Evaluation of the Efficacy of Dexamethasone-Eluting Electrode Array on the Post-Implant Cochlear Fibrotic Reaction by Three-Dimensional Immunofluorescence Analysis in Mongolian Gerbil Cochlea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrode Array
2.2. Cochlear Implant Surgery
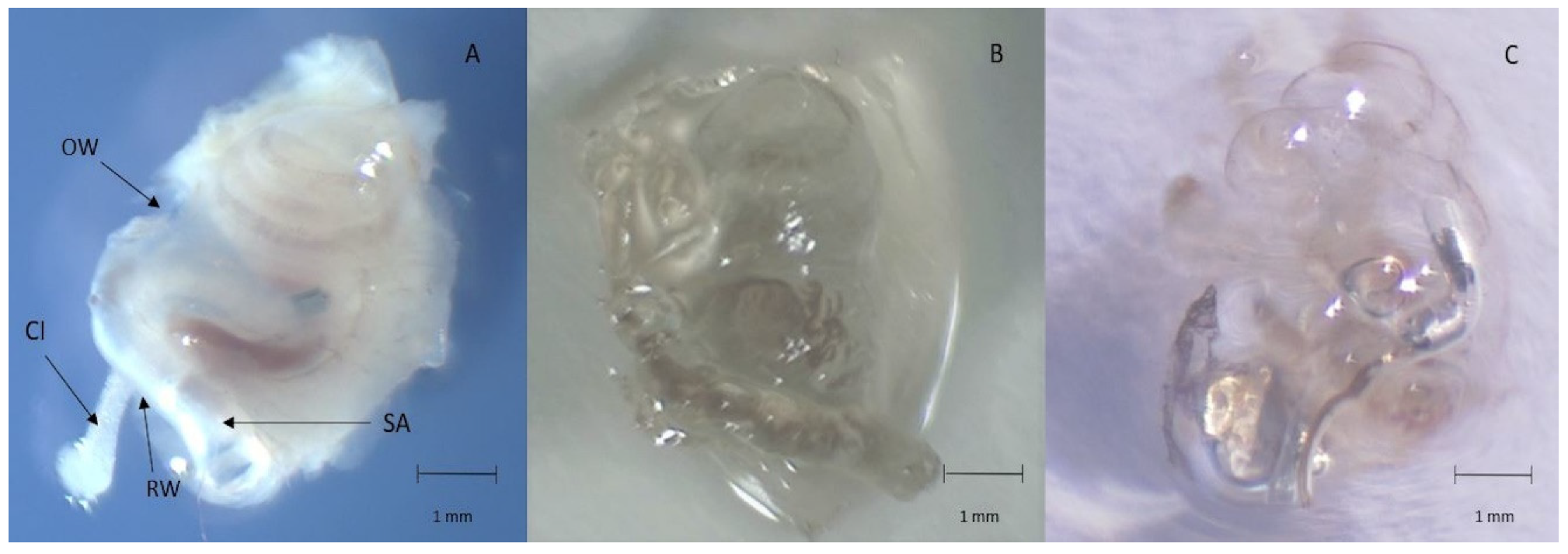
2.3. Tissue Preparation
2.4. Light-Sheet Microscopy
2.5. Three-Dimensional (3D) Analysis
2.6. Statistical Analysis
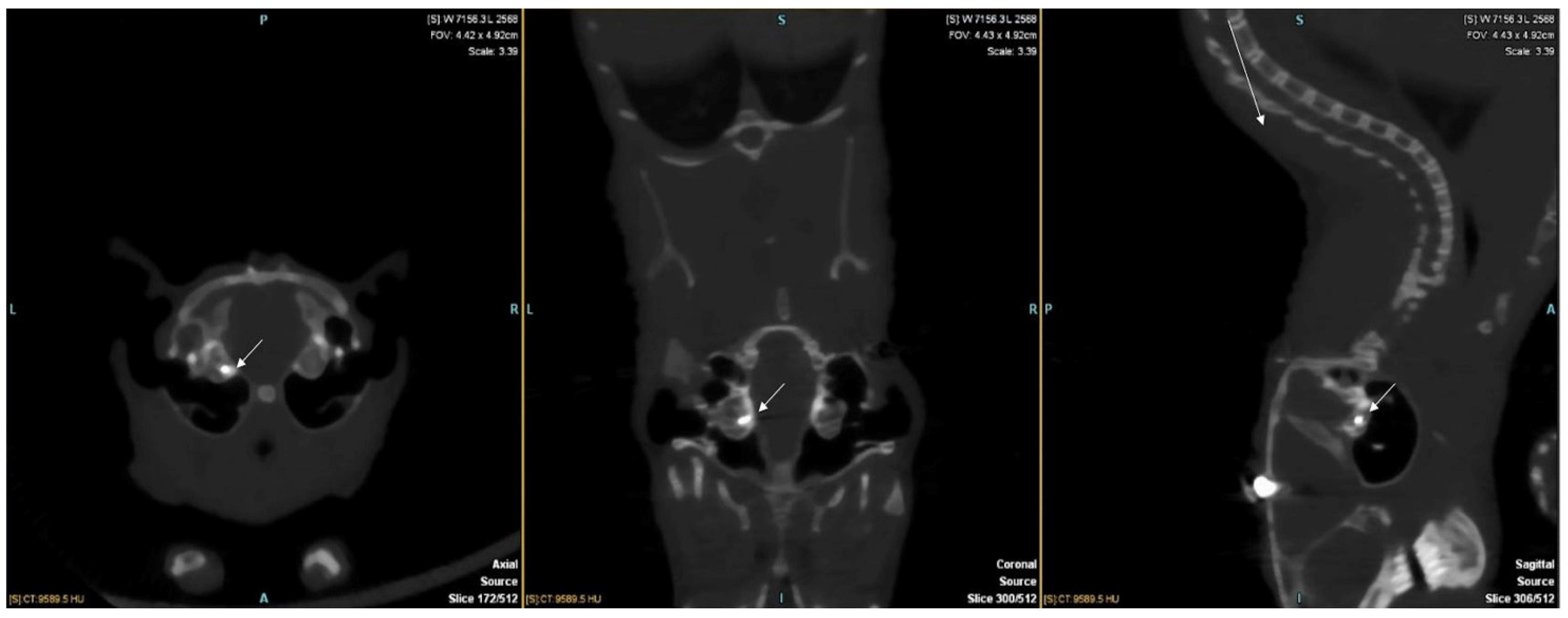
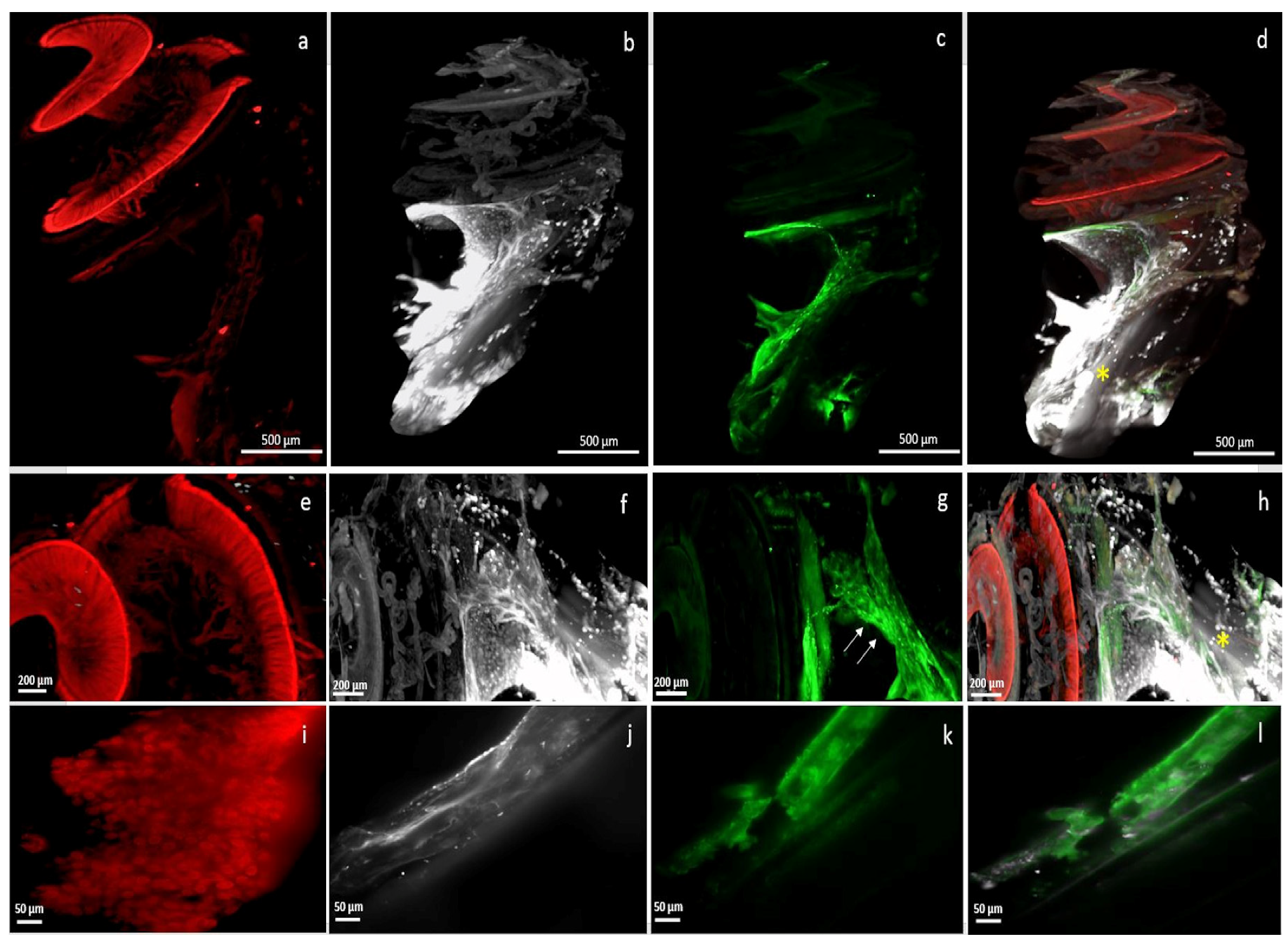
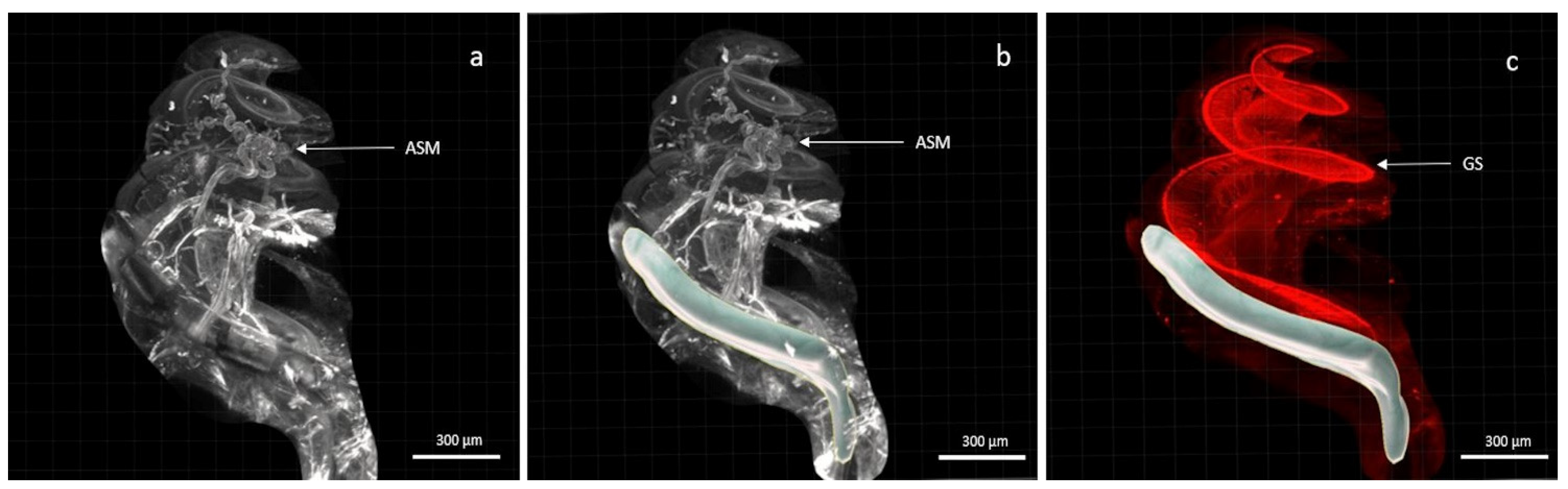
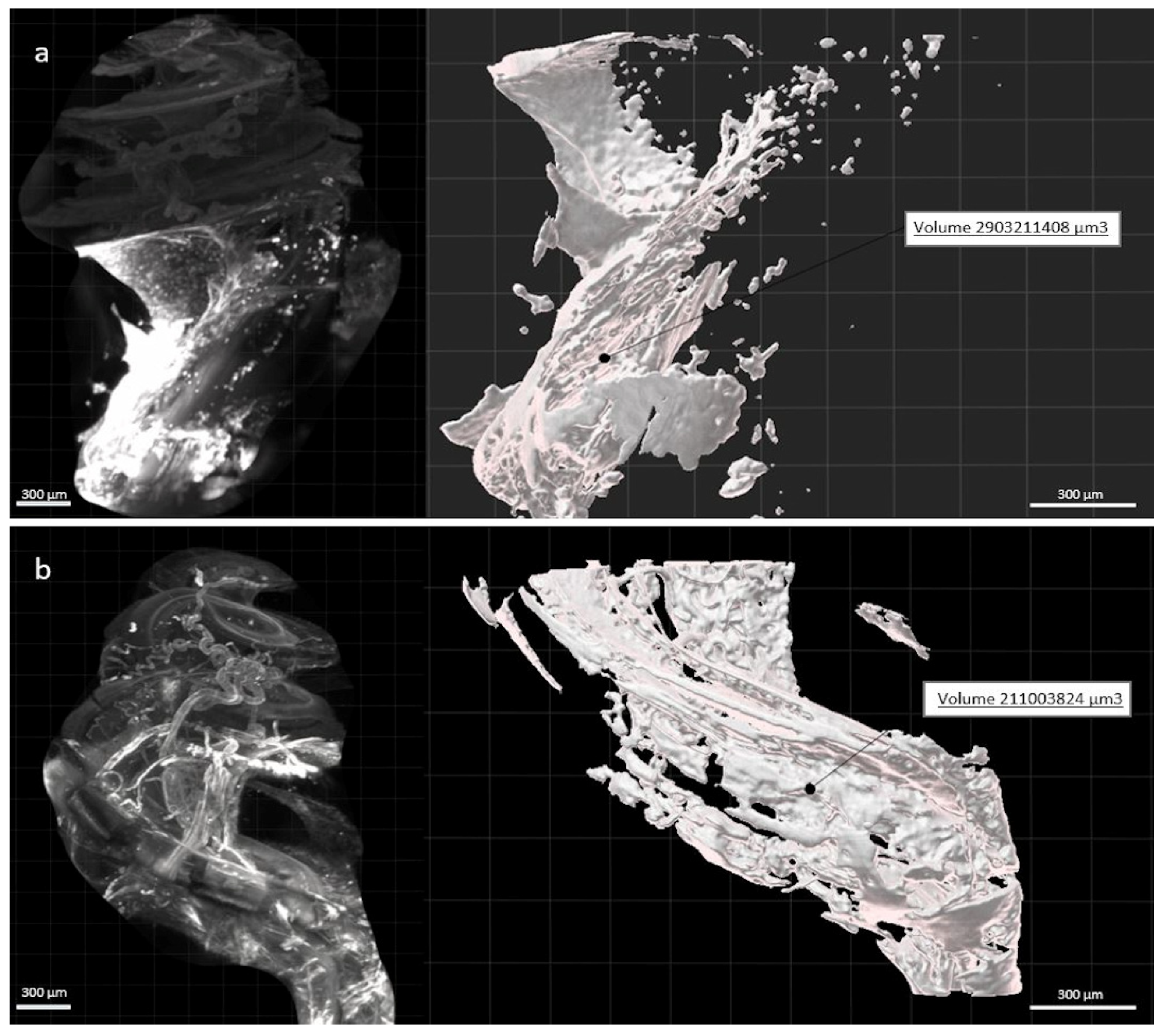
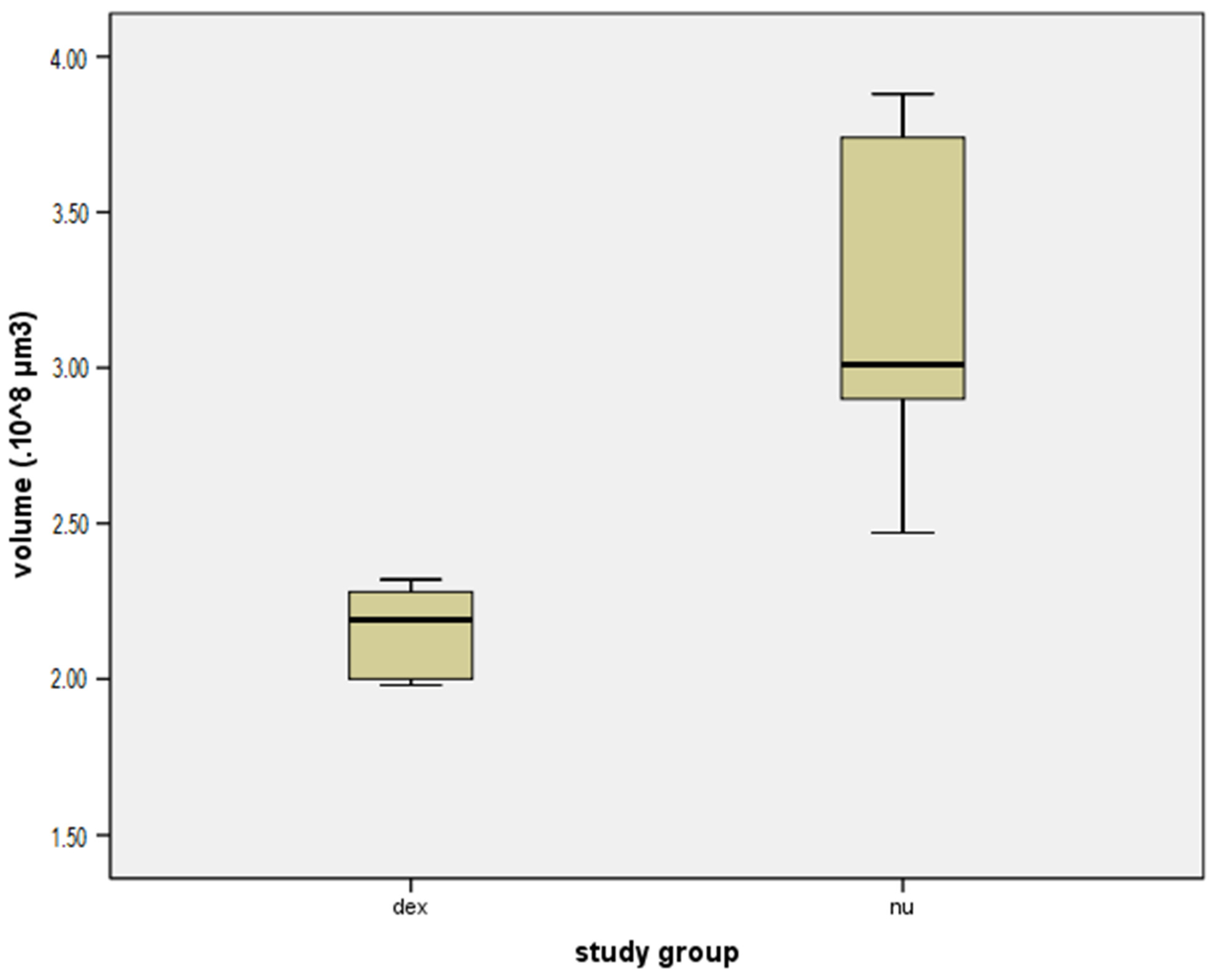
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Spoendlin, H. Degeneration behaviour of the cochlear nerve. Arch. Klin. Exp. Ohren Nasen Kehlkopfheilkd. 1971, 200, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Dodson, H.C.; Mohuiddin, A. Response of spiral ganglion neurones to cochlear hair cell destruction in the guinea pig. J. Neurocytol. 2000, 29, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Mudry, A.; Mills, M. The early history of the cochlear implant: A retrospective. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Lenarz, T.; Pau, H.-W.; Paasche, G. Cochlear implants. Curr. Pharm. Biotechnol. 2013, 14, 112–123. [Google Scholar]
- Clark, G.M.; Shepherd, R.K.; Franz, B.K.; Dowell, R.C.; Tong, Y.C.; Blamey, P.J.; Webb, R.L.; Pyman, B.C.; McNaughtan, J.; Bloom, D.M. The histopathology of the human temporal bone and auditory central nervous system following cochlear implantation in a patient. Correlation with psychophysics and speech perception results. Acta Otolaryngol. 1988, 105 (Suppl. 448), 1–65. [Google Scholar] [CrossRef]
- Seyyedi, M.; Nadol, J.B. Intracochlear inflammatory response to cochlear implant electrodes in humans. Otol. Neurotol. 2014, 35, 1545–1551. [Google Scholar] [CrossRef] [Green Version]
- Fayad, J.; Linthicum, F.H.; Otto, S.R.; Galey, F.R.; House, W.F. Cochlear implants: Histopathologic findings related to performance in 16 human temporal bones. Ann. Otol. Rhinol. Laryngol. 1991, 100, 807–811. [Google Scholar] [CrossRef]
