Impact of Renal Replacement Therapy on Mortality in Critically Ill Patients—The Nephrologist’s View within an Interdisciplinary Intensive Care Team
Abstract
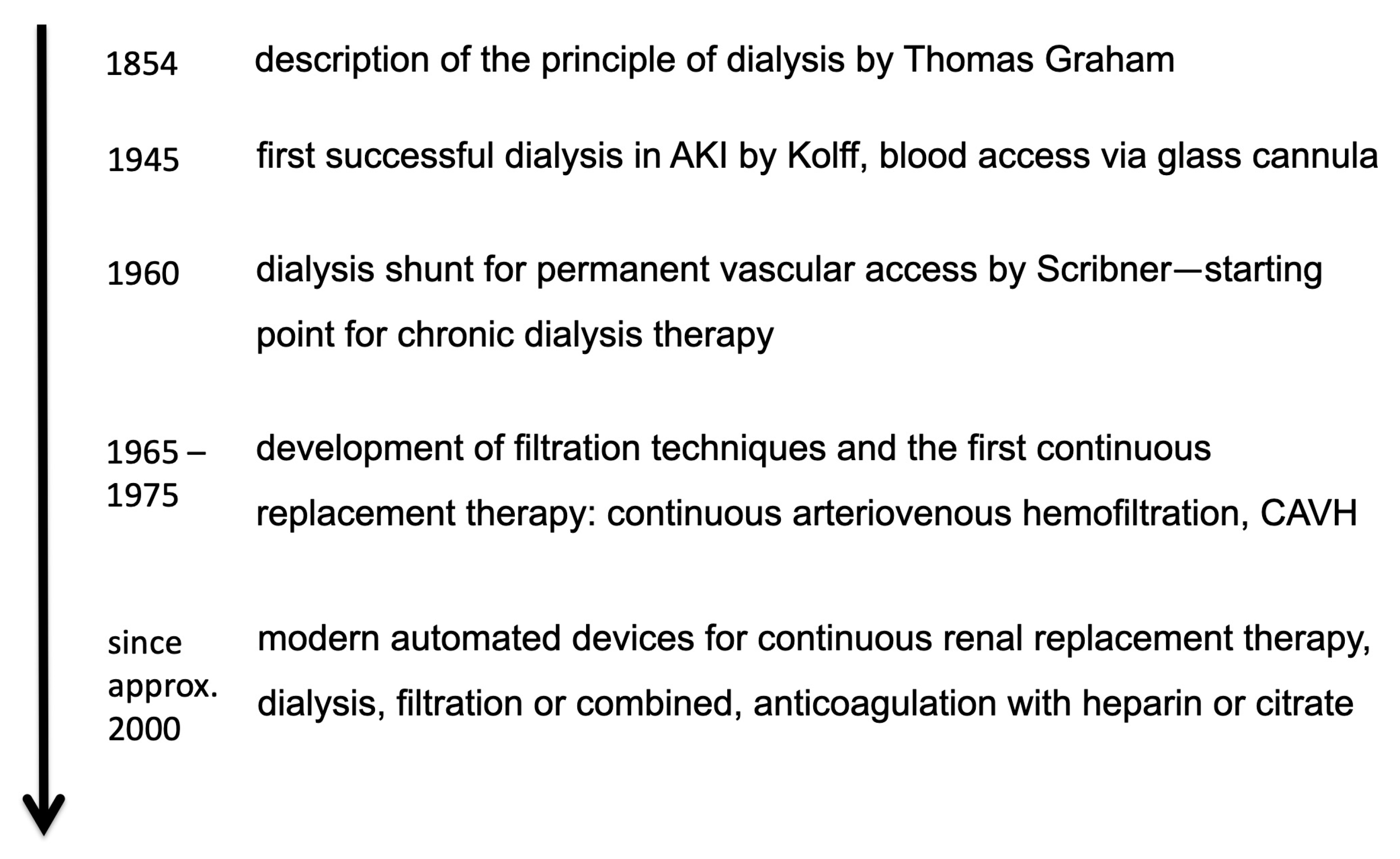
:1. History of Dialysis
- In the beginning, dialysis was considered as a short-term treatment in the case of severe AKI. Later, dialysis became an option for long-term treatment in the case of persistent kidney failure. Today, chronic dialysis is an established long-term therapy option.
