Current Lack of Evidence for an Effect of Physical Activity Intervention Combined with Pharmacological Treatment on Bone Turnover Biomarkers in People with Osteopenia and Osteoporosis: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Data Sources
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Quality Assessment
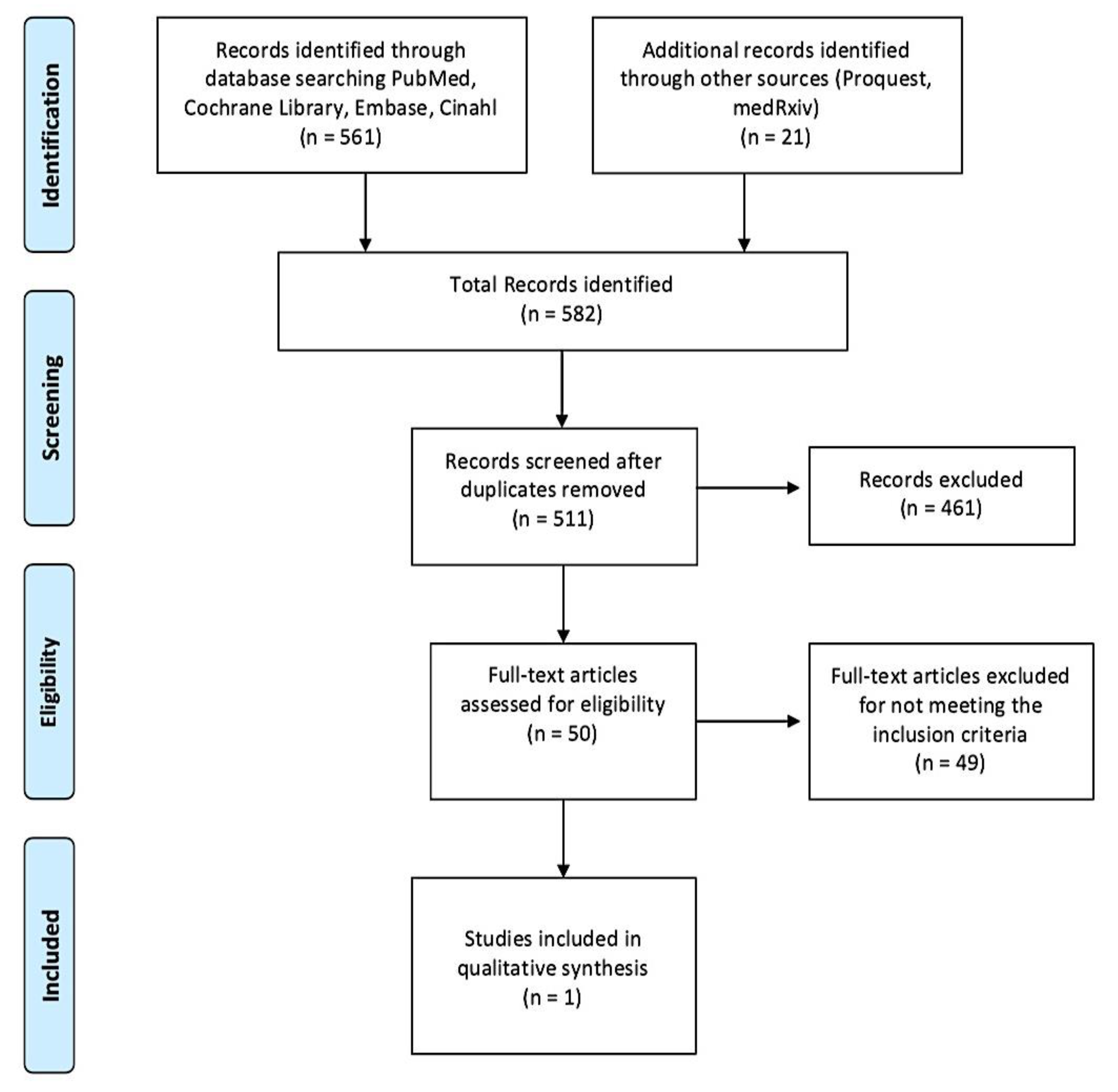
3. Results
3.1. Study Selection and Characteristics
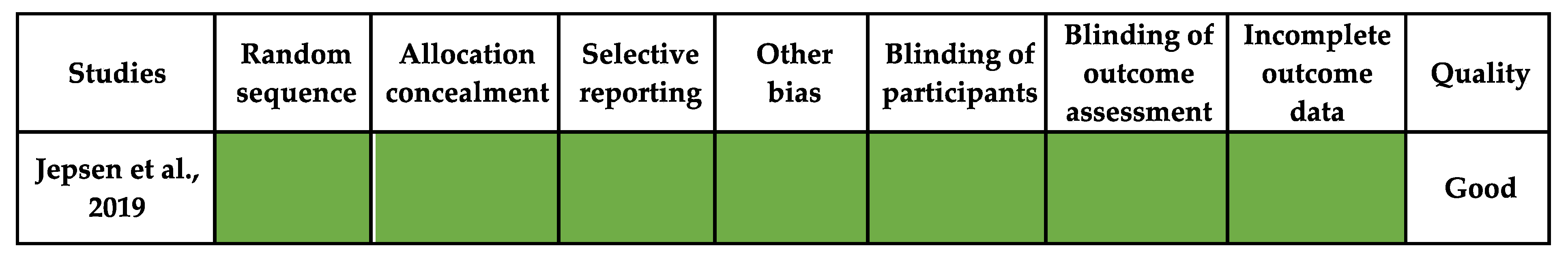
3.2. Risk of Bias
3.3. Data Extraction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anonymous. Consensus Development Conference: Diagnosis, Prophylaxis, and Treatment of Osteoporosis. Am. J. Med. 1993, 94, 646–650. [Google Scholar] [CrossRef]
- Kanis, J.A.; Melton, L.J., 3rd; Christiansen, C.; Johnston, C.C.; Khaltaev, N. The diagnosis of osteoporosis. J. Bone Miner. Res. 1994, 9, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Karaguzel, G.; Holick, M.F. Diagnosis and treatment of osteopenia. Rev. Endocr. Metab. Disord. 2010, 11, 237–251. [Google Scholar] [CrossRef]