- Linthicum, F.H.; Fayad, J.; Otto, S.R.; Galey, F.R.; House, W.F. Cochlear implant histopathology. Am. J. Otol. 1991, 12, 245–311. [Google Scholar] [CrossRef]
- Quesnel, A.M.; Nakajima, H.H.; Rosowski, J.J.; Hansen, M.R.; Gantz, B.J.; Nadol, J.B. Delayed loss of hearing after hearing preservation cochlear implantation: Human temporal bone pathology and implications for etiology. Hear. Res. 2016, 333, 225–234. [Google Scholar] [CrossRef] [Green Version]
- O’Leary, S.J.; Monksfield, P.; Kel, G.; Connolly, T.; Souter, M.A.; Chang, A.; Marovic, P.; O’Leary, J.S.; Richardson, R.; Eastwood, H. Relations between cochlear histopathology and hearing loss in experimental cochlear implantation. Hear. Res. 2013, 298, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Kamakura, T.; O’Malley, J.T.; Nadol, J.B. Preservation of Cells of the Organ of Corti and Innervating Dendritic Processes Following Cochlear Implantation in the Human: An Immunohistochemical Study. Otol. Neurotol. 2018, 39, 284–293. [Google Scholar] [CrossRef]
- Scheper, V.; Hoffmann, A.; Gepp, M.M.; Schulz, A.; Hamm, A.; Pannier, C.; Hubka, P.; Lenarz, T.; Schwieger, J. Stem Cell Based Drug Delivery for Protection of Auditory Neurons in a Guinea Pig Model of Cochlear Implantation. Front. Cell. Neurosci. 2019, 13, 177. [Google Scholar] [CrossRef]
- Boggess, W.J.; Baker, J.E.; Balkany, T.J. Loss of residual hearing after cochlear implantation. Laryngoscope 1989, 99, 1002–1005. [Google Scholar] [CrossRef] [PubMed]
- Wilk, M.; Hessler, R.; Mugridge, K.; Jolly, C.; Fehr, M.; Lenarz, T.; Scheper, V. Impedance Changes and Fibrous Tissue Growth after Cochlear Implantation Are Correlated and Can Be Reduced Using a Dexamethasone Eluting Electrode. PLoS ONE 2016, 11, e0147552. [Google Scholar] [CrossRef] [PubMed]
- Reich, U.; Warnecke, A.; Szczepek, A.J.; Mazurek, B.; Olze, H. Establishment of an experimental system to study the influence of electrical field on cochlear structures. Neurosci. Lett. 2015, 599, 38–42. [Google Scholar] [CrossRef]
- McCreery, D.B.; Yuen, T.G.; Agnew, W.F.; Bullara, L.A. Stimulus parameters affecting tissue injury during microstimulation in the cochlear nucleus of the cat. Hear. Res. 1994, 77, 105–115. [Google Scholar] [CrossRef]