- Nowadays, dialysis machines have been developed up to computer-controlled high-tech machines. These modern dialysis machines with several automatic monitoring and control functions ensure a safe and efficient dialysis treatment. Therefore, kidney replacement therapy can be carried out in practically any intensive care unit.
- Compared to the first dialysis, nowadays a variety of therapy options for different purposes are available, different choices of dialysis or filtration techniques can be chosen combined with a high variability of dialysate composition and flow rates.
2. AKI and Dialysis in Critically Ill Patients
- When to start dialysis?
- Which dialysis dose?
- Which modality of dialysis?
- Which anticoagulation?
- Special filters and adsorbers?
- Special extracorporeal elimination procedures
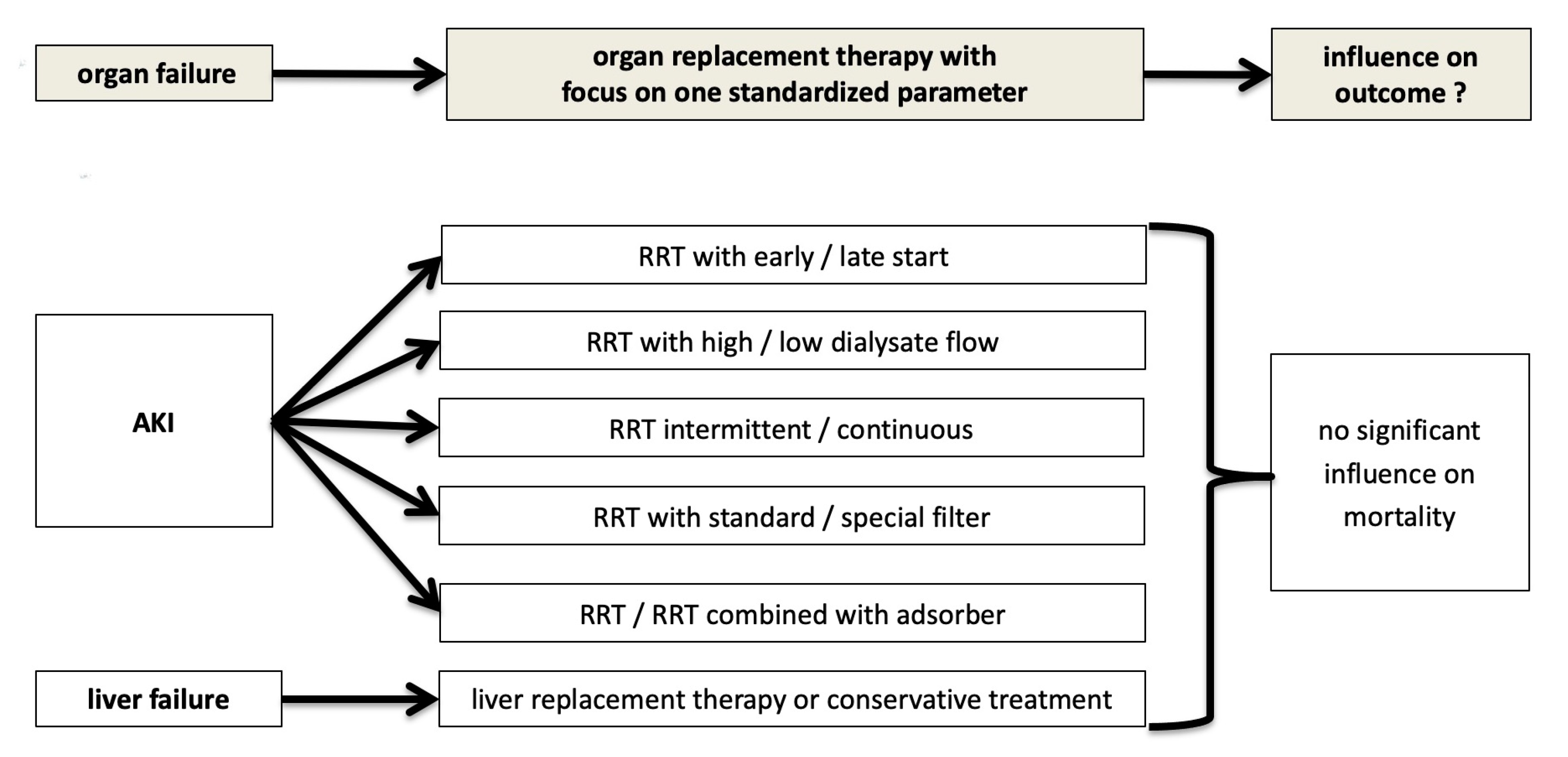
3. The Dilemma: Despite RRT in the Critically Ill with AKI Persistent High Mortality
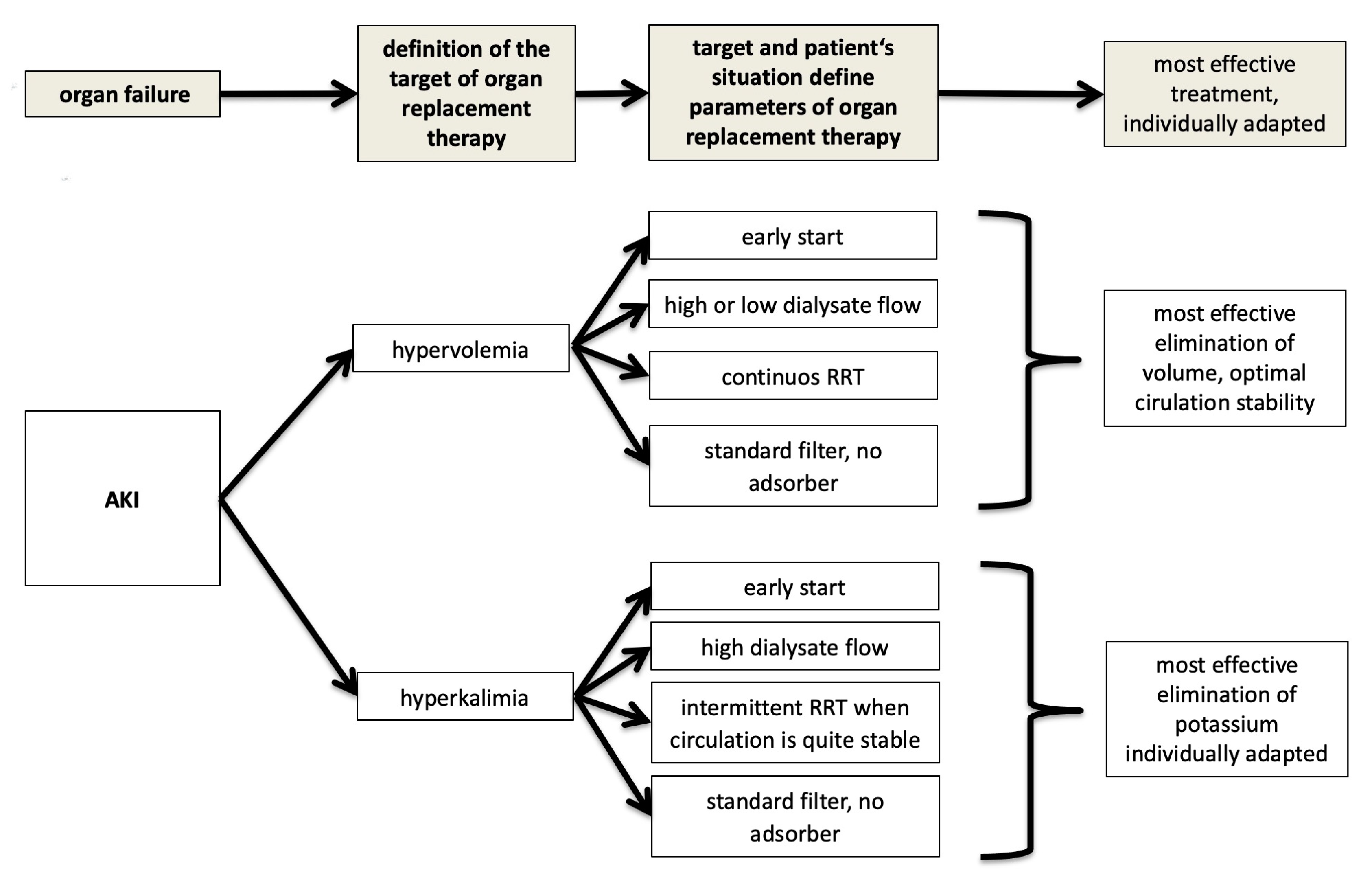
4. AKI and Dialysis in the Context of Multiple Organ Failure: The Nephrologist’s Attempt at Explanation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mehta, R.L.; Kellum, J.A.; Shah, S.V.; Molitoris, B.A.; Ronco, C.; Warnock, D.G.; Levin, A.; Acute Kidney Injury Network. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit. Care 2007, 11, R31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronco, C.; Bellomo, R.; Kellum, J.A. Acute kidney injury. Lancet 2019, 394, 1949–1964. [Google Scholar] [CrossRef]
- Griffin, B.R.; Liu, K.D.; Teixeira, J.P. Critical Care Nephrology: Core Curriculum 2020. Am. J. Kidney Dis. 2020, 75, 435–452. [Google Scholar] [CrossRef] [PubMed]
- Gaudry, S.; Hajage, D.; Schortgen, F.; Martin-Lefevre, L.; Pons, B.; Boulet, E.; Boyer, A.; Chevrel, G.; Lerolle, N.; Carpentier, D.; et al. Initiation Strategies for Renal-Replacement Therapy in the Intensive Care Unit. N. Engl. J. Med. 2016, 375, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Zarbock, A.; Kellum, J.A.; Schmidt, C.; Van Aken, H.; Wempe, C.; Pavenstadt, H.; Boanta, A.; Gerss, J.; Meersch, M. Effect of Early vs Delayed Initiation of Renal Replacement Therapy on Mortality in Critically Ill Patients With Acute Kidney Injury: The ELAIN Randomized Clinical Trial. JAMA 2016, 315, 2190–2199. [Google Scholar] [CrossRef] [Green Version]