- Orwoll, E.S.; Vanderschueren, D.; Boonen, S. Osteoporosis in men: Epidemiology, pathophysiology, and clinical characterization. In Osteoporosis, 4th ed.; Marcus, R., Feldman, D., Dempster, D.W., Luckey, M., Cauley, J.A., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 757–802. [Google Scholar]
- Cooper, C.; Ferrari, S. Compendium of Osteoporosis. International Osteoporosis Foundation. 2019. Available online: http://www.worldosteoporosisday.org/sites/default/WOD-2019/resources/compendium/2019-IOF-Compendium-of-Osteoporosis-WEB.pdf (accessed on 25 June 2021).
- Eriksen, E.F. Treatment of osteopenia. Rev. Endocr. Metab. Disord. 2012, 13, 209–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odén, A.; McCloskey, E.V.; Kanis, J.A.; Harvey, N.C.; Johansson, H. Burden of high fracture probability worldwide: Secular increases 2010–2040. Osteoporos. Int. 2015, 26, 2243–2248. [Google Scholar] [CrossRef]
- Kanis, J. Assessment of Osteoporosis at the Primary Health-Care Level, WHO Scientific Group Technical Report. 2007. Available online: https://www.sheffield.ac.uk/FRAX/pdfs/WHO_Technical_Report.pdf (accessed on 25 June 2021).