- Gstoettner, W.K.; Helbig, S.; Maier, N.; Kiefer, J.; Radeloff, A.; Adunka, O.F. Ipsilateral electric acoustic stimulation of the auditory system: Results of long-term hearing preservation. Audiol. Neurootol. 2006, 11 (Suppl. 1), 49–56. [Google Scholar] [CrossRef]
- Adunka, O.; Kiefer, J.; Unkelbach, M.H.; Lehnert, T.; Gstoettner, W. Development and evaluation of an improved cochlear implant electrode design for electric acoustic stimulation. Laryngoscope 2004, 114, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Adunka, O.; Unkelbach, M.H.; Mack, M.; Hambek, M.; Gstoettner, W.; Kiefer, J. Cochlear implantation via the round window membrane minimizes trauma to cochlear structures: A histologically controlled insertion study. Acta Otolaryngol. 2004, 124, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Vittoria, S.; Lahlou, G.; Torres, R.; Daoudi, H.; Mosnier, I.; Mazalaigue, S.; Ferrary, E.; Nguyen, Y.; Sterkers, O. Robot-based assistance in middle ear surgery and cochlear implantation: First clinical report. Eur. Arch. Otorhinolaryngol. 2021, 278, 77–85. [Google Scholar] [CrossRef]
- Roland, J.T. A model for cochlear implant electrode insertion and force evaluation: Results with a new electrode design and insertion technique. Laryngoscope 2005, 115, 1325–1339. [Google Scholar] [CrossRef]
- Torres, R.; Drouillard, M.; De Seta, D.; Bensimon, J.-L.; Ferrary, E.; Sterkers, O.; Bernardeschi, D.; Nguyen, Y. Cochlear Implant Insertion Axis Into the Basal Turn: A Critical Factor in Electrode Array Translocation. Otol. Neurotol. 2018, 39, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Rajan, G.P.; Kuthubutheen, J.; Hedne, N.; Krishnaswamy, J. The role of preoperative, intratympanic glucocorticoids for hearing preservation in cochlear implantation: A prospective clinical study. Laryngoscope 2012, 122, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Kuthubutheen, J.; Coates, H.; Rowsell, C.; Nedzelski, J.; Chen, J.M.; Lin, V. The role of extended preoperative steroids in hearing preservation cochlear implantation. Hear. Res. 2015, 327, 257–264. [Google Scholar] [CrossRef] [Green Version]
