Cerebellar Dysfunction in Adults with Prader Willi Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Behavioral Assessment
2.3. Motor Tests during fMRI
2.4. Functional MRI Acquisition
2.5. Functional MRI Preprocessing
Control of Potential Head Motion Effects
2.6. Statistical Analysis
2.6.1. Behavioral Data
2.6.2. Functional MRI Data
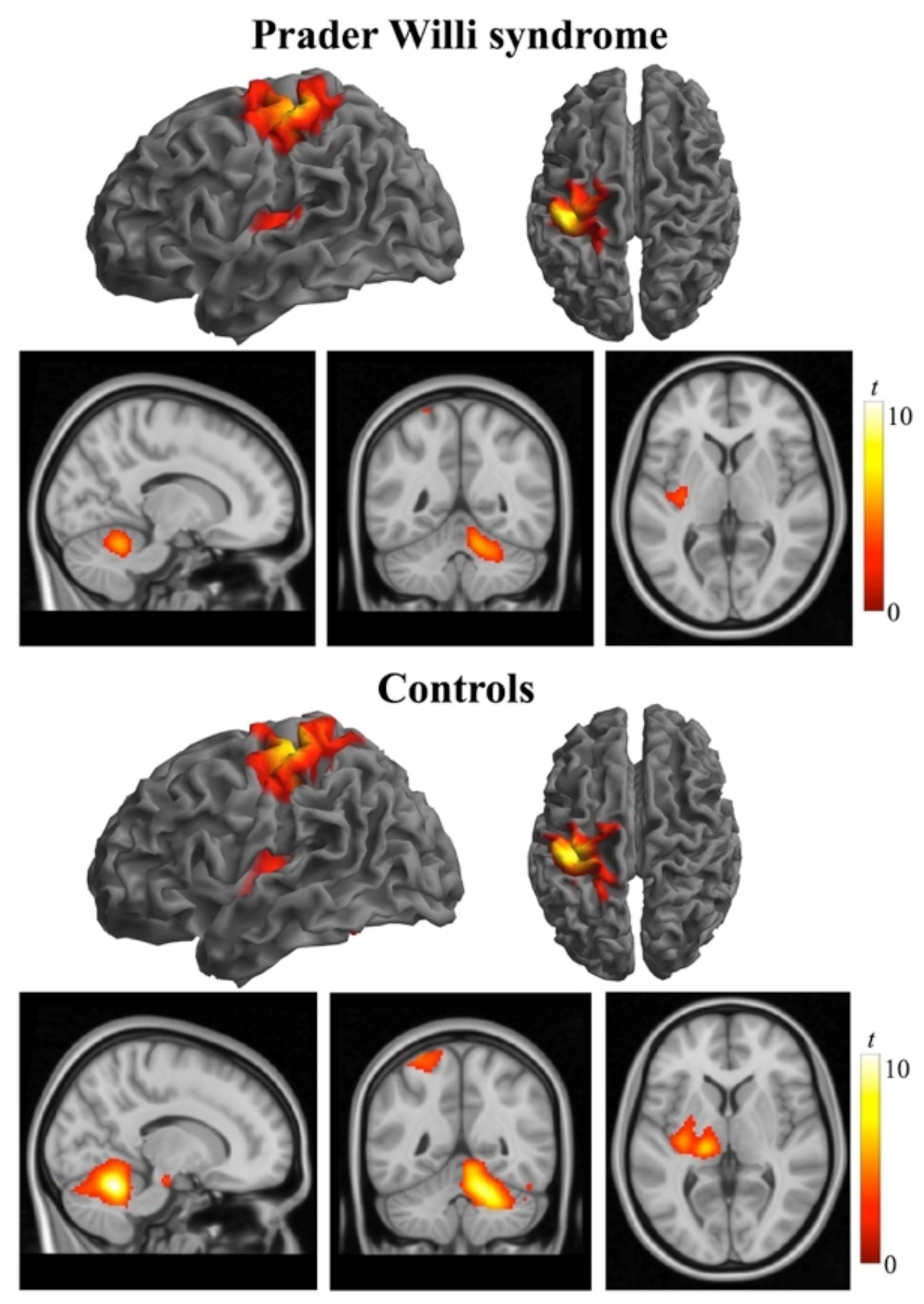
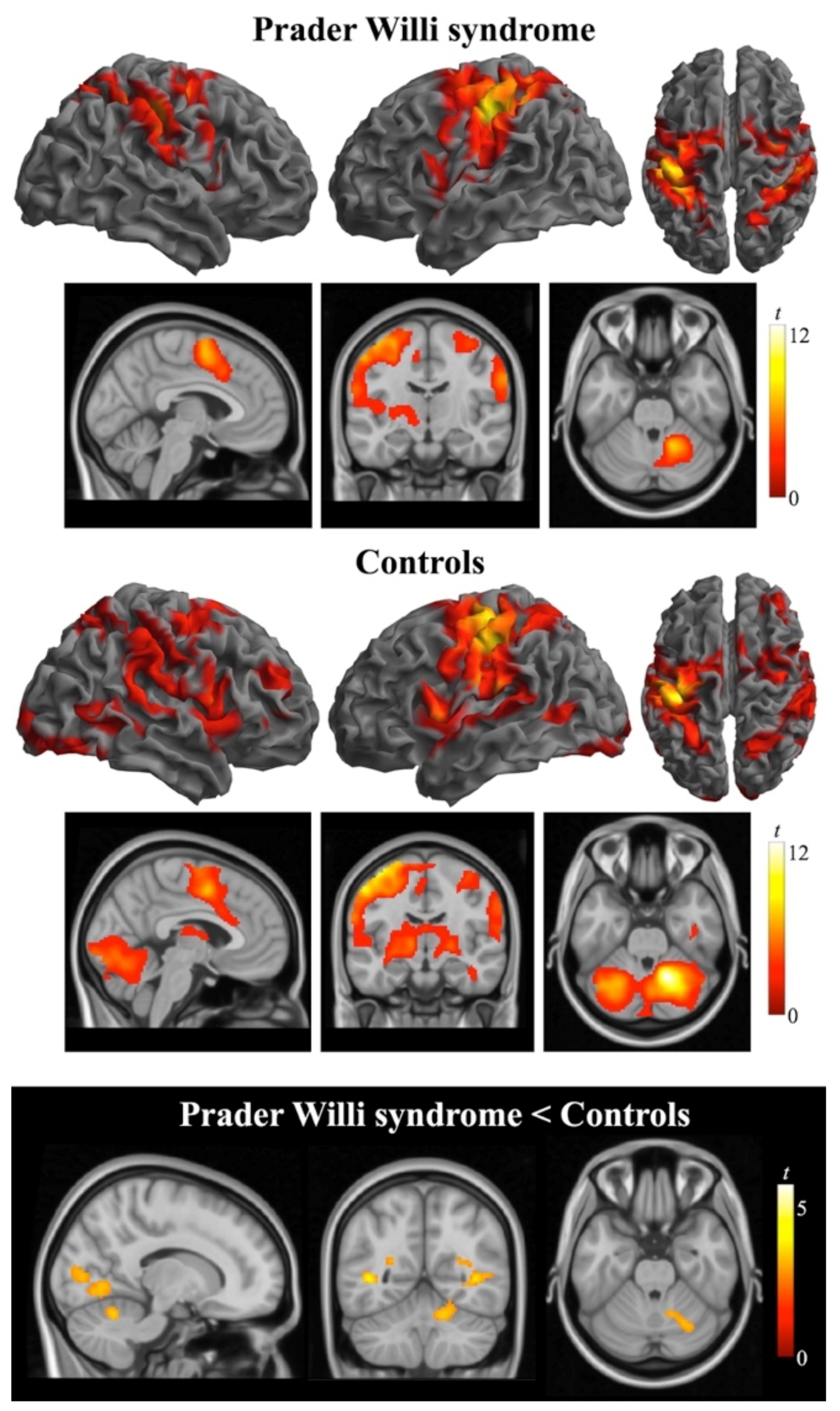
3. Results
3.1. Behavioral Data
3.2. Functional MRI Data
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cassidy, S.B.; Schwartz, S.; Miller, J.L.; Driscoll, D.J. Prader-Willi syndrome. Genet. Med. 2012, 14, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Goldstone, A.P.; Holland, A.J.; Hauffa, B.P.; Hokken-Koelega, A.C.; Tauber, M. Recommendations for the diagnosis and management of Prader-Willi syndrome. J. Clin. Endocrinol. Metab. 2008, 93, 4183–4197. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.G.; Miller, J.L.; Forster, J.L. Prader-Willi syndrome—Clinical genetics, diagnosis and treatment approaches: An update. Curr. Pediatr. Rev. 2019, 15, 207–244. [Google Scholar] [CrossRef] [PubMed]
- Novell-Alsina, R.; Esteb3a-Castillo, S.; Caixàs, A.; Gabau, E.; Giménez-Palop, O.; Pujol, J.; Deus, J.; Torrents-Rodas, D. Compulsions in Prader-Willi syndrome: Occurence and severity as a function of genetic subtype. Actas Esp. Psiquiatr. 2019, 47, 79–87. [Google Scholar]
- Pujol, J.; Blanco-Hinojo, L.; Esteba-Castillo, S.; Caixàs, A.; Harrison, B.J.; Bueno, M.; Deus, J.; Rigla, M.; Macià, D.; Llorente-Onaindia, J.; et al. Anomalous basal ganglia connectivity and obsessive-compulsive behavior in patients with Prader Willi syndrome. J. Psychiatry Neurosci. 2016, 41, 261–271. [Google Scholar] [CrossRef]
- Reus, L.; Zwarts, M.; van Vlimmeren, L.A.; Willemsen, M.A.; Otten, B.J.; Nijhuis-van der Sanden, M.W. Motor problems in Prader-Willi syndrome: A systematic review on body composition and neuromuscular functioning. Neurosci. Biobehav. Rev. 2011, 35, 956–969. [Google Scholar] [CrossRef] [PubMed]
- Reus, L.; van Vlimmeren, L.A.; Staal, J.B.; Otten, B.J.; Nijhuis-van der Sanden, M.W. The effect of growth hormone treatment or physical training on motor performance in Prader-Willi syndrome: A systematic review. Neurosci. Biobehav. Rev. 2012, 36, 1817–1838. [Google Scholar] [CrossRef] [PubMed]
