Abstract
Mental health disorders are ambiguously defined and diagnosed. The established diagnosis technique, which is based on structured interviews, questionnaires and data subjectively reported by the patients themselves, leaves the mental health field behind other medical areas. We support these statements with examples from major depressive disorder (MDD). The National Institute of Mental Health (NIMH) launched the Research Domain Criteria (RDoC) project in 2009 as a new framework to investigate psychiatric pathologies from a multidisciplinary point of view. This is a good step in the right direction. Contemporary psychiatry considers mental illnesses as diseases that manifest in the mind and arise from the brain, expressed as a behavioral condition; therefore, we claim that these syndromes should be characterized primarily using behavioral characteristics. We suggest the use of smartphones and wearable devices to passively collect quantified behavioral data from patients by utilizing digital biomarkers of mental disorder symptoms. Various digital biomarkers of MDD symptoms have already been detected, and apps for collecting this longitudinal behavioral data have already been developed. This quantified data can be used to determine a patient’s diagnosis and personalized treatment, and thereby minimize the diagnosis rate of comorbidities. As there is a wide spectrum of human behavior, such a fluidic and personalized approach is essential.
1. Introduction
The problematic heterogeneity of mental disorders is a well-discussed issue [1,2,3]. Olbert et al. [2] used combinatorial mathematics to show that two individual patients who have the same psychiatric diagnosis may not share any symptoms. In addition, two combinations of symptoms of the same disorder will often share less than half of the symptoms [2]. In order to conduct an in-depth exploration of the problem of and the potential digital solutions to the inconsistency in the characterization of psychiatric pathologies, in this article we focus on major depressive disorder (MDD).
MDD is a highly prevalent condition, with 6% of the adult population worldwide affected each year [4]. MDD has been recognized as a major risk factor for suicide by the World Health Organization [5]. In addition, MDD is associated with other life-threatening conditions, such as stroke [6]. As MDD is of particular medical importance, it is unfortunate that experts from the psychiatric community claim that MDD is poorly defined and diagnosed [7,8]. Santor et al. [8] mapped over 280 different depression scales to measure MDD. Another study that emphasized the unwanted heterogeneity of MDD was undertaken by Zimmerman et al. [9], who found that there are 227 possible ways to meet the DSM-IV diagnostic criteria for MDD, while only 170 different combinations occur among patients.
This evidence indicates the problematic diagnosis system established by the Diagnostic and Statistical Manual of Mental Disorders (DSM), which is routinely used by psychiatrists. The DSM-5 diagnosis method for MDD includes a list of nine symptoms applied as diagnostic criteria [10]. Patients meet the diagnostic criteria based on the number and duration of symptoms and signs. Threshold scores are used to classify and measure depression severity. The conventional way to determine those crucial scores is to use questionnaires completed by the psychiatrist (Figure 1a). Even though psychiatrists aim to determine scores objectively, this diagnosis technique, which is mostly based on data subjectively reported by the patients themselves, leaves the mental health field behind other medical areas. One of the consequences of using this problematic diagnosis technique is also reflected in the low remission rates of MDD patients, even after they are treated with different treatment options [11,12]. Other factors that are also likely to have an influence on remission rates include patients who do not routinely take their prescribed medications. In this article, we provide examples and evidence from the literature discussing MDD, but our ideas are also relevant to other mental health disorders.
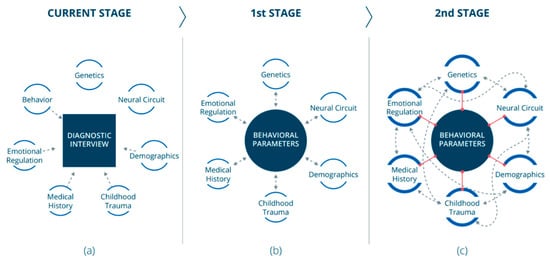
Figure 1.
Stages toward a paradigm shift: (a) Today, established diagnoses of mental disorders are based on interviews. Different disciplines (smaller circles) are investigated separately. Some of those investigated areas contribute (unidirectional dashed arrows) to the diagnosis. (b) At the first stage, behavioral data should become the field’s pillar. Data from other relevant areas (smaller circles) will be cross-referenced (dashed bidirectional arrows) with behavioral data. (c) At the second stage, a substantial understanding of the behavioral component could lead to finding correlations (red lines) between behavioral markers and markers from other areas (smaller circles). This would promote crosstalk (dashed bidirectional arrows) between the different areas and consequently expand our knowledge in those areas.