- Sweeney, A.D.; Carlson, M.L.; Zuniga, M.G.; Bennett, M.L.; Wanna, G.B.; Haynes, D.S.; Rivas, A. Impact of Perioperative Oral Steroid Use on Low-frequency Hearing Preservation After Cochlear Implantation. Otol. Neurotol. 2015, 36, 1480–1485. [Google Scholar] [CrossRef]
- von Ilberg, C.; Kiefer, J.; Tillein, J.; Pfenningdorff, T.; Hartmann, R.; Stürzebecher, E.; Klinke, R. Electric-acoustic stimulation of the auditory system. New technology for severe hearing loss. ORL J. Otorhinolaryngol. Relat. Spec. 1999, 61, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Canlon, B.; Meltser, I.; Johansson, P.; Tahera, Y. Glucocorticoid receptors modulate auditory sensitivity to acoustic trauma. Hear. Res. 2007, 226, 61–69. [Google Scholar] [CrossRef]
- Eshraghi, A.A.; Adil, E.; He, J.; Graves, R.; Balkany, T.J.; Van De Water, T.R. Local dexamethasone therapy conserves hearing in an animal model of electrode insertion trauma-induced hearing loss. Otol. Neurotol. 2007, 28, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.X.; Lin, Z.; Lei, D.; Bao, J. The Role of Glucocorticoids for Spiral Ganglion Neuron Survival. Brain Res. 2009, 1277, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Juhn, S.K. Barrier systems in the inner ear. Acta Otolaryngol. 1988, 105 (Suppl. 458), 79–83. [Google Scholar] [CrossRef] [PubMed]
- Juhn, S.K.; Rybak, L.P. Labyrinthine barriers and cochlear homeostasis. Acta Otolaryngol. 1981, 91, 529–534. [Google Scholar] [CrossRef]
- Salt, A.N.; Plontke, S.K. Principles of Local Drug Delivery to the Inner Ear. AUD 2009, 14, 350–360. [Google Scholar] [CrossRef] [Green Version]
- Borenstein, J.T. Intracochlear drug delivery systems. Expert Opin. Drug Deliv. 2011, 8, 1161–1174. [Google Scholar] [CrossRef] [Green Version]
- McCall, A.A.; Leary Swan, E.E.; Borenstein, J.T.; Sewell, W.F.; Kujawa, S.G.; McKenna, M.J. Drug Delivery for Treatment of Inner Ear Disease: Current State of Knowledge. Ear Hear. 2010, 31, 156–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, A.; Eastwood, H.; Sly, D.; James, D.; Richardson, R.; O’Leary, S. Factors influencing the efficacy of round window dexamethasone