Abstract
This study aimed to evaluate the long-term (24-month) efficacy and safety of a modified treat-and-extend (mTAE) regimen of aflibercept for macular edema (ME) due to branch retinal vein occlusion (BRVO). This was a prospective multicenter intervention study. We evaluated 50 eyes in 50 patients with ME due to BRVO enrolled between October 2016 and September 2017. The patients received intravitreal aflibercept (IVA) injections according to a mTAE regimen for 24 months. This study reports the secondary endpoints of best-corrected visual acuity (BCVA) and central subfield thickness (CST) at 24 months and compares them with previously reported primary endpoints. Compared with baseline BCVA and CST of 0.33 (0.27) and 488 (165) µm (mean (standard deviation)), respectively, BCVA and CST were significantly improved at 12 and 24 months (12 months: 0.059 (0.19) LogMAR and 299 (112) µm; 24 months: 0.034 (0.18) LogMAR and 272 (81) µm, respectively; both p < 0.0001). Over the 24-month period, the mean number of IVA injections and clinic visits was 7.4 (3.3) and 11.1 (2.0), respectively. The mTAE regimen of IVA injections for ME due to BRVO was effective for improving BCVA and reducing CST over 24 months. This regimen shows promise for reducing the number of injections and clinic visits.
1. Introduction
Branch retinal vein occlusion (BRVO) is one of the most common retinal vascular diseases. The prevalence of BRVO reported by studies conducted in the United States, Europe, Asia, and Australia is 0.4%, whereas in Japan it is 2.0% [1,2]. Anti-vascular endothelial growth factor (VEGF) therapy is currently the standard treatment for macular edema (ME), secondary to BRVO [3]. Dexamethasone intravitreal implant has been reported as another treatment option [4]. Many studies have demonstrated the efficacy of anti-VEGF for BRVO [5,6,7,8,9], but as many as 50% of patients still required intravitreal ranibizumab (IVR) injections 4 years after the first treatment [9]. We therefore consider treatment of chronic BRVO to be burdensome for both patients and healthcare workers. In addition, real-world data shows that treatment outcomes in actual practice were not as impressive as the seminal randomized controlled studies, possibly due to undertreatment [10]. In previous reports, most studies used a pro re nata (PRN) regimen, whereas some studies supported the use of a treat-and-extend (TAE) regimen; however, a more optimal regimen that avoids undertreatment and reduces the number of intravitreal injections and clinic visits has been sought.
To this end, we developed a modified treat-and extend (mTAE) regimen of intravitreal aflibercept (IVA) injections for ME due to BRVO and initiated a prospective multicenter intervention study to assess the effectiveness of the regimen. We previously reported the primary outcomes of best-corrected visual acuity (BCVA) and reduced central subfield thickness (CST) at 12 months after the initial injection and found that the mTAE regimen was effective and required fewer injections and clinic visits [11].
Here, to evaluate whether the efficacy and safety of the regimen is maintained and whether the reduced burden on patients and healthcare staff continues over the long term, we report the 24-month results of this mTAE regimen.
2. Material and Methods
We have described the materials and methods in detail in our previous report on outcomes at 12 months [11] and provide a brief summary here.
2.1. Study Design
This prospective multicenter intervention study involving patients with treatment-naïve ME due to BRVO commenced in October 2016 with the last patient completing the 1-year study in September 2018.
The study protocol was approved by the institutional review board of Jichi Medical University (B18-003). The study followed the tenets laid out in the Declaration of Helsinki. Written informed consent was obtained from all patients. The study was registered in the University Hospital Medical Information Network Clinical Trials registry prior to study commencement (27/10/2016, UMIN000024587).
2.2. Patients and Ophthalmological Examination
This study was conducted at 6 sites in Japan (Institution A, Jichi Medical University Hospital; B, Japan Community Health Care Organization Tokyo Shinjuku Medical Center; C, Ohkubo Eye Clinic; D, Takahashi Eye Clinic; E, Saito Eye Clinic; and F, Aoki Eye Clinic). The detailed inclusion and exclusion criteria are described in our previous report [10]. Briefly, the study included treatment-naïve patients aged ≥20 and <90 years with visual impairment due to ME secondary to BRVO who underwent a complete ophthalmic examination.
