999 |
| Number of administered red blood cell units, mean (±SD) | 0.6 (1.6) | 0.6 (1.6) | 0.6 (1.2) | 1.0 (3.0) | 0.6 (1.2) | 0.242 |
| Length of stay, median (±IQR) | 11.0 (5) | 11 (5) | 9 (7) | 11.5 (6) | 9.0 (7) | 0.188 |
| Unmatched-Population (n = 692) | PS-Matched-Population (n = 144) | |||||
|---|---|---|---|---|---|---|
| Overall n = 692 | iSAVR < 75 Years n = 604 | TAVR < 75 Years n = 88 | iSAVR < 75 Years n = 72 | TAVR < 75 Years n = 72 | p-Value | |
| VARC-2 Adverse Events | ||||||
| Myocardial infarction, n (%) | 2 (0.3) | 1 (0.2) | 1 (1.1) | 0 (0) | 1 (1.4) | n/a ‡ |
| Neurological adverse event, n (%) | 9 (1.3) | 7 (1.2) | 2 (2.3) | 2 (2.8) | 2 (2.8) | 0.999 |
| Major vascular access complication, n (%) | 5 (0.7) | 0 (0) | 5 (5.7) | 0 (0) | 4 (5.6) | n/a ‡ |
| Major bleeding complication, n (%) | 28 (4.0) | 24 (4.0) | 4 (4.6) | 6 (8.3) | 3 (4.2) | 0.508 |
| Postoperative dialysis, n (%) | 10 (1.4) | 8 (1.3) | 2 (2.3) | 2 (2.8) | 2 (2.8) | 0.999 |
| New-onset atrial fibrillation, n (%) | 84 (12.1) | 79 (13.1) | 5 (5.7) | 14 (19.4) | 5 (6.9) | 0.049 |
| AV-Block III, n (%) | 16 (2.3) | 11 (1.8) | 5 (5.7) | 0 (0) | 2 (2.8) | n/a ‡ |
| Pacemaker implantation, n (%) | 18 (2.6) | 13 (2.2) | 5 (5.7) | 0 (0) | 2 (2.8) | n/a ‡ |
| Reoperation for valvular dysfunction, n (%) | 2 (0.3) | 2 (0.3) | 0 (0) | 1 (1.4) | 0 (0) | n/a ‡ |
| Reoperation for bleeding/tamponade, n (%) | 16 (2.3) | 15 (2.5) | 1 (1.1) | 4 (5.6) | 1 (1.4) | 0.375 |
| Reoperation for other cardiac problem, n (%) | 5 (0.7) | 2 (0.3) | 3 (0.4) | 1 (1.4) | 2 (2.8) | 0.999 |
| Reoperation for non-cardiac problem, n (%) | 15 (2.2) | 10 (1.7) | 5 (5.7) | 0 (0) | 5 (6.9) | n/a ‡ |
| Postoperative sepsis, n (%) | 4 (0.6) | 4 (0.7) | 0 (0) | 2 (2.8) | 0 (0) | n/a ‡ |
| Pronounced wound infection, n (%) | 9 (1.3) | 9 (1.5) | 0 (0) | 5 (5.6) | 0 (0) | n/a ‡ |
| Prolonged ventilation > 6 h, n (%) | 64 (9.2) | 61 (10.1) | 3 (0.4) | 18 (25.0) | 2 (2.8) | <0.001 |
| Multi- organ dysfunction syndrome, n (%) | 10 (1.4) | 10 (1.7) | 0 (0) | 5 (6.9) | 0 (0) | n/a ‡ |
| In-hospital death, n (%) | 17 (2.5) | 16 (2.3) | 1 (1.1) | 10 (13.9) | 1 (1.4) | 0.012 |
| 30-day all-cause mortality, n (%) | 19 (2.7) | 16 (2.7) | 3 (3.4) | 9 (12.5) | 2 (2.8) | 0.022 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mach, M.; Poschner, T.; Hasan, W.; Kerbel, T.; Szalkiewicz, P.; Hasimbegovic, E.; Andreas, M.; Gross, C.; Strouhal, A.; Delle-Karth, G.; et al. Transcatheter versus Isolated Surgical Aortic Valve Replacement in Young High-Risk Patients: A Propensity Score-Matched Analysis. J. Clin. Med. 2021, 10, 3447. https://doi.org/10.3390/jcm10153447
Mach M, Poschner T, Hasan W, Kerbel T, Szalkiewicz P, Hasimbegovic E, Andreas M, Gross C, Strouhal A, Delle-Karth G, et al. Transcatheter versus Isolated Surgical Aortic Valve Replacement in Young High-Risk Patients: A Propensity Score-Matched Analysis. Journal of Clinical Medicine. 2021; 10(15):3447. https://doi.org/10.3390/jcm10153447
Chicago/Turabian StyleMach, Markus, Thomas Poschner, Waseem Hasan, Tillmann Kerbel, Philipp Szalkiewicz, Ena Hasimbegovic, Martin Andreas, Christoph Gross, Andreas Strouhal, Georg Delle-Karth, and et al. 2021. "Transcatheter versus Isolated Surgical Aortic Valve Replacement in Young High-Risk Patients: A Propensity Score-Matched Analysis" Journal of Clinical Medicine 10, no. 15: 3447. https://doi.org/10.3390/jcm10153447
APA StyleMach, M., Poschner, T., Hasan, W., Kerbel, T., Szalkiewicz, P., Hasimbegovic, E., Andreas, M., Gross, C., Strouhal, A., Delle-Karth, G., Grabenwöger, M., Adlbrecht, C., & Schober, A. (2021). Transcatheter versus Isolated Surgical Aortic Valve Replacement in Young High-Risk Patients: A Propensity Score-Matched Analysis. Journal of Clinical Medicine, 10(15), 3447. https://doi.org/10.3390/jcm10153447