- Hernlund, E.; Svedbom, A.; Ivergård, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jonsson, B.; Kanis, J.A. Osteoporosis in the European Union: Medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch. Osteoporos. 2013, 8, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavone, V.; Testa, G.; Giardina, S.M.C.; Vescio, A.; Restivo, D.A.; Sessa, G. Pharmacological Therapy of Osteoporosis: A Systematic Current Review of Literature. Front. Pharmacol. 2017, 8, 803. [Google Scholar] [CrossRef]
- Bilezikian, J.P.; Bouillon, R.; Clemens, T.; Compston, J.; Bauer, D.C.; Ebeling, P.R.; Engelke, K.; Goltzman, D.; Guise, T.; Jan de Beur, S.M. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism, 1st ed.; Wiley: Hoboken, NJ, USA, 2018; ISBN 978-1-119-26656-3. [Google Scholar]
- Kanis, J.A.; Johnell, O.; Black, D.M.; Downs, R.W.; Sarkar, S.; Fuerst, T.; Secrest, R.J.; Pavo, I. Effect of raloxifene on the risk of new vertebral fracture in postmenopausal women with osteopenia or osteoporosis: A reanalysis of the Multiple Outcomes of Raloxifene Evaluation trial. Bone 2003, 33, 293–300. [Google Scholar] [CrossRef]
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Reginster, J.Y.; Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis (ESCEO); The Committees of Scientific Advisors and National Societies of the International Osteoporosis Foundation (IOF). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2019, 30, 3–44. [Google Scholar] [CrossRef] [Green Version]
- International Osteoporosis Foundation. No More Broken Bones! Take Action for Prevention, Diagnosis & Treatment. 2020. Available online: https://www.osteoporosis.foundation/health-professionals/treatment#ref_bottom_1 (accessed on 25 June 2021).
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd ed.; Department of Health and Human Services: Washington, DC, USA, 2018. Available online: https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf (accessed on 25 June 2021).
- WHO. WHO Guidelines on Physical Activity and Sedentary Behaviour; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- McMillan, L.B.; Zengin, A.; Ebeling, P.R.; Scott, D. Prescribing Physical Activity for the Prevention and Treatment of Osteoporosis in Older Adults. Healthcare 2017, 5, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber-Rajek, M.; Mieszkowski, J.; Niespodziński, B.; Ciechanowska, K. Whole-body vibration exercise in postmenopausal osteoporosis. Prz. Menopauzalny 2015, 14, 41–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klarner, A.; von Stengel, S.; Kemmler, W.; Kladny, B.; Kalender, W. Effects of two different types of whole body vibration on neuromuscular performance and body composition in postmenopausal women. Dtsch. Med. Wochenschr. 2011, 136, 2133–2139. [Google Scholar] [CrossRef] [PubMed]
- Agostini, D.; Donati, S.Z.; Lucertini, F.; Annibalini, G.; Gervasi, M.; Ferri Marini, C.; Piccoli, G.; Stocchi, V.; Barbieri, E.; Sestili, P. Muscle and bone health in postmenopausal women: Role of protein and vitamin D supplementation combined with exercise training. Nutrients 2018, 10, 1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, X.; Chen, X.; Zhang, S.; Huang, M.; Shen, X.; Xu, J.; Zou, J. The Effect of Exercise on the Prevention of Osteoporosis and Bone Angiogenesis. BioMed Res. Int. 2019, 2019, 8171897. [Google Scholar] [CrossRef]
- Xu, J.; Lombardi, G.; Jiao, W.; Banfi, G. Effects of Exercise on Bone Status in Female Subjects, from Young Girls to Postmenopausal Women: An Overview of Systematic Reviews and Meta-Analyses. Sports Med. 2016, 46, 1165–1182. [Google Scholar] [CrossRef]
- Daly, R.M. Exercise and nutritional approaches to prevent frail bones, falls and fractures: An update. Climacteric 2017, 20, 119–124. [Google Scholar] [CrossRef]
- International Osteoporosis Foundation. 2020. Available online: https://www.osteoporosis.foundation/health-professionals/about-osteoporosis/bone-biology (accessed on 25 June 2021).