2.3. mTAE Regimen
Patients were treated with aflibercept according to the mTAE regimen we described in detail previously [11]. Supplementary Figures S1–S4 show an example of the administration methods of PRN and TAE regimens compared with our mTAE regimen. Briefly, after the first injection, all patients attended follow-up visits every 4 weeks. A loading dose was not administered. If there was any exudative lesion in the macula (ME and/or serous retinal detachment), the patient received a second injection and the TAE process commenced. At that time, no CST criteria were applied. After the second injection, if there was no exudative lesion, the patient received an IVA injection and the period to the next treatment was extended by 4 weeks at a time (no maximum interval was specified). This interval of 4 weeks was selected instead of 2 weeks because the degree of ME secondary to BRVO was considered to be not so severe, and 4 weeks was considered adequate to prevent retinal damage. If there was increased exudative change in optical coherence tomography findings at first recurrence, the patient received an injection and the visit interval was shortened by 4 weeks; if the exudative change was the same or less compared with the first recurrence, an injection was given and the interval to the next visit remained unchanged. Patients who maintained a dry macula after the first injection were followed up every 4 weeks until week 16, and thereafter, the visit interval could be extended to 3 months. If the first recurrence of exudative changes was observed after week 16, the TAE regimen was recommended at 3-month intervals. Patients who had no recurrence after week 16 were observed every 3 months.
2.4. Outcome Measures
The main outcome measures were mean changes in BCVA and CST, with the primary outcomes evaluated at 12 months [11] and the secondary outcomes evaluated at 24 months. We also examined the numbers of IVA injections and clinic visits over the 24-month period, as well as the distribution of IVA treatment intervals. In addition, we assessed the changes in BCVA and CST compared with baseline and 12-month values as well as calculated the numbers of IVA injections and clinic visits according to type of BRVO: macular BRVO (i.e., occlusion of only the vein inside the arcade vessels) and major BRVO (all BRVO with ME other than macular BRVO). Major BRVO is caused by occlusion of one of the four major branch retinal veins, and it involves the entire segment of the retina drained by the vein extending all the way to the peripheral retina. Macular BRVO is caused by occlusion of one of the veins from only the macular region (the part of the retina between the superior and inferior vascular arcades) [12].
2.5. Statistical Analysis
As in our evaluation of the primary outcomes [11], statistical analysis was performed using JMP Pro software version 14.1.0 (SAS Institute, Cary, NC, USA). The efficacy endpoints were analyzed in the full analysis set (FAS), which included all patients who received any study treatment and had data at baseline and at least 1 time point after baseline. Between 4 and 23 months, the last observation carried forward was used to impute the missing data. Major BRVO and macular BRVO were compared in 39 of 50 cases with per protocol set (PPS), excluding 11 that dropped out. Decimal BCVA was converted to logMAR. BCVA and CST were compared with those at the first injection by using a two-tailed paired t-test.
3. Results
3.1. Baseline Characteristics
Table 1 shows the baseline characteristics of the participants. As reported previously [11], among the 50 patients included in this study (24 men, 26 women), 11 dropped out (8 were lost to follow-up, 1 withdrew consent, 1 underwent cataract surgery, and 1 underwent pan-retinal photocoagulation). In total, 46 eyes were observed for 12 months, and 39 eyes were observed for 24 months. Mean age (SD) at the start of treatment was 66 (12) years (range 42–85 years). Mean baseline BCVA (logMAR) was 0.33 (0.27) and mean baseline CST was 488 (165) µm. Major BRVO was evident in 25 eyes and macular BRVO in 14 eyes. Figure 1 showed the study design and patient disposition.

Table 1.
Baseline characteristics.
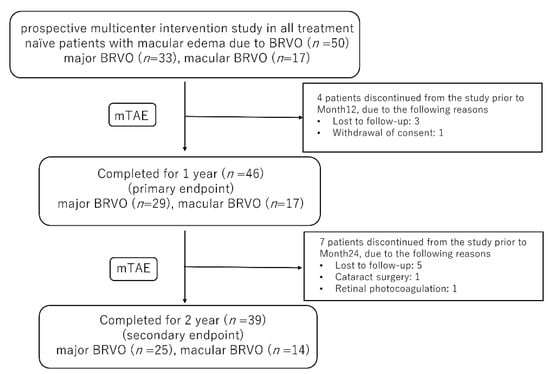
Figure 1.
