Primary Hyperparathyroidism in Pregnancy: Literature Review of the Diagnosis and Management
Abstract
1. Introduction
2. Materials and Methods
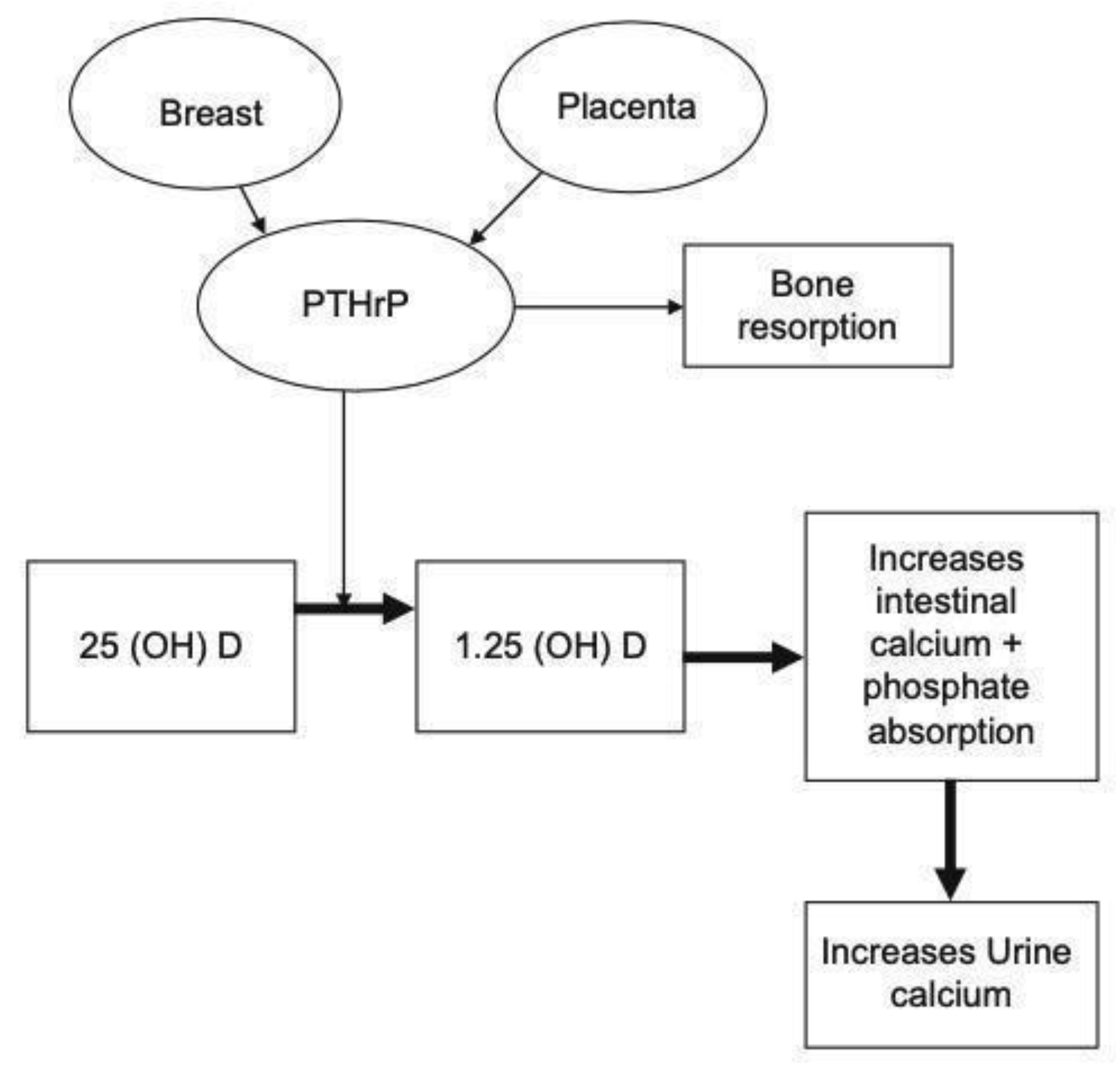
3. Impact of PHPT on Mother and Fetus during Pregnancy
4. Diagnosis of PHPT in Pregnancy
4.1. History
4.2. Physical Examination
4.3. Laboratory Investigations
- Calcium adjusted for Albumin Formula.
Corrected calcium (mg/dL) = measured total Ca(mg/dL) + 0.8 (4.0 − serum albumin (g/dL))
- 2.
- Calculations of Calcium to Creatinine Clearance Ratio (CCCR).