- Nagy, E.; Nagy-Finna, C.; Popoviciu, H.-V.; Kovács, B. Soluble Biomarkers of Osteoporosis and Osteoarthritis, from Pathway Mapping to Clinical Trials: An Update. Clin. Interv. Aging. 2020, 15, 501–518. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.P.; Albert, C.; Nassar, B.A.; Adachi, J.D.; Cole, D.; Davison, K.S.; Dooley, K.C.; Don-Wauchope, A.; Douville, P.; Hanley, D.A. Bone turnover markers in the management of postmenopausal osteoporosis. Clin. Biochem. 2009, 42, 929–942. [Google Scholar] [CrossRef]
- Kuo, T.-R.; Chen, C.-H. Bone biomarker for the clinical assessment of osteoporosis: Recent developments and future perspectives. Biomark. Res. 2017, 5, 18. [Google Scholar] [CrossRef] [Green Version]
- Glendenning, P.; Chubb, S.A.P.; Vasikaran, S. Clinical utility of bone turnover markers in the management of common metabolic bone diseases in adults. Clin. Chim. Acta 2018, 481, 161–170. [Google Scholar] [CrossRef]
- Migliorini, F.; Maffulli, N.; Spiezia, F.; Peretti, G.M.; Tingart, M.; Giorgino, R. Potential of biomarkers during pharmacological therapy setting for postmenopausal osteoporosis: A systematic review. J. Orthop. Surg. Res. 2021, 16, 351. [Google Scholar] [CrossRef]
- Faienza, M.F.; Lassandro, G.; Chiarito, M.; Valente, F.; Ciaccia, L.; Giordano, P. How Physical Activity across the Lifespan Can Reduce the Impact of Bone Ageing: A Literature Review. Int. J. Environ. Res. Public Health 2020, 17, 1862. [Google Scholar] [CrossRef] [Green Version]
- Arazi, H.; Samadpour, M.; Eghbali, E. The effects of concurrent training (Aerobic resistance) and milk consumption on some markers of bone mineral density in women with osteoporosis. BMC Womens Health 2018, 18, 202. [Google Scholar] [CrossRef] [PubMed]
- Roghani, T.; Torkaman, G.; Movasseghe, S.; Hedayati, M.; Goosheh, B.; Bayat, N. Effects of short-term aerobic exercise with and without external loading on bone metabolism and balance in postmenopausal women with osteoporosis. Rheumatol. Int. 2013, 33, 291–298. [Google Scholar] [CrossRef] [PubMed]
- El-Mekawy, H.E.-S.; Dein, L.S.E. Exercise Programs for Treating Post Menopausal Osteoporotic Women; Which is Best? Indian J. Physiother. Occup. Ther. 2012, 6, 301–305. [Google Scholar]
- Khosla, S.; Shane, E. A Crisis in the Treatment of Osteoporosis. J. Bone Miner. Res. 2016, 31, 1485–1487. [Google Scholar] [CrossRef] [Green Version]
- Tarantino, U.; Iolascon, G.; Cianferotti, L.; Masi, L.; Marcucci, G.; Giusti, F.; Marini, F.; Parri, S.; Feola, M.; Rao, C.; et al. Clinical guidelines for the prevention and treatment of osteoporosis: Summary statements and recommendations from the Italian Society for Orthopaedics and Traumatology. J. Orthop. Traumatol. 2017, 18, 3–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Compston, J.; Cooper, A.; Cooper, C.; Gittoes, N.; Gregson, C.; Harvey, N.; Hope, S.; Kanis, J.A.; McCloskey, E.V.; Vine, N.; et al. National Osteoporosis Guideline Group (NOGG). UK clinical guideline for the prevention and treatment of osteoporosis. Arch. Osteoporos. 2017, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Yates, C.J.; Chauchard, M.A.; Liew, D.; Bucknill, A.; Wark, J.D. Bridging the osteoporosis treatment gap: Performance and cost-effectiveness of a fracture liaison service. J. Clin. Densitom. 2015, 18, 150–156. [Google Scholar] [CrossRef]
- Vestergaard, P.; Mosekilde, L.; Langdahl, B. Fracture prevention in postmenopausal women. BMJ Clin. Evid. 2011, 2011, 1109. [Google Scholar]