- Karvellas, C.J.; Farhat, M.R.; Sajjad, I.; Mogensen, S.S.; Leung, A.A.; Wald, R.; Bagshaw, S.M. A comparison of early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury: A systematic review and meta-analysis. Crit. Care 2011, 15, R72. [Google Scholar] [CrossRef] [Green Version]
- Ronco, C.; Bellomo, R.; Homel, P.; Brendolan, A.; Dan, M.; Piccinni, P.; La Greca, G. Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: A prospective randomised trial. Lancet 2000, 356, 26–30. [Google Scholar] [CrossRef]
- Network, V.N.A.R.F.T.; Palevsky, P.M.; Zhang, J.H.; O’Connor, T.Z.; Chertow, G.M.; Crowley, S.T.; Choudhury, D.; Finkel, K.; Kellum, J.A.; Paganini, E.; et al. Intensity of renal support in critically ill patients with acute kidney injury. N. Engl. J. Med. 2008, 359, 7–20. [Google Scholar] [CrossRef]
- Investigators, R.R.T.S.; Bellomo, R.; Cass, A.; Cole, L.; Finfer, S.; Gallagher, M.; Lo, S.; McArthur, C.; McGuinness, S.; Myburgh, J.; et al. Intensity of continuous renal-replacement therapy in critically ill patients. N. Engl. J. Med. 2009, 361, 1627–1638. [Google Scholar] [CrossRef] [Green Version]
- Bouchard, J.; Soroko, S.B.; Chertow, G.M.; Himmelfarb, J.; Ikizler, T.A.; Paganini, E.P.; Mehta, R.L.; Program to Improve Care in Acute Renal Disease Study Group. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009, 76, 422–427. [Google Scholar] [CrossRef] [Green Version]
- Woodward, C.W.; Lambert, J.; Ortiz-Soriano, V.; Li, Y.; Ruiz-Conejo, M.; Bissell, B.D.; Kelly, A.; Adams, P.; Yessayan, L.; Morris, P.E.; et al. Fluid Overload Associates With Major Adverse Kidney Events in Critically Ill Patients With Acute Kidney Injury Requiring Continuous Renal Replacement Therapy. Crit. Care Med. 2019, 47, e753–e760. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Y.; Bellomo, R. Renal replacement therapy in the ICU: Intermittent hemodialysis, sustained low-efficiency dialysis or continuous renal replacement therapy? Curr. Opin. Crit. Care 2018, 24, 437–442. [Google Scholar] [CrossRef]