- Caixàs, A.; Blanco-Hinojo, L.; Pujol, J.; Deus, J.; Giménez-Palop, O.; Torrents-Rodas, D.; Coronas, R.; Novell, R.; Esteba-Castillo, S. Altered gesture imitation and brain anatomy in adult Prader-Willi syndrome patients. J. Int. Neuropsychol. Soc. 2021, 1–13. [Google Scholar] [CrossRef]
- Defloor, T.; Van Borsel, J.; Curfs, L. Articulation in Prader-Willi syndrome. J. Commun. Disord. 2002, 35, 261–282. [Google Scholar] [CrossRef]
- Hsu, W.L.; Chiu, V.J.; Chang, W.H.; Lin, M.C.; Wei, J.T.; Tzeng, I.S. Hand strength and dexterity in patients with Prader-Willi syndrome: A pilot intervention study. J. Int. Med. Res. 2018, 46, 4669–4677. [Google Scholar] [CrossRef]
- Capodaglio, P.; Menegoni, F.; Vismara, L.; Cimolin, V.; Grugni, G.; Galli, M. Characterisation of balance capacity in Prader-Willi patients. Res. Dev. Disabil. 2011, 32, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Cimolin, V.; Cau, N.; Galli, M.; Pau, M.; Parisio, C.; Saezza, A.; Grugni, G.; Capodaglio, P. Gait strategy and body composition in patients with Prader-Willi syndrome. Eat. Weight Disord. 2021, 26, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Galli, M.; Cimolin, V.; Vismara, L.; Grugni, G.; Camerota, F.; Celletti, C.; Albertini, G.; Rigoldi, C.; Capodaglio, P. The effects of muscle hypotonia and weakness on balance: A study on Prader-Willi and Ehlers-Danlos syndrome patients. Res. Dev. Disabil. 2011, 32, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Capodaglio, P.; Vismara, L.; Menegoni, F.; Baccalaro, G.; Galli, M.; Grugni, G. Strength characterization of knee flexor and extensor muscles in Prader-Willi and obese patients. BMC Musculoskelet. Disord. 2009, 10, 47. [Google Scholar] [CrossRef]
- Chiu, V.; Tsai, L.; Wei, J.; Tzeng, I.; Wu, H. Motor performance in Prader-Willi syndrome patients and its potential influence on caregiver’s quality of live. PeerJ 2017, 5, e4097. [Google Scholar] [CrossRef]
- Lafortuna, C.L.; Minocci, A.; Capodaglio, P.; Gondoni, L.A.; Sartorio, A.; Vismara, L.; Rizzo, G.; Grugni, G. Skeletal muscle characteristics and motor performance after 2-year growth hormone treatment in adults with Prader-Willi syndrome. J. Clin. Endocrinol. Metab. 2014, 99, 1816–1824. [Google Scholar] [CrossRef]