The study design and patient disposition. BRVO, branch retinal vein occlusion, mTAE, modified treat-and-extend.
3.2. BCVA and CST Outcomes
Mean BCVA was significantly improved at 1, 12, and 24 months (0.11 (0.16), 0.059 (0.18), and 0.034 (0.18), respectively) compared with baseline (0.33 (0.27); all p < 0.0001; Figure 2).
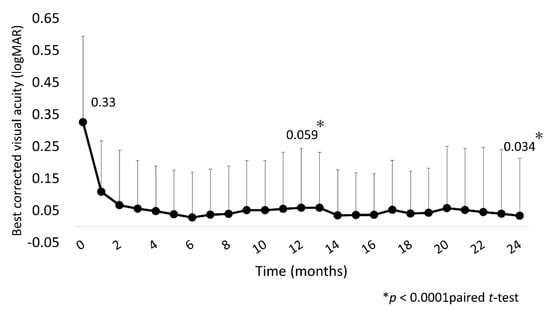
Figure 2.
Mean BCVA (logMAR) over 24 months. Mean BCVA improved significantly at 1 month after IVA injection, with the improvement continuing through to 24 months. BCVA, best-corrected visual acuity; IVA, intravitreal aflibercept.
In three eyes, the final BCVA was less than logMAR0.3: in two of these eyes, the BCVA was equivalent to that at the first visit, and in one eye, it declined due to cataract progression. The mean CST was significantly decreased after IVA injections at 1, 12, and 24 months (248 (36) µm, 299 (112) µm, and 272 (81) µm, respectively) compared with baseline (480 (165) µm; all p < 0.0001; Figure 3).
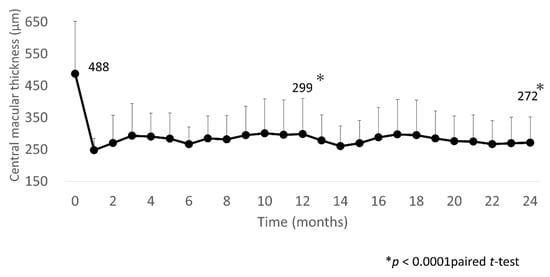
Figure 3.
Mean CST over 24 months. Mean CST improved significantly at 1 month after IVA injection, with the improvement sustained through to 24 months. CST, central subfield thickness; IVA, intravitreal aflibercept.
3.3. IVA Injections and Clinic Visits
The mean number of IVA injections over the 24 months was 7.4 (3.3), with significantly fewer injections administered in the second year compared with the first year (2.9 (1.4) vs. 4.7 (2.0); p = 0.024, paired t-test). Five eyes received only one injection (Figure 4).
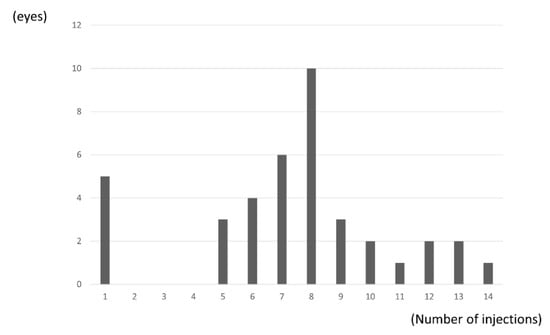
Figure 4.
Distribution of number of injections. Five eyes (13%) received only one injection. Mean number of IVA injections over the 24 months was 7.4 (3.3).
The mean number of clinic visits was 11.1 (2.0) over the 24 months, with significantly fewer visits occurring in the second year than in the first year (3.9 (1.4) vs. 7.1 (1.0); p < 0.0001, paired t-test). (Figure 5).