- Morris, H.A.; Eastell, R.; Jorgensen, N.R.; Cavalier, E.; Vasikaran, S.; Chubb, S.A.P.; Kanis, J.A.; Cooper, C.; Makris, K.; IFCC-IOF Working Group for Standardisation of Bone Marker Assays (WG-BMA). Clinical usefulness of bone turnover marker concentrations in osteoporosis. Clin. Chim. Acta 2017, 467, 34–41. [Google Scholar] [CrossRef]
- Fischbacher, M.; Weeks, B.K.; Beck, B.R. The influence of antiresorptive bone medication on the effect of high- intensity resistance and impact training on osteoporotic fracture risk in postmenopausal women with low bone mass: Protocol for the MEDEX-OP randomised controlled trial. BMJ Open 2019, 9, e029895. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.; Green, S. Cochrane Reviewers’ Handbook 5.0.1; Wiley, The Cochrane Library: Chichester, UK, 2008. [Google Scholar]
- Greenhalgh, T.; Peacock, R. Effectiveness and efficiency of search methods in systematic reviews of complex evidence: Audit of primary sources. BMJ 2005, 331, 1064–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jepsen, D.B.; Ryg, J.; Hansen, S.; Jørgensen, N.R.; Gram, J.; Masud, T. The combined effect of Parathyroid hormone (1–34) and whole-body Vibration exercise in the treatment of postmenopausal OSteoporosis (PaVOS study): A randomized controlled trial. Osteoporos. Int. 2019, 30, 1827–1836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corrie, H.; Brooke-Wavell, K.; Mansfield, N.J.; Cowley, A.; Morris, R.; Masud, T. Effects of vertical and side-alternating vibration training on fall risk factors and bone turnover in older people at risk of falls. Age Ageing 2015, 44, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Sözen, T.; Özışık, L.; Başaran, N.Ç. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Ensrud, K.E.; Thompson, D.E.; Cauley, J.A.; Nevitt, M.C.; Kado, D.M.; Hochberg, M.C.; Santora, A.C., II; Black, D.M. Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass. Fracture Intervention Trial Research Group. J. Am. Geriatr. Soc. 2000, 48, 241–249. [Google Scholar] [CrossRef]
- Johnell, O.; Kanis, J.A.; Odén, A.; Sernbo, I.; Redlund-Johnell, I.; Petterson, C.; De Laet, C.; Jönsson, B. Mortality after osteoporotic fractures. Osteoporos. Int. 2004, 15, 38–42. [Google Scholar] [CrossRef]
- Gillespie, L.D.; Robertson, M.C.; Gillespie, W.J.; Lamb, S.E.; Gates, S.; Cumming, R.G.; Rowe, B.H. Interventions for preventing falls in older people living in the community. Cochrane Database Syst. Rev. 2009, 2, CD007146. [Google Scholar] [CrossRef]
- Forsén, L.; Arstad, C.; Sandvig, S.; Schuller, A.; Røed, U.; Søgaard, A.J. Prevention of hip fracture by external hip protectors: An intervention in 17 nursing homes in two municipalities in Norway. Scand. J. Public. Health 2003, 31, 261–266. [Google Scholar] [CrossRef]
- Kistler-Fischbacher, M.; Weeks, B.K.; Beck, B.R. The effect of exercise intensity on bone in postmenopausal women (part 1): A systematic review. Bone 2021, 143, 115696. [Google Scholar] [CrossRef]
- Berry, S.D.; Miller, R.R. Falls: Epidemiology, pathophysiology, and relationship to fracture. Curr. Osteoporos. Rep. 2008, 6, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Sherrington, C.; Fairhall, N.J.; Wallbank, G.K.; Tiedemann, A.; Michaleff, Z.A.; Howard, K.; Clemson, L.; Hopewell, S.; Lamb, S.E. Exercise for preventing falls in older people living in the community. Cochrane Database Syst. Rev. 2019, 1, CD012424. [Google Scholar] [CrossRef]