From a historical viewpoint, we can observe the conceptual changes that the classification system for mental conditions has undergone. The first DSM versions were focused on collecting statistical data [13]. In the DSM-III, there was a paradigm shift to include empirically based data with the goal of producing non-biased diagnosis criteria [13]. The DSM-5 combines etiological and neurobiological research results into the definitions of mental disorders in order to improve the diagnosis process [14,15]. This approach is consistent with the Research Domain Criteria (RDoC) project of the National Institute of Mental Health (NIMH).
The NIMH launched the RDoC project in 2009, which proposed a new framework to investigate psychiatric pathologies [16]. The project’s idea is that the integration of data from different disciplines, such as genomics, neuroimaging and the clinical field, can provide a better understanding of psychiatric pathologies. Thomas R. Insel [17] explained that today’s diagnostic systems, ICD and DSM, create a common language in psychiatry but rely on observable symptoms. This approach limits physicians from performing examinations to obtain a specific diagnosis, an option that exists in other medical fields [17]. This approach also leads physicians to routinely diagnose comorbidities within patients [18,19]. In addition, Thomas R. Insel [17] mentioned that medical diagnoses that rely only on symptoms, which are reported by the patients themselves, are not only heterogeneous and imprecise, but the subsequent treatment focuses on symptom relief and prevention.
Addressing these problems that accompany the conservative and common practice of psychiatric diagnosis requires a paradigm shift. Although it is known that various aspects (e.g., genetics, chronic medical conditions and deprivation) influence the development of a mental disorder within an individual [20], mental disorders—including MDD—are behavioral conditions. It is also known that contemporary psychiatry considers mental illnesses as diseases that manifest in the mind and arise from the brain, expressing themselves as behavioral conditions [21]. Therefore, we claim that mental syndromes should be characterized primarily using behavioral characteristics. This approach does not fully correspond with the RDoC project’s view since we argue that behavioral data should be the field’s pillar. Data from other areas, such as emotional regulation and genetics, are also essential for understanding the core of the psychiatric disorder, and must therefore be cross-referenced with behavioral data (Figure 1b). As we will emphasize in the following sections, we support the use of digital devices to collect behavioral data for the purpose of psychiatric assessment.
2. The Digital Revolution in Mental Health
2.1. An Active Digital Collection of Behavioral Data
Digitally completing a singular self-reported questionnaire is a current technique in the field [22]. Furthermore, it has been shown that smartphones and wearable devices can also be utilized for repeated self-reported questionnaires [23,24,25]. This method is called ecological momentary assessment (EMA). The advantage of this method is the frequent number of times in a short period that patients can be repetitively asked to answer diagnostic questions while in a natural setting, as opposed to periodical assessment at the clinic. Torous et al. [25] developed an app with an interface that allows users to answer the Patient Health Questionnaire-9 (PHQ-9). Suhara, Xu and Pentland [24] developed the Cognition Kit app, which asks patients to report their moods and perform digital cognitive tasks [24].
The data collected using these apps correlates with the data collected using traditional methods [23,24,25]. Although these apps show progress in the data collection method in MDD patients, they are based on conventional methods of symptom-based self-reported diagnosis.