- Miller, J.L.; Couch, J.; Schwenk, K.; Long, M.; Towler, S.; Theriaque, D.W.; He, G.; Liu, Y.; Driscoll, D.J.; Leonard, C.M. Early childhood obesity is associated with compromised cerebellar development. Dev. Neurospsychol. 2009, 34, 272–283. [Google Scholar] [CrossRef]
- Ogura, K.; Fujii, T.; Abe, N.; Hosokai, Y.; Shinohara, M.; Takahashi, S.; Mori, E. Small gray matter volume in orbitofrontal cortex in Prader-Willi syndrome: A voxel-based MRI study. Hum. Brain Mapp. 2011, 32, 1059–1066. [Google Scholar] [CrossRef]
- Titomanlio, L.; De Brasi, D.; Romano, A.; Genesio, R.; Diano, A.A.; Del Giudice, E. Partial cerebellar hypoplasia in a patient with Prader-Willi syndrome. Acta Paediatr. 2006, 95, 861–863. [Google Scholar] [CrossRef]
- Yamada, K.; Watanabe, M.; Suzuki, K.; Suzuki, Y. Cerebellar volumes associate with behavioral phenotypes in Prader-Willi syndrome. Cerebellum 2020, 19, 778–787. [Google Scholar] [CrossRef]
- Blanco-Hinojo, L.; Pujol, J.; Esteba-Castillo, S.; Martínez-Vilavella, G.; Giménez-Palop, O.; Gabau, E.; Casamitjana, L.; Deus, J.; Novell, R.; Caixàs, A. Lack of response to disgusting food in the hypothalamus and related structures in Prader-Willi syndrome. Neuroimage Clin. 2019, 21, 101662. [Google Scholar] [CrossRef]
- Kim, S.E.; Jin, D.K.; Cho, S.S.; Kim, J.H.; Hong, S.D.; Paik, K.H.; Oh, Y.J.; Kim, A.H.; Kwon, E.K.; Choe, Y.H. Regional cerebral glucose metabolic abnormality in Prader-Wili syndrome: A 18F-FDG PET study under sedation. J. Nucl. Med. 2006, 47, 1088–1092. [Google Scholar]
- Mantoulan, C.; Payoux, P.; Diene, G.; Glattard, M.; Rogé, B.; Molinas, C.; Sevely, A.; Zilbovicius, M.; Celsis, P.; Tauber, M. PET scan perfusion imaging in the Prader-Willi syndrome: New insights into the psychiatric and social disturbances. J. Cereb. Blood Flow Metab. 2011, 31, 275–282. [Google Scholar] [CrossRef]
- Ogura, K.; Fujii, T.; Abe, N.; Hosokai, Y.; Shinohara, M.; Fukuda, H.; Mori, E. Regional cerebral blood flow and abnormal eating behavior in Prader-Willi syndrome. Brain Dev. 2013, 35, 427–434. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, H.; Qiu, S.; Tian, J.; Wen, X.; Miller, J.L.; von Deneen, K.M.; Zhou, Z.; Gold, M.S.; Liu, Y. Altered functional brain networks in Prader-Willi syndrome. NMR Biomed. 2013, 26, 622–629. [Google Scholar] [CrossRef]