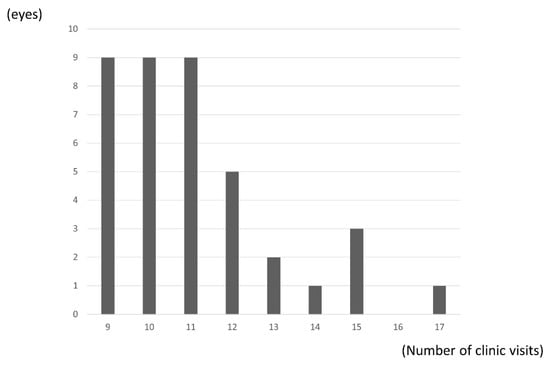
Figure 5.
Distribution of number of clinic visits. Mean number of clinic visits was 10.1 (2.0) over the 24 months.
3.4. Time to First Recurrence from First Injection
Recurrence occurred most frequently at 3 months (15 eyes). Recurrence of exudative changes was apparent in eight eyes at 1 month and in two eyes at 7 months. These two eyes showed no recurrence of exudative change after the TAE regimen was started. Five eyes showed no recurrence over the 24 months, three with major BRVO, and two with macular BRVO.
3.5. Injection Frequency
The IVA injection interval could be extended to more than 5 months in 21 eyes (54%), including the 5 eyes that showed no recurrence over the 24 months (Figure 6).
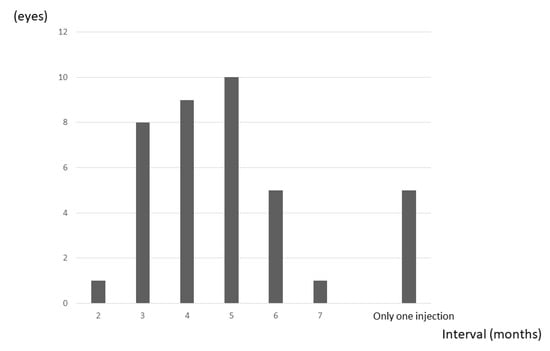
Figure 6.
Frequency of maximum dosing interval. Five eyes (13%) had no recurrence. The IVA injection interval could be extended to more than 5 months in 21 eyes (54%) (including all 5 eyes showing no recurrence).
3.6. Major and Macular BRVO
Table 2 shows the comparison between major BRVO and macular BRVO.

Table 2.
Comparison of major BRVO and macular BRVO.
Figure 7 shows the improvements in BCVA and CST found in 25 eyes with major BRVO and 14 eyes with macular BRVO.
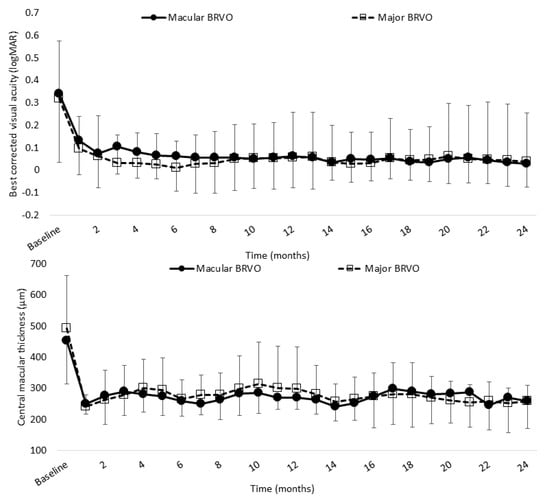
Figure 7.
Mean BCVA (logMAR) and CST of major BRVO and macular BRVO over 24 months. Mean BCVA in major and macular BRVO was significantly improved at 12 and 24 months (0.085 (0.20) and 0.061 (0.23), 0.0017 (0.14) and −0.015 (0.11), respectively) compared with baseline (0.33 (0.26) and 0.30 (0.30), respectively; both p < 0.0001, paired t-test). Mean CST in major and macular BRVO was significantly decreased at 12 and 24 months (299 (135) µm and 257 (52) µm, (270 (129) µm and 259 (70) µm, respectively) compared with baseline (494 (168) µm and 454 (141) µm, respectively; both p < 0.0001, paired t-test). BCVA, best-corrected visual acuity; BRVO, branch retinal vein occlusion; CST, central subfield thickness.