2.2. Digital Phenotyping
To perform the characterization of MDD via behavioral features, patient behavior needs to be quantified objectively. At present, patients self-report about their own behavior. Physicians try their best to objectively fill the diagnosis questionnaires and determine the quantified scores. Nevertheless, the existing data are still biased. In addition, the reported behavioral data are not longitudinal and are collected during meetings between patients and their physicians. Most of the time, this is based on retrospective recollection. To overcome these limitations, we suggest the use of smartphones and wearable devices to collect quantified behavioral data from patients passively. This process falls under the definition of digital phenotyping, as defined in 2016 by Onnela and Rauch [26]. The utilization of smartphones and wearable devices exists in many other medical areas. For example, the monitoring of glucose levels among diabetes patients was revolutionized by developing and producing low-cost continuous glucose monitoring sensors [27]. Recently, decision-making using data recorded by these devices was approved by the FDA [27]. Another example of data collection via wearable devices is that done with the Apple Watch or other fitness bands that can passively measure pulse rate and detect pulse irregularity, which can signal atrial fibrillation or flutter [28]. Embracing smartphones and wearable devices as sensors for collecting quantified behavioral data could generate a quantified, continuous and objective database of patients’ behavior. Applying a computational algorithm to this database could enable different mental disorders to be subtyped into subcategories using data-driven analysis and combined with other relevant dimensions. This computational system could help determine better disease diagnoses and treatments for new patients. Of course, there are limitations to this digital approach. Since personal data about patients are collected in face-to-face meetings, unique nuances can be overlooked; a professional clinician has the potential to detect information that a machine would miss.
Torous, Onnela and Keshavan [29] previously suggested the use of digital phenotyping to collect data as part of the RDoC project. Our idea is novel in that we suggest that digital-behavioral biomarkers can not only assist in the diagnosis of MDD and other mental disorders, but that a new characterization of subtypes of such disorders should be based on them.
Our vision of embracing smartphones and wearable devices to collect data among patients suffering from mental health disorders is very reasonable since the use of these devices is growing tremendously, and 50% of all smartphones and tablets have a mobile health app downloaded [30].
The digital phenotyping field is growing. In 2018, it was suggested that actions recorded by smartphones, such as typing and scrolling patterns, can reflect the results of tests conducted by psychiatrists to assess mental health patients [31]. Potential behavioral biomarkers can be researched, relying on the information available in the DSM and ICD. Later, new biomarkers can be detected through the new data that will be gathered. Several studies performed in recent years have already produced promising results regarding digital biomarkers that can be used for MDD characterization and diagnosis. These biomarkers include the utilization of a variety of data types to detect depressive behavior. Mundt et al. and Zhang et al. [32,33,34] were able to extract features from voice samples and use them to measure depression symptoms. Actigraphy was used to measure patterns of motor activity and was employed for the development of digital biomarkers [35,36,37]. Tonon et al. [37] were able to use light exposure measurements to differentiate between melancholic depression and non-melancholic depression patients. Jacobson et al. [36] were able to draw associations between light exposure and depression severity. Saeb et al. [38] found a correlation between the severity of depression symptoms and a number of features that were extracted from mobile phone global positioning systems (GPS) and smartphones’ normal usage (usage duration and frequency). Dagum [39] analyzed the human–computer interaction of normal smartphone usage to identify digital biomarkers associated with cognitive function. Mandryk and Birk [40] conducted a literature review and suggested five categories of potential biomarkers that can be deduced from data recorded from individuals playing computer games.
Several apps for the passive collection of behavioral data via smartphones have already been developed [40]. The Mobilyze! app, developed in 2011, includes machine learning models for predicting patients’ moods [41]. Marzano et al. [42] developed a prototype system app that was tested in a small trial and was shown to accumulate both quantitative and qualitative data. Beiwe is a research platform that can passively collect various behavioral data types from users’ smartphones and transfer this data to another server for analysis. This app is currently only available for use by a small group of researchers [43].
A designated algorithm for analyzing the collected data is necessary for further development. Lydon-Staley et al. [44] created a computational algorithm that applied network science methodologies to a longitudinal behavioral database to learn about interactions between psychiatric symptoms. As a result, Lydon-Staley et al. [44] offered a robust framework to capture dynamic symptom networks.
The link between emotions and emotion regulation in psychopathology has been demonstrated in the past [41]. The transition between different emotional states and moods is substantial in psychopathology research. Therefore, digital biomarkers for emotional changes are essential. Research by Pratap et al. [43] found that mobility and smartphones’ normal usage has the potential to predict an individual’s mood state changes using personalized models. Further important work was published recently by Sultana, Al-Jefri and Lee [45], in which machine learning algorithms were used to analyze varied data recorded from individuals’ smartphones and smartwatches to determine emotional states and transitions.
Combining omics data together with the collected behavioral data could be another essential strategy to achieve better disease diagnoses. As genetic and proteomic biomarkers for MDD are routinely revealed [42,46,47], this option is becoming a reality. Olmert et al. [22] launched the Delta Trial, where blood spot samples and psychiatric digital questionnaire answers were collected from patients. Both proteomic biomarkers and symptomatic behavioral data were collected digitally and used to differentiate between bipolar disorder and MDD patients [22].