- Wald, R.; Shariff, S.Z.; Adhikari, N.K.; Bagshaw, S.M.; Burns, K.E.; Friedrich, J.O.; Garg, A.X.; Harel, Z.; Kitchlu, A.; Ray, J.G. The association between renal replacement therapy modality and long-term outcomes among critically ill adults with acute kidney injury: A retrospective cohort study. Crit. Care Med. 2014, 42, 868–877. [Google Scholar] [CrossRef]
- Oudemans-van Straaten, H.M.; Bosman, R.J.; Koopmans, M.; van der Voort, P.H.; Wester, J.P.; van der Spoel, J.I.; Dijksman, L.M.; Zandstra, D.F. Citrate anticoagulation for continuous venovenous hemofiltration. Crit. Care Med. 2009, 37, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Pinnick, R.V.; Wiegmann, T.B.; Diederich, D.A. Regional citrate anticoagulation for hemodialysis in the patient at high risk for bleeding. N. Engl. J. Med. 1983, 308, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Lohr, J.W.; Slusher, S.; Diederich, D. Safety of regional citrate hemodialysis in acute renal failure. Am. J. Kidney Dis. 1989, 13, 104–107. [Google Scholar] [CrossRef]
- Bai, M.; Zhou, M.; He, L.; Ma, F.; Li, Y.; Yu, Y.; Wang, P.; Li, L.; Jing, R.; Zhao, L.; et al. Citrate versus heparin anticoagulation for continuous renal replacement therapy: An updated meta-analysis of RCTs. Intensive Care Med. 2015, 41, 2098–2110. [Google Scholar] [CrossRef] [PubMed]
- Zarbock, A.; Kullmar, M.; Kindgen-Milles, D.; Wempe, C.; Gerss, J.; Brandenburger, T.; Dimski, T.; Tyczynski, B.; Jahn, M.; Mulling, N.; et al. Effect of Regional Citrate Anticoagulation vs Systemic Heparin Anticoagulation During Continuous Kidney Replacement Therapy on Dialysis Filter Life Span and Mortality Among Critically Ill Patients With Acute Kidney Injury: A Randomized Clinical Trial. JAMA 2020, 324, 1629–1639. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, F.J.; Oster, J.R.; Perez, G.O.; Materson, B.J.; Lopez, R.A.; Al-Reshaid, K. Metabolic alkalosis induced by regional citrate hemodialysis. ASAIO Trans. 1989, 35, 22–25. [Google Scholar]
- Khadzhynov, D.; Schelter, C.; Lieker, I.; Mika, A.; Staeck, O.; Neumayer, H.H.; Peters, H.; Slowinski, T. Incidence and outcome of metabolic disarrangements consistent with citrate accumulation in critically ill patients undergoing continuous venovenous hemodialysis with regional citrate anticoagulation. J. Crit. Care 2014, 29, 265–271. [Google Scholar] [CrossRef]