Mean BCVA in major and macular BRVO was significantly improved at 12 and 24 months after the initial injection (12 months: 0.058 (0.18) and 0.037 (0.20); 24 months: 0.061 (0.20) and 0.029 (0.15), respectively) compared with baseline (0.32 (0.25) and 0.34 (0.30), respectively; both p < 0.0001, paired t-test). Mean CST in major and macular BRVO was significantly decreased at 12 and 24 months (12 months: 299 (135) µm and 258 (52) µm; 24 months: 270 (38) µm and 259 (90) µm, respectively) compared with baseline (494 (168) µm and 454 (141) µm, respectively; both p < 0.0001, paired t-test).
There was no significant difference in either BCVA or CST at 24 months between major and macular BRVO (p = 0.84 and p = 0.53, respectively; paired t-test). There was also no significant difference between major and macular BRVO in the mean number of IVA injections administered (7.5 (3.6) and 7.1 (3.0), respectively; p = 0.69, paired t-test) or in the mean number of clinic visits required (11.3 (2.2) and 10.6 (1.6), respectively; p = 0.31, paired t-test). The mean time from awareness of symptoms to diagnosis was significantly shorter in major BRVO than in macular BRVO (1.5 (1.6) and 3.3 (3.8), respectively; p = 0.041, paired t-test).
3.7. Safety over the 24 Months
No serious ocular complications associated with IVA injections were observed. Cataract progressed in only one eye and was not a complication of the IVA injection. BCVA was improved in the eye after cataract surgery. No serious systemic adverse events were noted in any eyes over the 24 months.
4. Discussion
In this study, we demonstrated the long-term effectiveness of the mTAE regimen of IVA injections for improving VA and reducing CST in ME due to BRVO. To our knowledge, this is the first prospective study of an mTAE regimen using aflibercept that combines the best of the PRN and TAE regimens and reports long-term outcomes. Considering the relatively good baseline VA in the present study (0.33 LogMAR unit, which is equivalent to 68.5 ETDRS letters), we believe that the improvement in BCVA and the reduction in CST values are comparable with those in previous studies, such as the VIBRANT study, a randomized controlled trial conducted using aflibercept in a fixed protocol for regulatory approval [13], and the BRIGHTER study, which confirmed the efficacy of less treatment-intensive treatment regimen using IVR injections, that is, one injection plus PRN [14]. Over 2 years, our mTAE regimen maintained BCVA and CST with fewer IVA injections compared with the PRN regimen of IVR injections used in the BRIGHTER study (7.4 (3.34) vs. 11.4 (5.81) injections, respectively). Moreover, our regimen also resulted in fewer clinic visits (11.1 (2.0) vs. 24, respectively). In the second year of our regimen, there were fewer IVA injections and clinic visits compared with the first year (2.9 injections and 3.9 visits). In previous studies, the number of injections after the second year also decreased compared with the first year [9,15]. Analysis of real-world data of 2530 eyes from 48 real-world studies demonstrated that the mean baseline VA in the pooled data was 54.0 letters and the change in VA at 12 months was 14.6 letters, with a change in CST of -181.7 mm, suggesting that visual and anatomical gains were not as impressive as those in the seminal randomized control study [10]. This is, at least in part, due to reduced injection frequency. To address such real-world problems, in the field of exudative age-related macular degeneration, a TAE approach, rather than a PRN approach, is recommended, considering it in terms of predictability and lower treatment burden, which will improve service capacity [16]. We believe such advantages of TAE rationalize its use for the treatment of ME due to BRVO as well. This is especially relevant for countries such as Japan given that Ogura et al. reported that most retina specialists adopt PRN regimens regardless of ME severity [17].