3. Toward Personalized Psychiatry
Since MDD is only one example among many poorly defined and diagnosed mental disorders, an innovative and fluidic approach to all mental health is necessary. Naming a set of symptoms reported by a patient does not yield satisfying outcomes. There is a wide spectrum of human behavior that cannot be expressed in one or two pathologies. The standard diagnosis of comorbidities is evidence of this incorrect classification of mental health disorders. Though personalized medicine exists in other fields [48,49,50], psychiatry has been left behind. However, innovative personal psychiatry tools for the treatment of MDD do exist [51,52,53,54]. Predictix is a decision-support tool that calculates the likely effectiveness of several antidepressant medications for individual patients, based on environmental and genetic input [54]. In an article that was accepted for publication recently, Taliaz et al. [55] successfully used data from the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study to analyze the response patterns of patients to antidepressant medications using machine learning algorithms. Expanding the applied database of such a tool with data of different mental pathologies could allow the optimum treatment to be predicted according to an individual’s specific parameters instead of prescribing medications per diagnosed condition. Furthermore, diagnoses will be shifted depending on the training and the background of the doctor, as was recently argued by Perugi and Barbuti [19].
Analysis of longitudinal behavior allows for a better comparison between a patient’s behavior in the present and their behavior in the past, as well as in a demographic context. Sleep disorders demonstrate the importance of this feature. Insomnia is one of the most common symptoms of MDD [56]. However, many studies have shown that sleep quantity and quality differ among people of different ages [57,58,59,60]. Therefore, an integration of this demographic information is crucial for an accurate diagnosis. A pilot clinical study to ascertain the feasibility of this approach is recommended.
In the future, we also believe that behavioral data could provide a reference for identifying biomarkers from other fields. A thorough understanding of the behavioral characteristics of mental disorders has the potential to find correlations between behavioral features and features from other areas (Figure 1c) and promote crosstalk between the different areas, which would result in further expanding our knowledge in those areas (Figure 1c).
As we have shown in this article, technologically, we already have the knowledge to collect the necessary behavioral data in order to learn precisely about individuals’ behavior. Therefore, personalized psychiatry should be pursued and become a standard soon.
4. Conclusions
Mental disorders are ambiguously defined. The currently available methods used to diagnose mental disorders are limited, as they rely on patients’ subjective self-reports. In this article, we supported these statements with examples from MDD. We suggest the use of smartphones and wearable devices to collect longitudinal data of patients’ behavior passively. Several designated apps to collect this type of data have already been developed, and digital biomarkers of MDD symptoms have already been identified. Algorithms and computational methods for analyzing continuous behavioral data have also been published. We believe all this gathered available knowledge should be integrated into one tool that can assist in diagnosing mental health patients and help bring a much-needed paradigm shift in psychiatric treatment approaches. With a better understanding of behavioral data, an integrated tool may have the potential to identify new characteristics of disorder subtypes and promote crosstalk, expanding our knowledge between and within other fields. Furthermore, quantified longitudinal behavioral data can be used to determine a patient’s diagnosis and to personalize treatment over time. This may help minimize the incorrect classification of mental health disorders and the associated diagnoses of comorbidities. Of course, rules and restrictions to preserve patients’ privacy must be established. Slavich et al. [61] suggested some guidelines for the field of speech analysis that can be generalized to other data types as well. These guidelines include informing patients of precisely what data are collected using their wearable device and enabling patients to easily stop the recording action of their device [61].
We support the RDoC framework to investigate psychiatric pathologies based on integrating biological and behavioral data from various disciplines. However, as there is a wide spectrum of human behavior that cannot be expressed in one or two pathologies, today’s medical diagnoses (that rely mainly on observable symptoms) are limited. For this reason, we believe that mental syndromes should be characterized primarily using behavioral characteristics, and that behavioral data should be the field’s pillar.