- Klingele, M.; Stadler, T.; Fliser, D.; Speer, T.; Groesdonk, H.V.; Raddatz, A. Long-term continuous renal replacement therapy and anticoagulation with citrate in critically ill patients with severe liver dysfunction. Crit. Care 2017, 21, 294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronco, C.; Tetta, C.; Mariano, F.; Wratten, M.L.; Bonello, M.; Bordoni, V.; Cardona, X.; Inguaggiato, P.; Pilotto, L.; d’Intini, V.; et al. Interpreting the mechanisms of continuous renal replacement therapy in sepsis: The peak concentration hypothesis. Artif. Organs 2003, 27, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Zuccari, S.; Damiani, E.; Domizi, R.; Scorcella, C.; D’Arezzo, M.; Carsetti, A.; Pantanetti, S.; Vannicola, S.; Casarotta, E.; Ranghino, A.; et al. Changes in Cytokines, Haemodynamics and Microcirculation in Patients with Sepsis/Septic Shock Undergoing Continuous Renal Replacement Therapy and Blood Purification with CytoSorb. Blood Purif. 2020, 49, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Douvris, A.; Malhi, G.; Hiremath, S.; McIntyre, L.; Silver, S.A.; Bagshaw, S.M.; Wald, R.; Ronco, C.; Sikora, L.; Weber, C.; et al. Interventions to prevent hemodynamic instability during renal replacement therapy in critically ill patients: A systematic review. Crit. Care 2018, 22, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kribben, A.; Gerken, G.; Haag, S.; Herget-Rosenthal, S.; Treichel, U.; Betz, C.; Sarrazin, C.; Hoste, E.; Van Vlierberghe, H.; Escorsell, A.; et al. Effects of fractionated plasma separation and adsorption on survival in patients with acute-on-chronic liver failure. Gastroenterology 2012, 142, 782–789.e3. [Google Scholar] [CrossRef]
- Klingele, M.; Allmendinger, C.; Thieme, S.; Baerens, L.; Fliser, D.; Jan, B. Therapeutic apheresis within immune-mediated neurological disorders: Dosing and its effectiveness. Sci. Rep. 2020, 10, 7925. [Google Scholar] [CrossRef]
- Holst, L.B.; Haase, N.; Wetterslev, J.; Wernerman, J.; Guttormsen, A.B.; Karlsson, S.; Johansson, P.I.; Aneman, A.; Vang, M.L.; Winding, R.; et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N. Engl. J. Med. 2014, 371, 1381–1391. [Google Scholar] [CrossRef] [Green Version]