- Schmidt, R.T.; Toews, J.V. Grip strength as measured by the Jamar dynamometer. Arch Phys. Med. Rehabil. 1970, 51, 321–327. [Google Scholar]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar]
- Berg, K.; Wood-Dauphinee, S.L.; Williams, J.I.; Maki, B. Measuring balance in the elderly: Validation of an instrument. Can. J. Public Health 1992, 83 (Suppl. 2), S7–S11. [Google Scholar]
- Martino, J.; Gabarrós, A.; Deus, J.; Juncadella, M.; Acebes, J.J.; Torres, A.; Pujol, J. Intrasurgical mapping of complex motor function in the superior frontal gyrus. Neuroscience 2011, 179, 131–142. [Google Scholar] [CrossRef]
- Pujol, J.; Conesa, G.; Deus, J.; López-Obarrio, L.; Isamat, F.; Capdevila, A. Clinical application of functional magnetic resonance imaging in presurgical identification of the central sulcus. J. Neurosurg. 1998, 88, 863–869. [Google Scholar] [CrossRef]
- Power, J.D.; Mitra, A.; Laumann, T.O.; Snyder, A.Z.; Schlaggar, B.L.; Petersen, S.E. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 2014, 84, 320–341. [Google Scholar] [CrossRef] [PubMed]
- Pujol, J.; Macià, D.; Blanco-Hinojo, L.; Martínez-Vilavella, G.; Sunyer, J.; de la Torre, R.; Caixàs, A.; Martín-Santos, R.; Deus, J.; Harrison, B.J. Does motion-related brain functional connectivity reflect both artifacts and genuine neural activity? Neuroimage 2014, 101, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Trampisch, U.S.; Franke, J.; Jedamzik, N.; Hinrichs, T.; Platen, P. Optimal Jamar dynamometer handle position to assess maximal isometric hand grip strength in epidemiological studies. J. Hand. Surg. Am. 2012, 37, 2368–2373. [Google Scholar] [CrossRef] [PubMed]
- Allison, J.D.; Meador, K.J.; Loring, D.W.; Figueroa, R.E.; Wright, J.C. Functional MRI cerebral activation and deactivation during finger movement. Neurology 2000, 54, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Catalan, M.J.; Honda, M.; Weeks, R.A.; Cohen, L.G.; Hallett, M. The functional neuroanatomy of simple and complex sequential finger movements: A PET study. Brain 1998, 121, 253–264. [Google Scholar] [CrossRef]