Author Contributions
Study initiation, D.T.; conceptualization, D.T.; methodology, D.T. and D.S.; formal analysis, D.T. and D.S.; investigation, D.T. and D.S.; resources, D.T. and D.S.; data curation, D.T. and D.S.; writing—original draft preparation, D.T.; writing—review and editing, D.T.; visualization, D.T. and D.S.; supervision, D.T.; project administration, D.T.; funding acquisition, D.T. Both authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Union, grant number 874082, project name Predictix Horizon 2020. The APC was funded by Daniel Souery.
Acknowledgments
The authors would like to thank Amit Spinrad, Roy Schurr, Sne Morag and Roni Zoller for the research and scientific discussions, and to Lihi Levin for the research and writing.
Conflicts of Interest
Dekel Taliaz is the founder and CEO of Taliaz, and reports stock ownership in Taliaz. Daniel Souery is on the scientific advisory board of Taliaz and has received consulting fees from Taliaz.
References
- Newson, J.J.; Hunter, D.; Thiagarajan, T.C. The Heterogeneity of Mental Health Assessment. Front. Psychiatry 2020, 11, 76. [Google Scholar] [CrossRef] [Green Version]
- Olbert, C.M.; Gala, G.J.; Tupler, L.A. Quantifying Heterogeneity Attributable to Polythetic Diagnostic Criteria: Theoretical Framework and Empirical Application. J. Abnorm. Psychol. 2014, 123, 452. [Google Scholar] [CrossRef]
- Polanczyk, G.V.; Salum, G.A.; Sugaya, L.S.; Caye, A.; Rohde, L.A. Annual Research Review: A Meta-Analysis of the Worldwide Prevalence of Mental Disorders in Children and Adolescents. J. Child Psychol. Psychiatry 2015, 56, 345–365. [Google Scholar] [CrossRef]
- Bromet, E.; Andrade, L.H.; Hwang, I.; Sampson, N.A.; Alonso, J.; De Girolamo, G.; De Graaf, R.; Demyttenaere, K.; Hu, C.; Iwata, N. Cross-National Epidemiology of DSM-IV Major Depressive Episode. BMC Med. 2011, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Suicide. Available online: https://www.who.int/health-topics/suicide (accessed on 15 March 2021).
- Whooley, M.A.; Wong, J.M. Depression and Cardiovascular Disorders. Annu. Rev. Clin. Psychol. 2013, 9, 327–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fried, E.I. Moving Forward: How Depression Heterogeneity Hinders Progress in Treatment and Research. Expert Rev. Neurother. 2017, 17, 423–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santor, D.A.; Gregus, M.; Welch, A. FOCUS ARTICLE: Eight Decades of Measurement in Depression. Meas. Interdiscip. Res. Perspect. 2006, 4, 135–155. [Google Scholar] [CrossRef]
- Zimmerman, M.; Ellison, W.; Young, D.; Chelminski, I.; Dalrymple, K. How Many Different Ways Do Patients Meet the Diagnostic Criteria for Major Depressive Disorder? Compr. Psychiatry 2015, 56, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Association, A.P. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Pub: Arlington, WV, USA, 2013; ISBN 978-0-89042-557-2. [Google Scholar]
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; Niederehe, G.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D. Acute and Longer-Term Outcomes in Depressed Outpatients Requiring One or Several Treatment Steps: A STAR* D Report. Am. J. Psychiatry 2006, 163, 1905–1917. [Google Scholar] [CrossRef]
- Thase, M.E.; Friedman, E.S.; Biggs, M.M.; Wisniewski, S.R.; Trivedi, M.H.; Luther, J.F.; Fava, M.; Nierenberg, A.A.; McGrath, P.J.; Warden, D. Cognitive Therapy versus Medication in Augmentation and Switch Strategies as Second-Step Treatments: A STAR* D Report. Am. J. Psychiatry 2007, 164, 739–752. [Google Scholar] [CrossRef]
- Kendler, K.S.; First, M.B. Alternative Futures for the DSM Revision Process: Iteration v. Paradigm Shift. Br. J. Psychiatry 2010, 197, 263–265. [Google Scholar] [CrossRef] [Green Version]
- Kupfer, D.J.; Regier, D.A. Neuroscience, Clinical Evidence, and the Future of Psychiatric Classification in DSM-5. Am. J. Psychiatry 2011, 168, 672–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regier, D.A.; Narrow, W.E.; Kuhl, E.A.; Kupfer, D.J. The Conceptual Development of DSM-V. Am. J. Psychiatry 2009, 166, 645–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NIMH. About RDoC. Available online: https://www.nimh.nih.gov/research/research-funded-by-nimh/rdoc/about-rdoc.shtml (accessed on 15 March 2021).