We believe that the “modified” TAE regimen in this study contributes to avoiding overtreatment. Specifically, in this study, the time of the first recurrence varied, highlighting the need to individualize the administration method for each patient. Five eyes showed no recurrence at all. As such, we believe that choosing a “standard” TAE regimen from the beginning might have led to overtreatment in such patients, and our mTAE regimen may prevent this from occurring. Importantly, the IVA injection interval could be extended to more than 5 months in 21 eyes (54%) in this study (including in the 5 eyes that showed no recurrence). Our results may also indicate that IVA injections can be stopped if the injection interval can be extended to 4 months because patients in this study for whom the injection interval could be extended to 4 months or longer did not have recurrence or had only small exudative changes over the entire observation period. For patients who require frequent injections, it is important that the administration regimen and the interval between hospital visits place as low a burden as possible. In this aspect, the injection interval could not be extended beyond 3 months in nine eyes (23.1%). In the RETAIN study, 50% of patients still required IVR injections 4 years after the first treatment [9]. Thus, although anti-VEGF therapy can maintain good VA, roughly one-third to half of the patients need injections over the long term. Additionally, chronic BRVO may require additional treatment such as steroids and laser photocoagulation [18,19], further highlighting the importance for retina specialists to secure time for patient management by reducing outpatient visits. In this study, five cases that showed no recurrence were followed up every 3 months after month 4, so the number of clinic visits increased to 11 times. For example, if the first recurrence occurred in month 4 and no recurrence thereafter, the administration period was steadily extended because there is no upper limit on the administration period. As a result, the number of clinic visits was nine. The number of clinic visits would be further reduced if the follow-up period for one-injection cases can be extended.
Based on previous studies, it is generally assumed that major BRVO is more aggressive than macular BRVO. A previous study found that macular BRVO responded better than major BRVO to treatment, with clearer improvement of vision and fewer injections [20]. Similar results are reported for conbercept, which had a better short-term effect in patients with macular BRVO than in those with major BRVO [21]. In the present study, however, neither the change in BCVA nor CST over 24 months was significantly different between major and macular BRVO. Only the mean time from awareness of symptoms to diagnosis was significantly shorter in major BRVO, which may reflect patients being more likely to notice symptoms when lesions are large. Our study suggests that aflibercept is equally effective for both major and macular BRVO; however, further studies are warranted to confirm whether the treatment efficacy of aflibercept is equivalent for both major and macular BRVO.
There are some limitations to this study. First, the study was designed as a single-arm trial and the sample size was relatively small. Second, only Japanese patients were included. Therefore, further prospective studies with larger study populations and longer follow-up are needed to confirm the usefulness of our regimen.
5. Conclusions
In this prospective study, an mTAE regimen of IVA injections for treating ME due to BRVO effectively improved BCVA and reduced CST over a 2-year period. We believe that this mTAE regimen of aflibercept may be useful in actual clinical practice, by improving VA and structural outcomes as well as reducing the number of required injections and hospital visits, thereby also helping to reduce burden on both patients and healthcare workers.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jcm10143162/s1, Figure S1: The case of first retreatment criteria at month 3, Figure S2: The case of first TAE starting criteria at month 4, Figure S3: The case of first retreatment criteria at month 10, Figure S4: (a) PRN regimen. (b) TAE regimen.
Author Contributions
Y.A., H.T., and Y.Y. designed and conducted the study. Y.A and Y.Y. collected and analyzed the data and wrote the main manuscript text. S.I., S.S., X.T., Y.I., and S.T. contributed to data collection and interpretation and critically reviewed the manuscript. H.K. supported and supervised the study. All authors have read and agreed to the published version of the manuscript.
Funding
This study received financial support from Bayer Yakuhin. The funding organization had no role in the design and conduct of this research.
Institutional Review Board Statement
The study was registered in the University Hospital Medical Information Network Clinical Trials Registry prior to study commencement (October 27, 2016, UMIN000024587).
Informed Consent Statement
Written informed consent was obtained from all patients.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its Supplementary Information files. All data set files are available from the Figshare database (https://doi.org/10.6084/m9.figshare.8982500, accessed on 6 May 2021).
Acknowledgments
The authors thank Yuji Takahashi, Shinsuke Aoki, Shinichiro Saito, Yuma Shibahara, Yukiko Sanui, Ryota Takahashi, Masaki Onda, Akira Ohkubo, Yujiro Fujino, Ichiya Sano, and Yuka Kasuya for recruitment and treatment of the patients. The authors also thank Chihiro Mayama for management of the Japan Community Healthcare Organization Tokyo Shinjuku Medical Center. ThinkSCIENCE Inc., Japan, provided language editing assistance.