- Omel’chenko, A.N.; Rozhkova, Z.Z. Characteristics of fMRI patterns during the performance of hand and finger movements of different complexity. Neurophysiology 2016, 48, 23–30. [Google Scholar] [CrossRef]
- Ivry, R.B.; Spencer, R.M. The neural representation of time. Curr. Opin. Neurobiol. 2004, 14, 225–232. [Google Scholar] [CrossRef]
- Witt, S.T.; Laird, A.R.; Meyerand, M.E. Functional neuroimaging correlates of finger-tapping task variations: An ALE meta-analysis. Neuroimage 2008, 42, 343–356. [Google Scholar] [CrossRef]
- Stoodley, C.J.; Schmahmann, J.D. Functional topography of the human cerebellum. Handb. Clin. Neurol. 2018, 154, 59–70. [Google Scholar]
- Ebner, T.J.; Hewitt, A.L.; Popa, L.S. What features of limb movements are encoded in the discharge of cerebellar neurons? Cerebellum 2011, 10, 683–693. [Google Scholar] [CrossRef][Green Version]
- Lisberger, S.G.; Thach, W.T. The cerebellum. In Principles of Neural Science, 5th ed.; Kandel, E.R., Schwartz, J.H., Jessell, T.M., Siegelbaum, S.A., Hudspeth, A.J., Eds.; McGraw-Hill: New York, NY, USA, 2013; pp. 960–981. [Google Scholar]
- Manto, M.; Bower, J.M.; Conforto, A.B.; Delgado-García, J.M.; da Guarda, S.N.; Gerwig, M.; Habas, C.; Hagura, N.; Irvy, R.B.; Mariën, P.; et al. Consensus paper: Roles of the cerebellum in motor control—The diversity of ideas on cerebellar involvement in movement. Cerebellum 2012, 11, 457–487. [Google Scholar] [CrossRef]
- Grodd, W.; Hülsmann, E.; Lotze, M.; Wildgruber, D.; Erb, M. Sensorimotor mapping of the human cerebellum: fMRI evidence of somatotopic organization. Hum. Brain Mapp. 2001, 13, 55–73. [Google Scholar] [CrossRef]
- Groiss, S.J.; Ugawa, Y. Cerebellum. Handb. Clin. Neurol. 2013, 116, 643–653. [Google Scholar]
- Manto, M. Cerebellar motor syndrome from children to the elderly. Handb. Clin. Neurol. 2018, 154, 151–166. [Google Scholar]
- Baumann, O.; Borra, R.J.; Bower, J.M.; Cullen, K.E.; Habas, C.; Irvy, R.B.; Leggio, M.; Mattingley, J.B.; Molinari, M.; Moulton, E.A.; et al. Consensus paper: The role of the cerebellum in perceptual processes. Cerebellum 2015, 14, 197–220. [Google Scholar] [CrossRef]
- Mostofsky, S.H.; Powell, S.K.; Simmonds, D.J.; Goldberg, M.C.; Caffo, B.; Pekar, J.J. Decreased connectivity and cerebellar activity in autism during motor task performance. Brain 2009, 132 Pt 9, 2413–2425. [Google Scholar] [CrossRef]