protection of residual hearing post-cochlear implant surgery. Hear. Res. 2009, 255, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Stathopoulos, D.; Chambers, S.; Enke, Y.L.; Timbol, G.; Risi, F.; Miller, C.; Cowan, R.; Newbold, C. Development of a safe dexamethasone-eluting electrode array for cochlear implantation. Cochlear Implant. Int. 2014, 15, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Risoud, M.; Sircoglou, J.; Dedieu, G.; Tardivel, M.; Vincent, C.; Bonne, N.-X. Imaging and cell count in cleared intact cochlea in the Mongolian gerbil using laser scanning confocal microscopy. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2017, 134, 221–224. [Google Scholar] [CrossRef]
- Risoud, M.; Tardivel, M.; Lemesre, P.-E.; Bonne, N.-X.; Vincent, C. Optimised immunofluorescence method on cleared intact Mongolian gerbil cochlea. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2020, 137, 145–150. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, G.H.; Rubel, E.W. Three-dimensional imaging of the intact mouse cochlea by fluorescent laser scanning confocal microscopy. Hear. Res. 2008, 243, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montgomery, S.C.; Cox, B.C. Whole Mount Dissection and Immunofluorescence of the Adult Mouse Cochlea. J. Vis. Exp. 2016, 107, e53561. [Google Scholar] [CrossRef]
- Gardella, D.; Hatton, W.J.; Rind, H.B.; Rosen, G.D.; Von Bartheld, C.S. Differential tissue shrinkage and compression in the z-axis: Implications for optical disector counting in vibratome-, plastic- and cryosections. J. Neurosci. Methods 2003, 124, 45–59. [Google Scholar] [CrossRef]
- Kopecky, B.J.; Duncan, J.S.; Elliott, K.L.; Fritzsch, B. Three-dimensional reconstructions from optical sections of thick mouse inner ears using confocal microscopy. J. Microsc. 2012, 248, 292–298. [Google Scholar] [CrossRef] [Green Version]
- Farhadi, M.; Jalessi, M.; Salehian, P.; Ghavi, F.F.; Emamjomeh, H.; Mirzadeh, H.; Imani, M.; Jolly, C. Dexamethasone eluting cochlear implant: Histological study in animal model. Cochlear Implant. Int. 2013, 14, 45–50. [Google Scholar] [CrossRef]