- Insel, T.R. The NIMH Research Domain Criteria (RDoC) Project: Precision Medicine for Psychiatry. Am. J. Psychiatry 2014, 171, 395–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuthbert, B.N.; Insel, T.R. Toward the Future of Psychiatric Diagnosis: The Seven Pillars of RDoC. BMC Med. 2013, 11, 126. [Google Scholar] [CrossRef] [Green Version]
- Perugi, G.; Barbuti, M. There Are No Patients without Comorbidity. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2021, 50, 104–106. [Google Scholar] [CrossRef]
- Haggerty, R.J.; Mrazek, P.J. Reducing Risks for Mental Disorders: Frontiers for Preventive Intervention Research; National Academies Press: Washington, DC, USA, 1994. [Google Scholar]
- Andreasen, N.C. Linking Mind and Brain in the Study of Mental Illnesses: A Project for a Scientific Psychopathology. Science 1997, 275, 1586–1593. [Google Scholar] [CrossRef] [Green Version]
- Olmert, T.; Cooper, J.D.; Han, S.Y.S.; Barton-Owen, G.; Farrag, L.; Bell, E.; Friend, L.V.; Ozcan, S.; Rustogi, N.; Preece, R.L. A Combined Digital and Biomarker Diagnostic Aid for Mood Disorders (the Delta Trial): Protocol for an Observational Study. JMIR Res. Protoc. 2020, 9, e18453. [Google Scholar] [CrossRef]
- Cormack, F.; McCue, M.; Taptiklis, N.; Skirrow, C.; Glazer, E.; Panagopoulos, E.; van Schaik, T.A.; Fehnert, B.; King, J.; Barnett, J.H. Wearable Technology for High-Frequency Cognitive and Mood Assessment in Major Depressive Disorder: Longitudinal Observational Study. JMIR Ment. Health 2019, 6, e12814. [Google Scholar] [CrossRef] [Green Version]
- Suhara, Y.; Xu, Y.; Pentland, A. Deepmood: Forecasting Depressed Mood Based on Self-Reported Histories via Recurrent Neural Networks. In Proceedings of the 26th International Conference on World Wide Web, Perth, Australia, 3 July 2017; pp. 715–724. [Google Scholar]
- Torous, J.; Staples, P.; Shanahan, M.; Lin, C.; Peck, P.; Keshavan, M.; Onnela, J.-P. Utilizing a Personal Smartphone Custom App to Assess the Patient Health Questionnaire-9 (PHQ-9) Depressive Symptoms in Patients with Major Depressive Disorder. JMIR Ment. Health 2015, 2, e3889. [Google Scholar] [CrossRef] [Green Version]
- Onnela, J.-P.; Rauch, S.L. Harnessing Smartphone-Based Digital Phenotyping to Enhance Behavioral and Mental Health. Neuropsychopharmacology 2016, 41, 1691–1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vettoretti, M.; Cappon, G.; Acciaroli, G.; Facchinetti, A.; Sparacino, G. Continuous Glucose Monitoring: Current Use in Diabetes Management and Possible Future Applications. J. Diabetes Sci. Technol. 2018, 12, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Turakhia, M.P.; Desai, M.; Hedlin, H.; Rajmane, A.; Talati, N.; Ferris, T.; Desai, S.; Nag, D.; Patel, M.; Kowey, P.; et al. Rationale and Design of a Large-Scale, App-Based Study to Identify Cardiac Arrhythmias Using a Smartwatch: The Apple Heart Study. Am. Heart J. 2019, 207, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Torous, J.; Onnela, J.-P.; Keshavan, M. New Dimensions and New Tools to Realize the Potential of RDoC: Digital Phenotyping via Smartphones and Connected Devices. Transl. Psychiatry 2017, 7, e1053. [Google Scholar] [CrossRef]
- Brietzke, E.; Hawken, E.R.; Idzikowski, M.; Pong, J.; Kennedy, S.H.; Soares, C.N. Integrating Digital Phenotyping in Clinical Characterization of Individuals with Mood Disorders. Neurosci. Biobehav. Rev. 2019, 104, 223–230. [Google Scholar] [CrossRef]