- Uusi-Rasi, K.; Kannus, P.; Cheng, S.; Sievänen, H.; Pasanen, M.; Heinonen, A.; Nenonen, A.; Halleen, J.; Fuerst, T.; Genant, H.; et al. Effect of alendronate and exercise on bone and physical performance of postmenopausal women: A randomized controlled trial. Bone 2003, 33, 132–143. [Google Scholar] [CrossRef]
- Newstead, A.; Smith, K.I.; Bruder, J.; Keller, C. The effect of a jumping exercise intervention on bone mineral density in postmenopausal women. J. Ger. Phys. Ther. 2004, 27, 47–52. [Google Scholar] [CrossRef]
- Kendler, D.; Bauer, D.C.; Davison, K.; Dian, L.; Hanley, D.A.; Harris, S.; McClung, M.R.; Miller, P.; Schousboe, J.; Yuen, C. Vertebral fractures: Clinical importance and management. Am. J. Med. 2016, 129, 221.e1–221.e10. [Google Scholar] [CrossRef] [Green Version]
| Drug Class | Mechanism of Action | Characteristics | References |
|---|---|---|---|
| Anti-Resorptive | |||
| Biphosphonates | Interaction with specific intracellular pathways in osteoclasts, resulting in cellular toxicity | First-line pharmacological therapy for most post-menopausal women at risk for fractures. Efficacy in reducing the risk of vertebral, non-vertebral and hip | Pavone, 2017 [10]; Kanis, 2019 [13]; IOF, 2020 [14]; Compston, 2019 [15] |
| Denosumab | Inactivation of osteoclasts, reduction of osteoclasts’ differentiation | Strong efficacy in reducing spine and hip fractures. First-line therapy in patients intolerant to oral BP or having renal failure | Pavone, 2017 [10]; Kanis, 2019 [13]; Compston 2019 [15]. |
| Menopausal Hormone Therapy /Hormone Replacement Therapy | Increase of bone mineral density at all skeletal sites in early and late postmenopausal women | Therapy to prevent bone loss and reduce fracture risk in women at high risk of fracture when alternate therapies are not appropriate | Pavone, 2017 [10]; Kanis, 2019 [13]; IOF, 2020 [14]. |
| Selective Estrogen Receptor Modulators | Interaction with bone estrogen receptors, increasing trabecular bone mass | Contraindicated for prevention or treatment of OP in premenopausal women. Option treatment for younger postmenopausal women with osteopenia and osteoporosis without pronounced vasomotor menopausal symptoms, who are at risk for vertebral but not hip fractures | Pavone, 2017 [10]; Kanis, 2019 [13]; IOF, 2020 [14]. |
| Anabolic | |||
| Teriparatide | Activation of osteoblasts by binding the parathyroid hormone receptor; stimulation of bone formation on active remodelling sites, particularly in the trabecular compartment | Daily administration of subcutaneous injection for 18–24 months reduces the risk of vertebral and non-vertebral fracture in osteoporotic women | Pavone, 2017 [10]; Kanis, 2019 [13]; IOF, 2020 [14]. |
| Abaloparatide | Selective activation of the parathyroid hormone receptor | Increase of BMD at the lumbar spine, femoral neck, and total hip; reduced risk of new vertebral fractures in postmenopausal patients with osteoporosis. It has been approved only in the USA | Compston, 2019 [15]; IOF, 2020 [14]. |
| Romosozumab | Inhibition of sclerostin; Increase of bone formation and decrease of bone resorption | A starting treatment in women with high risk of fracture reduces the incidence of new vertebral fractures. The effects are reversible when the treatment is stopped, hence the therapy will need to be administered in sequence with an anti-resorptive drug | IOF, 2020 [14]. |