- Bas, E.; Goncalves, S.; Adams, M.; Dinh, C.T.; Bas, J.M.; Van De Water, T.R.; Eshraghi, A.A. Spiral ganglion cells and macrophages initiate neuro-inflammation and scarring following cochlear implantation. Front. Cell. Neurosci. 2015, 9, 303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neal, C.; Kennon-McGill, S.; Freemyer, A.; Shum, A.; Staecker, H.; Durham, D. Hair Cell Counts in a Rat Model of Sound Damage: Effects of Tissue 1 Preparation & Identification of Regions of Hair Cell Loss. Hear. Res. 2015, 328, 120–132. [Google Scholar] [PubMed] [Green Version]
- Hutson, K.A.; Pulver, S.H.; Ariel, P.; Naso, C.; Fitzpatrick, D.C. Light sheet microscopy of the gerbil cochlea. J. Comp. Neurol. 2021, 529, 757–785. [Google Scholar] [CrossRef]
- Brody, K.M.; Hampson, A.J.; Cho, H.; Johnson, P.; O’Leary, S.J. A new method for three-dimensional immunofluorescence study of the cochlea. Hear. Res. 2020, 392, 107956. [Google Scholar] [CrossRef] [PubMed]
- Malfeld, K.; Armbrecht, N.; Volk, H.A.; Lenarz, T.; Scheper, V. In Situ 3D-Imaging of the Inner Ear Synapses with a Cochlear Implant. Life 2021, 11, 301. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.-H.; Oghalai, J.S. Predicting the effect of post-implant cochlear fibrosis on residual hearing. Hear. Res. 2005, 205, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, J.; Baker, R.S.; Wood, M. Clinical Case Study Review: Steroid-Responsive Change in Electrode Impedance. Otol. Neurotol. 2013, 34, 227–232. [Google Scholar] [CrossRef]
- Bae, S.-C.; Shin, Y.-R.; Chun, Y.-M. Cochlear Implant Surgery Through Round Window Approach Is Always Possible. Ann. Otol. Rhinol. Laryngol. 2019, 128, 38S–44S. [Google Scholar] [CrossRef]
- Freni, F.; Gazia, F.; Slavutsky, V.; Perello Scherdel, E.; Nicenboim, L.; Posada, R.; Portelli, D.; Galletti, B.; Galletti, F. Cochlear Implant Surgery: Endomeatal Approach versus Posterior Tympanotomy. Int. J. Environ. Res. Public Health 2020, 17, 4187. [Google Scholar] [CrossRef]