- Insel, T.R. Digital Phenotyping: A Global Tool for Psychiatry. World Psychiatry 2018, 17, 276. [Google Scholar] [CrossRef] [Green Version]
- Mundt, J.C.; Vogel, A.P.; Feltner, D.E.; Lenderking, W.R. Vocal Acoustic Biomarkers of Depression Severity and Treatment Response. Biol. Psychiatry 2012, 72, 580–587. [Google Scholar] [CrossRef] [Green Version]
- Mundt, J.C.; Snyder, P.J.; Cannizzaro, M.S.; Chappie, K.; Geralts, D.S. Voice Acoustic Measures of Depression Severity and Treatment Response Collected via Interactive Voice Response (IVR) Technology. J. Neurolinguistics 2007, 20, 50–64. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Duvvuri, R.; Chandra, K.K.L.; Nguyen, T.; Ghomi, R.H. Automated Voice Biomarkers for Depression Symptoms Using an Online Cross-Sectional Data Collection Initiative. Depress. Anxiety 2020, 37, 657–669. [Google Scholar] [CrossRef]
- Jacobson, N.C.; Weingarden, H.; Wilhelm, S. Digital Biomarkers of Mood Disorders and Symptom Change. NPJ Digit. Med. 2019, 2, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, N.C.; Weingarden, H.; Wilhelm, S. Using Digital Phenotyping to Accurately Detect Depression Severity. J. Nerv. Ment. Dis. 2019, 207, 893–896. [Google Scholar] [CrossRef]
- Tonon, A.C.; Fuchs, D.F.P.; Gomes, W.B.; Levandovski, R.; de Almeida Fleck, M.P.; Hidalgo, M.P.L.; da Silva Alencastro, L. Nocturnal Motor Activity and Light Exposure: Objective Actigraphy-Based Marks of Melancholic and Non-Melancholic Depressive Disorder. Brief Report. Psychiatry Res. 2017, 258, 587–590. [Google Scholar] [CrossRef]
- Saeb, S.; Zhang, M.; Karr, C.J.; Schueller, S.M.; Corden, M.E.; Kording, K.P.; Mohr, D.C. Mobile Phone Sensor Correlates of Depressive Symptom Severity in Daily-Life Behavior: An Exploratory Study. J. Med. Internet Res. 2015, 17, e175. [Google Scholar] [CrossRef]
- Dagum, P. Digital Biomarkers of Cognitive Function. NPJ Digit. Med. 2018, 1, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Mandryk, R.L.; Birk, M.V. The Potential of Game-Based Digital Biomarkers for Modeling Mental Health. JMIR Ment. Health 2019, 6, e13485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, J.J.; Jazaieri, H. Emotion, Emotion Regulation, and Psychopathology: An Affective Science Perspective. Clin. Psychol. Sci. 2014, 2, 387–401. [Google Scholar] [CrossRef]
- Marzano, L.; Bardill, A.; Fields, B.; Herd, K.; Veale, D.; Grey, N.; Moran, P. The Application of MHealth to Mental Health: Opportunities and Challenges. Lancet Psychiatry 2015, 2, 942–948. [Google Scholar] [CrossRef] [Green Version]
- Pratap, A.; Atkins, D.C.; Renn, B.N.; Tanana, M.J.; Mooney, S.D.; Anguera, J.A.; Areán, P.A. The Accuracy of Passive Phone Sensors in Predicting Daily Mood. Depress. Anxiety 2019, 36, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Lydon-Staley, D.M.; Barnett, I.; Satterthwaite, T.D.; Bassett, D.S. Digital Phenotyping for Psychiatry: Accommodating Data and Theory with Network Science Methodologies. Curr. Opin. Biomed. Eng. 2019, 9, 8–13. [Google Scholar] [CrossRef]
- Sultana, M.; Al-Jefri, M.; Lee, J. Using Machine Learning and Smartphone and Smartwatch Data to Detect Emotional States and Transitions: Exploratory Study. JMIR MHealth UHealth 2020, 8, e17818. [Google Scholar] [CrossRef]