- Pro, C.I.; Yealy, D.M.; Kellum, J.A.; Huang, D.T.; Barnato, A.E.; Weissfeld, L.A.; Pike, F.; Terndrup, T.; Wang, H.E.; Hou, P.C.; et al. A randomized trial of protocol-based care for early septic shock. N. Engl. J. Med. 2014, 370, 1683–1693. [Google Scholar] [CrossRef] [Green Version]
- Vincent, J.L. Recent negative clinical trials in septic patients: Maybe a good thing? Minerva Anestesiol. 2015, 81, 122–124. [Google Scholar]
- Mariano, F.; Hollo, Z.; Depetris, N.; Malvasio, V.; Mella, A.; Bergamo, D.; Pensa, A.; Berardino, M.; Stella, M.; Biancone, L. Coupled-plasma filtration and adsorption for severe burn patients with septic shock and acute kidney injury treated with renal replacement therapy. Burns 2020, 46, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Flaatten, H.; Darmon, M. A nephrologist should be consulted in all cases of acute kidney injury in the ICU: Yes. Intensive Care Med. 2017, 43, 874–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Askenazi, D.J.; Heung, M.; Connor, M.J., Jr.; Basu, R.K.; Cerda, J.; Doi, K.; Koyner, J.L.; Bihorac, A.; Golestaneh, L.; Vijayan, A.; et al. Optimal Role of the Nephrologist in the Intensive Care Unit. Blood Purif. 2017, 43, 68–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klingele, M.; Baerens, L. Impact of Renal Replacement Therapy on Mortality in Critically Ill Patients—The Nephrologist’s View within an Interdisciplinary Intensive Care Team. J. Clin. Med. 2021, 10, 3379. https://doi.org/10.3390/jcm10153379
Klingele M, Baerens L. Impact of Renal Replacement Therapy on Mortality in Critically Ill Patients—The Nephrologist’s View within an Interdisciplinary Intensive Care Team. Journal of Clinical Medicine. 2021; 10(15):3379. https://doi.org/10.3390/jcm10153379
Chicago/Turabian StyleKlingele, Matthias, and Lea Baerens. 2021. "Impact of Renal Replacement Therapy on Mortality in Critically Ill Patients—The Nephrologist’s View within an Interdisciplinary Intensive Care Team" Journal of Clinical Medicine 10, no. 15: 3379. https://doi.org/10.3390/jcm10153379
APA StyleKlingele, M., & Baerens, L. (2021). Impact of Renal Replacement Therapy on Mortality in Critically Ill Patients—The Nephrologist’s View within an Interdisciplinary Intensive Care Team. Journal of Clinical Medicine, 10(15), 3379. https://doi.org/10.3390/jcm10153379