- Müller, R.A.; Kleinhans, N.; Pierce, K.; Kemmotsu, N.; Courchesne, E. Functional MRI of motor sequence acquisition: Effects of learning stage and performance. Brain Res. Con. Brain Res. 2002, 14, 277–293. [Google Scholar] [CrossRef]
- Coffman, K.A.; Dum, R.P.; Strick, P.L. Cerebellar vermis is a target of projections from the motor areas in the cerebral cortex. Proc. Natl. Acad. Sci. USA 2011, 108, 16068–16073. [Google Scholar] [CrossRef]
- Hayashi, M.; Itoh, M.; Kabasawa, Y.; Hayashi, H.; Satoh, J.; Morimatsu, Y. A neuropathological study of a case of the Prader-Willi syndrome with an interstitial deletion of the proximal long arm of chromosome 15. Brain Dev. 1992, 14, 58–62. [Google Scholar] [CrossRef]
- Stevenson, D.A.; Anaya, T.M.; Clayton-Smith, J.; Hall, B.D.; Van Allen, M.I.; Zori, R.T.; Zackai, E.H.; Frank, G.; Clericuzio, C.L. Unexpected death and critical illness in Prader-Willi syndrome: Report of ten individuals. Am. J. Med. Genet. A 2004, 124A, 158–164. [Google Scholar] [CrossRef]
- Lukoshe, A.; White, T.; Schmidt, M.N.; van der Lugt, A.; Hokken-Koelega, A.C. Divergent structural brain abnormalities between different genetic subtypes of children with Prader-Willi syndrome. J. Neurodev. Disord. 2013, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Honea, R.A.; Holsen, L.M.; Lepping, R.J.; Perea, R.; Butler, M.G.; Brooks, W.M.; Savage, C.R. The neuroanatomy of genetic subtype differences in Prader-Willi syndrome. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2012, 159B, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Civardi, C.; Vicentini, R.; Grugni, G.; Cantello, R. Corticospinal physiology in patients with Prader-Willi syndrome: A transcranial magnetic stimulation study. Arch. Neurol. 2004, 61, 1585–1589. [Google Scholar] [CrossRef] [PubMed]
- Naro, A.; Milardi, D.; Cacciola, A.; Russo, M.; Sciarrone, F.; La Rosa, G.; Bramanti, A.; Bramanti, P.; Calabrò, R.S. What do we know about the influence of the cerebellum on walking ability? Promising findings from transcranial alternating current stimulation. Cerebellum 2017, 16, 859–867. [Google Scholar] [CrossRef]