- Slavutsky, V.; Nicenboim, L. Preliminary results in cochlear implant surgery without antromastoidectomy and with atraumatic electrode insertion: The endomeatal approach. Eur. Arch. Otorhinolaryngol. 2009, 266, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Vivero, R.J.; Joseph, D.E.; Angeli, S.; He, J.; Chen, S.; Eshraghi, A.A.; Balkany, T.J.; Van De Water, T.R. Dexamethasone base conserves hearing from electrode trauma-induced hearing loss. Laryngoscope 2008, 118, 2028–2035. [Google Scholar] [CrossRef]
- Hendricks, J.L.; Chikar, J.A.; Crumling, M.A.; Raphael, Y.; Martin, D.C. Localized cell and drug delivery for auditory prostheses. Hear. Res. 2008, 242, 117–131. [Google Scholar] [CrossRef] [Green Version]
- Eshraghi, A.A.; Yang, N.W.; Balkany, T.J. Comparative study of cochlear damage with three perimodiolar electrode designs. Laryngoscope 2003, 113, 415–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sircoglou, J.; Gehrke, M.; Tardivel, M.; Siepmann, F.; Siepmann, J.; Vincent, C. Trans-Oval-Window Implants, A New Approach for Drug Delivery to the Inner Ear: Extended Dexamethasone Release From Silicone-based Implants. Otol. Neurotol. 2015, 36, 1572–1579. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, M.; Sircoglou, J.; Vincent, C.; Siepmann, J.; Siepmann, F. How to adjust dexamethasone mobility in silicone matrices: A quantitative treatment. Eur. J. Pharm. Biopharm. 2016, 100, 27–37. [Google Scholar] [CrossRef]
- Mond, H.G.; Stokes, K.B. The Steroid-Eluting Electrode: A 10-Year Experience. Pacing Clin. Electrophysiol. 1996, 19, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Paasche, G.; Bockel, F.; Tasche, C.; Lesinski-Schiedat, A.; Lenarz, T. Changes of postoperative impedances in cochlear implant patients: The short-term effects of modified electrode surfaces and intracochlear corticosteroids. Otol. Neurotol. 2006, 27, 639–647. [Google Scholar] [CrossRef]
- Wrzeszcz, A.; Steffens, M.; Balster, S.; Warnecke, A.; Dittrich, B.; Lenarz, T.; Reuter, G. Hydrogel coated and dexamethasone releasing cochlear implants: Quantification of fibrosis in guinea pigs and evaluation of insertion forces in a human cochlea model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 169–178. [Google Scholar] [CrossRef]