| Parameter | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | OP people (T-score ≤ 2.5) Osteopenic people (1 < T-score < 2.5) Aged 45–80+ | Population with secondary OP Absence of OP diagnosis Different diseases |
| Intervention | PA combined with pharmacological treatment | Absence of PA and pharmacological treatment |
| Comparator | Standard pharmacological treatment No exercise intervention | Participants receiving different PA |
| Outcome | Bone biomarkers evaluation, physical performance or other indices of physical performance | No information about bone biomarkers and PA |
| Study design | Experimental or observational study with original primary data | Study Protocol or other papers without original data |
| Timing | English Language 10-year publication date limit (January 2011) | Not in English Language Published before January 2011 |
| Study | Study Design | Sample | Intervention | Outcomes | Results |
|---|---|---|---|---|---|
| Jepsen et al., 2019, [46] Odense, Denmark | RCT | N:35 age:53–81 EG:17 CG:18 | Duration: 12 weeks Type of intervention: EG: Whole-body vibration (WBV) training protocol 12 min × 3 session/week, rest ratio 1:1 min with a frequency of 30 Hz and amplitude of 1 mm + Teriparatide (20 μg/day) CG: Teriparatide (20 μg/day) | Primary outcome: CTX, P1NP Sclerostin Secondary outcome: BMD lumbar spine (L1-L4) and total hip, bone microarchitecture distal radius and tibia | Primary outcomeresults Statistically significant improvement in CTX: EG: p < 0.05 CG: p < 0.05 Statistically significant improvement in P1NP: EG: p < 0.05 CG: p < 0.05 No statistically significant improvement in Sclerostin Secondary outcome results Statistically significant improvement in BMD lumbar spine of both group at 6 and 12 months EG: 6.47% ± 3.40; 8.90% ± 5.48 CG: 3.48% ± 4.39; 6.65% ± 5.57 No statistically significant improvement in BMD total hip No statistically significant improvement in bone microarchitecture |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marini, S.; Barone, G.; Masini, A.; Dallolio, L.; Bragonzoni, L.; Longobucco, Y.; Maffei, F. Current Lack of Evidence for an Effect of Physical Activity Intervention Combined with Pharmacological Treatment on Bone Turnover Biomarkers in People with Osteopenia and Osteoporosis: A Systematic Review. J. Clin. Med. 2021, 10, 3442. https://doi.org/10.3390/jcm10153442
Marini S, Barone G, Masini A, Dallolio L, Bragonzoni L, Longobucco Y, Maffei F. Current Lack of Evidence for an Effect of Physical Activity Intervention Combined with Pharmacological Treatment on Bone Turnover Biomarkers in People with Osteopenia and Osteoporosis: A Systematic Review. Journal of Clinical Medicine. 2021; 10(15):3442. https://doi.org/10.3390/jcm10153442
Chicago/Turabian StyleMarini, Sofia, Giuseppe Barone, Alice Masini, Laura Dallolio, Laura Bragonzoni, Yari Longobucco, and Francesca Maffei. 2021. "Current Lack of Evidence for an Effect of Physical Activity Intervention Combined with Pharmacological Treatment on Bone Turnover Biomarkers in People with Osteopenia and Osteoporosis: A Systematic Review" Journal of Clinical Medicine 10, no. 15: 3442. https://doi.org/10.3390/jcm10153442
APA StyleMarini, S., Barone, G., Masini, A., Dallolio, L., Bragonzoni, L., Longobucco, Y., & Maffei, F. (2021). Current Lack of Evidence for an Effect of Physical Activity Intervention Combined with Pharmacological Treatment on Bone Turnover Biomarkers in People with Osteopenia and Osteoporosis: A Systematic Review. Journal of Clinical Medicine, 10(15), 3442. https://doi.org/10.3390/jcm10153442