| Prader Willi (n = 23) | Control (n = 22) | |
|---|---|---|
| Age, year | 30.6 ± 10.1 (18–53) | 29.8 ± 9.2 (19–46) |
| Sex, M/F | 11/12 | 10/12 |
| Body mass index, kg/m2 | 34.9 ± 7.4 (23.3–53.2) | 22.0 ± 1.8 (18.7–25.2) |
| Laterality (R/L) | 16/7 | 17/4 * |
| IQ K-BIT—Total score | 70.3 ± 14.3 (48–99) # | |
| Genetic diagnosis, no. | ||
| Type I deletion | 8 | |
| Type II deletion | 6 | |
| Atypical deletion | 2 | |
| Uniparental disomy | 4 | |
| Imprinting defect | 3 | |
| Motor function | ||
| Hand grip strength, max. kg | ||
| Handle position 1 | 13.7 ± 5.8 (6–32) | 28.2 ± 8.8 (17–54) * |
| Handle position 2 | 17.4 ± 5.8 (10–34) | 33.8 ± 10.6 (20–58) * |
| Handle position 3 | 16.3 ± 5.4 (9–33) | 31.2 ± 9.7 (19–52) * |
| Handle position 4 | 14.2 ± 5.3 (6–30) | 27.3 ± 8.8 (15–43) * |
| Handle position 5 | 12.0 ± 4.7 (6–23) | 22.6 ± 8.5 (12–40) * |
| Timed Up and Go, sec | 10.5 ± 6.6 (6.3–38.4) | 5.2 ± 0.6 (3.9–6.5) * |
| Berg Balance Scale (range 0–56) | 51.5 ± 6.3 (25–56) | 55.7 ± 0.5 (55–56) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco-Hinojo, L.; Casamitjana, L.; Pujol, J.; Martínez-Vilavella, G.; Esteba-Castillo, S.; Giménez-Palop, O.; Freijo, V.; Deus, J.; Caixàs, A. Cerebellar Dysfunction in Adults with Prader Willi Syndrome. J. Clin. Med. 2021, 10, 3320. https://doi.org/10.3390/jcm10153320
Blanco-Hinojo L, Casamitjana L, Pujol J, Martínez-Vilavella G, Esteba-Castillo S, Giménez-Palop O, Freijo V, Deus J, Caixàs A. Cerebellar Dysfunction in Adults with Prader Willi Syndrome. Journal of Clinical Medicine. 2021; 10(15):3320. https://doi.org/10.3390/jcm10153320
Chicago/Turabian StyleBlanco-Hinojo, Laura, Laia Casamitjana, Jesus Pujol, Gerard Martínez-Vilavella, Susanna Esteba-Castillo, Olga Giménez-Palop, Valentín Freijo, Joan Deus, and Assumpta Caixàs. 2021. "Cerebellar Dysfunction in Adults with Prader Willi Syndrome" Journal of Clinical Medicine 10, no. 15: 3320. https://doi.org/10.3390/jcm10153320
APA StyleBlanco-Hinojo, L., Casamitjana, L., Pujol, J., Martínez-Vilavella, G., Esteba-Castillo, S., Giménez-Palop, O., Freijo, V., Deus, J., & Caixàs, A. (2021). Cerebellar Dysfunction in Adults with Prader Willi Syndrome. Journal of Clinical Medicine, 10(15), 3320. https://doi.org/10.3390/jcm10153320