- Wrzeszcz, A.; Reuter, G.; Nolte, I.; Lenarz, T.; Scheper, V. Spiral ganglion neuron quantification in the guinea pig cochlea using Confocal Laser Scanning Microscopy compared to embedding methods. Hear. Res. 2013, 306, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Reynaud, E.G.; Kržič, U.; Greger, K.; Stelzer, E.H.K. Light sheet-based fluorescence microscopy: More dimensions, more photons, and less photodamage. HFSP J. 2008, 2, 266–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roehm, P.C.; Hansen, M.R. Strategies to preserve or regenerate spiral ganglion neurons. Curr. Opin. Otolaryngol. Head Neck Surg. 2005, 13, 294–300. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toulemonde, P.; Risoud, M.; Lemesre, P.E.; Beck, C.; Wattelet, J.; Tardivel, M.; Siepmann, J.; Vincent, C. Evaluation of the Efficacy of Dexamethasone-Eluting Electrode Array on the Post-Implant Cochlear Fibrotic Reaction by Three-Dimensional Immunofluorescence Analysis in Mongolian Gerbil Cochlea. J. Clin. Med. 2021, 10, 3315. https://doi.org/10.3390/jcm10153315
Toulemonde P, Risoud M, Lemesre PE, Beck C, Wattelet J, Tardivel M, Siepmann J, Vincent C. Evaluation of the Efficacy of Dexamethasone-Eluting Electrode Array on the Post-Implant Cochlear Fibrotic Reaction by Three-Dimensional Immunofluorescence Analysis in Mongolian Gerbil Cochlea. Journal of Clinical Medicine. 2021; 10(15):3315. https://doi.org/10.3390/jcm10153315
Chicago/Turabian StyleToulemonde, Philippine, Michaël Risoud, Pierre Emmanuel Lemesre, Cyril Beck, Jean Wattelet, Meryem Tardivel, Juergen Siepmann, and Christophe Vincent. 2021. "Evaluation of the Efficacy of Dexamethasone-Eluting Electrode Array on the Post-Implant Cochlear Fibrotic Reaction by Three-Dimensional Immunofluorescence Analysis in Mongolian Gerbil Cochlea" Journal of Clinical Medicine 10, no. 15: 3315. https://doi.org/10.3390/jcm10153315
APA StyleToulemonde, P., Risoud, M., Lemesre, P. E., Beck, C., Wattelet, J., Tardivel, M., Siepmann, J., & Vincent, C. (2021). Evaluation of the Efficacy of Dexamethasone-Eluting Electrode Array on the Post-Implant Cochlear Fibrotic Reaction by Three-Dimensional Immunofluorescence Analysis in Mongolian Gerbil Cochlea. Journal of Clinical Medicine, 10(15), 3315. https://doi.org/10.3390/jcm10153315