Conflicts of Interest
Hidenori Takahashi: Lecturer’s fees from Novartis Pharmaceuticals, Kowa Pharmaceutical, Senju Pharmaceutical, Alcon Pharmaceuticals, Santen Pharmaceutical, and Pfizer; grants from Alcon Pharmaceuticals, Senju Pharmaceuticals, and Bayer Yakuhin; consultant’s fee from Novartis Pharmaceuticals; one patent and two patents pending; and founder of DeepEyeVision Inc; all outside this work. Yuji Inoue: Lecturer’s fees from Kowa Pharmaceuticals, Novartis Pharmaceuticals, Bayer Yakuhin, and Santen Pharmaceuticals, outside this work. Hidetoshi Kawashima: Lecturer’s fees from Kowa Pharmaceutical, Novartis Pharmaceuticals, and Santen Pharmaceutical, outside this work. Yasuo Yanagi: Lecturer’s fees and grants from Santen Pharmaceutical, Novartis Pharmaceuticals Alcon Pharmaceuticals; advisory board member for Bayer Pharmaceuticals and Roche; and Consultant for Santen Pharmaceuticals. All remaining authors declare no conflicts of interest.
References
- Rogers, S.; McIntosh, R.L.; Cheung, N.; Lim, L.; Wang, J.J.; Mitchell, P.; Kowalski, J.W.; Nguyen, H.; Wong, T.Y. International Eye Disease Consortium. The prevalence of retinal vein occlusion: Pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology 2010, 117, 313–319. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, M.; Kiyohara, Y.; Arakawa, S.; Hata, Y.; Yonemoto, K.; Doi, Y.; Iida, M.; Ishibashi, T. Prevalence and systemic risk factors for retinal vein occlusion in a general Japanese population: The Hisayama study. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3205–3209. [Google Scholar] [CrossRef] [Green Version]
- Mitry, D.; Bunce, C.; Charteris, D. Anti-vascular endothelial growth factor for macular oedema secondary to branch retinal vein occlusion. Cochrane Database Syst. Rev. 2013, 2013, 009510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuppermann, B.D.; Blumenkranz, M.S.; Haller, J.A.; Williams, G.A.; Weinberg, C.C.; Whitcup, S.M.; Dexamethasone, D.D.S. Phase II Study Group. Randomized controlled study of an intravitreous dexamethasone drug delivery system in patients with persistent macular edema. Arch. Ophthalmol. 2007, 125, 309–317. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Hafiz, G.; Shah, S.M.; Nguyen, Q.D.; Ying, H.; Do, D.V.; Quinlan, E.; Zimmer-Galler, I.; Haller, J.A.; Solomon, S.D.; et al. Ranibizumab for macular edema due to retinal vein occlusions: Implication of VEGF as a critical stimulator. Mol. Ther. 2008, 16, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A.; Brown, D.M.; Awh, C.C.; Lee, S.Y.; Gray, S.; Saroj, N.; Murahashi, W.Y.; Rubio, R.G. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology 2011, 118, 1594–1602. [Google Scholar] [CrossRef]
- Tadayoni, R.; Waldstein, S.M.; Boscia, F.; Gerding, H.; Pearce, I.; Priglinger, S.; Wenzel, A.; Barnes, E.; Gekkieva, M.; Pilz, S.; et al. Individualized stabilization criteria-driven ranibizumab versus laser in branch retinal vein occlusion: Six-month results of BRIGHTER. Ophthalmology 2016, 123, 1332–1344. [Google Scholar] [CrossRef] [Green Version]
- Miwa, Y.; Muraoka, Y.; Osaka, R.; Ooto, S.; Murakami, T.; Suzuma, K.; Takahashi, A.; Iida, Y.; Yoshimura, N.; Tsujikawa, A. Ranibizumab for macular edema after branch retinal vein occlusion. Retina 2017, 37, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Campochiro, P.A.; Sophie, R.; Pearlman, J.; Brown, D.M.; Boyer, D.S.; Heier, J.S.; Marcus, D.M.; Feiner, L.; Patel, A.; RETAIN Study Group. Long-term outcomes inpatients with retinal vein occlusion treated with ranibizumab. The RETAIN study. Ophthalmology 2014, 121, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Ang, J.L.; Moye, S.A.; Kim, L.N.; Nguyen, V.; Hunt, A.; Barthelmes, D.; Gillies, M.C.; Mehta, H. A systematic review of real-world evidence of the management of macular oedema secondary to branch retinal vein occlusion. Eye (Lond.) 2020, 34, 1770–1796. [Google Scholar] [CrossRef]