- Burns, M.N.; Begale, M.; Duffecy, J.; Gergle, D.; Karr, C.J.; Giangrande, E.; Mohr, D.C. Harnessing Context Sensing to Develop a Mobile Intervention for Depression. J. Med. Internet Res. 2011, 13, e55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torous, J.; Kiang, M.V.; Lorme, J.; Onnela, J.-P. New Tools for New Research in Psychiatry: A Scalable and Customizable Platform to Empower Data Driven Smartphone Research. JMIR Ment. Health 2016, 3, e5165. [Google Scholar] [CrossRef]
- Ellsworth, R.E.; Decewicz, D.J.; Shriver, C.D.; Ellsworth, D.L. Breast Cancer in the Personal Genomics Era. Curr. Genomics 2010, 11, 146–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priyadharshini, V.S.; Teran, L.M. Personalized Medicine in Respiratory Disease: Role of Proteomics. Adv. Protein Chem. Struct. Biol. 2016, 102, 115–146. [Google Scholar]
- Xu, J.; Yang, P.; Xue, S.; Sharma, B.; Sanchez-Martin, M.; Wang, F.; Beaty, K.A.; Dehan, E.; Parikh, B. Translating Cancer Genomics into Precision Medicine with Artificial Intelligence: Applications, Challenges and Future Perspectives. Hum. Genet. 2019, 138, 109–124. [Google Scholar] [CrossRef] [Green Version]
- Winner, J.G.; Carhart, J.M. A Prospective, Randomized, Double-Blind Study Assessing the Clinical Impact of Integrated Pharmacogenomic Testing for Major Depressive Disorder. Discov. Med. 2013, 16, 219–227. [Google Scholar]
- Kim, K.; Magness, J.W.; Nelson, R.; Baron, V.; Brixner, D.I. Clinical Utility of Pharmacogenetic Testing and a Clinical Decision Support Tool to Enhance the Identification of Drug Therapy Problems Through Medication Therapy Management in Polypharmacy Patients. J. Manag. Care Spec. Pharm. 2018, 24, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Pérez, V.; Salavert, A.; Espadaler, J.; Tuson, M.; Saiz-Ruiz, J.; Sáez-Navarro, C.; Bobes, J.; Baca-García, E.; Vieta, E.; Olivares, J.M. Efficacy of Prospective Pharmacogenetic Testing in the Treatment of Major Depressive Disorder: Results of a Randomized, Double-Blind Clinical Trial. BMC Psychiatry 2017, 17, 1–13. [Google Scholar] [CrossRef] [Green Version]
- PREDICTIX Genetics. Available online: https://www.predictix.ai/predictix-genetics (accessed on 4 July 2021).
- Taliaz, D.; Spinrad, A.; Barzilay, R.; Barnett-Itzhaki, Z.; Averbuch, D.; Teltsh, O.; Schurr, R.; Darki-Morag, S.; Lerer, B. Optimizing Prediction of Response to Antidepressant Medications Using Machine Learning and Integrated Genetic, Clinical, and Demographic Data. Transl. Psychiatry 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Mendelson, W. Human Sleep and Its Disorders; Springer Science & Business Media: Boston, MA, USA, 2012. [Google Scholar]
- Conte, F.; Arzilli, C.; Errico, B.M.; Giganti, F.; Iovino, D.; Ficca, G. Sleep Measures Expressing ‘Functional Uncertainty’in Elderlies’ Sleep. Gerontology 2014, 60, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, I.; Carlson, V.R. Sleep Variables as a Function of Age in Man. Arch. Gen. Psychiatry 1968, 18, 239–250. [Google Scholar] [CrossRef]
- Klerman, E.B.; Dijk, D.-J. Age-Related Reduction in the Maximal Capacity for Sleep—Implications for Insomnia. Curr. Biol. 2008, 18, 1118–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webb, W.B.; Campbell, S.S. The First Night Effect Revisited with Age as a Variable. Waking Sleep. 1979, 3, 319–324. [Google Scholar]
- Slavich, G.M.; Taylor, S.; Picard, R.W. Stress Measurement Using Speech: Recent Advancements, Validation Issues, and Ethical and Privacy Considerations. Stress 2019, 22, 408–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).