- Arai, Y.; Takahashi, H.; Inoda, S.; Sakamoto, S.; Tan, X.; Inlue, Y.; Tominaga, S.; Kawashima, H.; Yanagi, Y. Efficacy of modified treat-and-extend aflibercept regimen for macular edema due to branch retinal vein occlusion: 1-year prospective study. J. Clin. Med. 2020, 239, 2360. [Google Scholar] [CrossRef] [PubMed]
- Hayreh, S.S.; Zimmerman, M.B. Fundus changes in branch retinal vein occlusion. Retina 2015, 35, 1016–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, W.L.; Boyer, D.S.; Heier, J.S.; Brown, D.M.; Haller, J.A.; Vitti, R.; Kazmi, H.; Berliner, A.J.; Erickson, K.; Chu, K.W.; et al. Intravitreal aflibercept for macular edema following branch retinal vein occlusion: 52-week results of the VIBRANT study. Ophthalmology 2016, 123, 330–336. [Google Scholar] [CrossRef]
- Tadayoni, R.; Waldstein, S.M.; Boscia, F.; Gerding, H.; Gekkieva, M.; Barnes, E.; Gupta, A.D.; Wenzel, A.; Pearce, I.; BRIGHTER Study Group. Sustained benefits of ranibizumab with or without laser in branch retinal vein occlusion. 24-month results of the BRIGHTER study. Ophthalmology 2017, 124, 1778–1787. [Google Scholar] [CrossRef] [Green Version]
- Chatziralli, I.; Theodossiadis, G.; Chatzirallis, A.; Parikakis, E.; Mitropoulos, P.; Theodossiadis, P. Ranibizumab for retinal vein occulusion: Predictive factors and long-term outcomes in real-life data. Retina 2018, 38, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.H.; Downey, L.; Devonport, H.; Gale, R.P.; Kotagiri, A.; Mahmood, S.; Mehta, H.; Narendran, N.; Patel, P.J.; Parmar, N.; et al. Recommendation by a UK expert panel on an aflibercept treat-and-extend pathway for the treatment of neovascular age-related macular degeneration. Eye (Lond.). 2020, 34, 1825–1834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogura, Y.; Kondo, M.; Kadonosono, K.; Shimura, M.; Kamei, M.; Tshujikawa, A. Current practice in the management of branch retinal vein occlusion in Japan: Survey results of retina specialists in Japan. Jpn. J. Ophthalmol. 2016, 63, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Haller, J.A.; Bandello, F.; Belfort, R., Jr.; Blumenkranz, M.S.; Gillies, M.; Heier, J.; Loewenstein, A.; Yoon, Y.H.; Jacques, M.L.; Jiao, J.; et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology 2010, 117, 1134–1146. [Google Scholar] [CrossRef]
- Sakimoto, S.; Kamei, M.; Sakaguchi, H.; Suzuki, M.; Matsumura, N.; Nishida, K.; Nishida, K. Direct photocoagulation to leakage points to treat chronic macular edema associated with branch retinal vein occlusion: A pilot study. Clin. Ophthalmol. 2014, 7, 2055–2060. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Li, Y.; Fang, S.F.; Wang, H. Efficacy of intravitreal lucentis injection on major and macular branch retinal vein occlusion. BMC Ophthalmol. 2020, 20, 274. [Google Scholar] [CrossRef]
- Tao, Y.; Huang, C.; Liu, M.; Sun, L.; Li, L.; Wei, Y.; Yu, X.; Wang, H. Short-term effect of intravitreal conbercept injection on major and macular branch retinal vein occlusion. J. Int. Med. Res. 2019, 47, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).