4.4. Localization
5. Clinical Management of PHPT in Pregnancy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, A.A.; Hanley, D.A.; Rizzoli, R.; Bollerslev, J.; Young, J.; Rejnmark, L.; Thakker, R.; D’Amour, P.; Paul, T.; Van Uum, S.; et al. Primary hyperparathyroidism: Review and recommendations on evaluation, diagnosis, and management. A Canadian and international consensus. Osteoporos. Int. 2017, 28, 1–19. [Google Scholar] [CrossRef]
- Hirsch, D.; Kopel, V.; Nadler, V.; Levy, S.; Toledano, Y.; Tsvetov, G. Pregnancy Outcomes in Women With Primary Hyperparathyroidism. J. Clin. Endocrinol. Metab. 2015, 100, 2115–2122. [Google Scholar] [CrossRef]
- Kelly, T.R. Primary hyperparathyroidism during pregnancy. Surgery 1991, 110, 1028–1034. [Google Scholar]
- Kort, K.C.; Schiller, H.J.; Numann, P.J. Hyperparathyroidism and pregnancy. Am. J. Surg. 1999, 177, 66–68. [Google Scholar] [CrossRef]
- Nadarasa, K.; Bailey, M.; Chahal, H.; Raja, O.; Bhat, R.; Gayle, C.; Grossman, A.B.; Druce, M.R. The use of cinacalcet in pregnancy to treat a complex case of parathyroid carcinoma. Endocrinol. Diabetes Metab. Case Rep. 2014, 2014, 140056. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.G.; Elston, M.S.; Gill, A.J.; Marsh, D.; Beer, I.; Wolmarans, L.; Conaglen, J.V.; Meyer-Rochow, G.Y. Hypercalcaemia due to parathyroid carcinoma presenting in the third trimester of pregnancy. Aust. N. Z. J. Obstet. Gynaecol. 2011, 52, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Montoro, M.N.; Paler, R.J.; Goodwin, T.M.; Mestman, J.H. Parathyroid carcinoma during pregnancy. Obstet. Gynecol. 2000, 96, 5. [Google Scholar]
- Parham, G.P.; Orr, J.W. Hyperparathyroidism secondary to parathyroid carcinoma in pregnancy. A case report. J. Reprod. Med. 1987, 32, 123–125. [Google Scholar] [PubMed]
- Hess, H.M.; Dickson, J.; Fox, H.E. Hyperfunctioning parathyroid carcinoma presenting as acute pancreatitis in pregnancy. J. Reprod. Med. 1980, 25, 83–87. [Google Scholar]
- Panchani, R.; Varma, T.; Goyal, A.; Tripathi, S. Parathyroid carcinoma masquerading as morning sickness in pregnancy. Indian J. Endocrinol. Metab. 2013, 17, 198–200. [Google Scholar] [CrossRef]
- Baretic, M.; Brzac, H.T.; Dobrenić, M.; Jakovčević, A. Parathyroid carcinoma in pregnancy. World J. Clin. Cases 2014, 2, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Palmieri-Sevier, A.; Palmieri, G.M.A.; Baumgartner, C.J.; Britt, L.G. Case Report: Long-Term Remission of Parathyroid Cancer: Possible Relation to Vitamin D and Calcitriol Therapy. Am. J. Med. Sci. 1993, 306, 309–312. [Google Scholar] [CrossRef]
- Marini, F. Molecular genetics in primary hyperparathyroidism: The role of genetic tests in differential diagnosis, disease prevention strategy, and therapeutic planning. A 2017 update. Clin. Cases Miner. Bone Metab. 2017, 14, 60–70. [Google Scholar] [CrossRef]
- Davis, C.; Nippita, T. Hyperparathyroidism in pregnancy. BMJ Case Rep. 2020, 13, e232653. [Google Scholar] [CrossRef] [PubMed]
- Dale, A.G.; Holbrook, B.D.; Sobel, L.; Rappaport, V.J. Hyperparathyroidism in Pregnancy Leading to Pancreatitis and Preeclampsia with Severe Features. Case Rep. Obstet. Gynecol. 2017, 2017, 1–3. [Google Scholar] [CrossRef]
- Parks, J.; Coe, F.; Favus, M. Hyperparathyroidism in Nephrolithiasis. Arch. Intern. Med. 1980, 140, 1479–1481. [Google Scholar] [CrossRef] [PubMed]
- DiMarco, A.N.; Meeran, K.; Christakis, I.; Sodhi, V.; Nelson-Piercy, C.; Tolley, N.S.; Palazzo, F.F. Seventeen Cases of Primary Hyperparathyroidism in Pregnancy: A Call for Management Guidelines. J. Endocr. Soc. 2019, 3, 1009–1021. [Google Scholar] [CrossRef]
- Norman, J.; Politz, D.; Politz, L. Hyperparathyroidism during pregnancy and the effect of rising calcium on pregnancy loss: A call for earlier intervention. Clin. Endocrinol. 2009, 71, 104–109. [Google Scholar] [CrossRef]
- Delmonico, F.L.; Neer, R.M.; Cosimi, A.; Barnes, A.B.; Russell, P.S. Hyperparathyroidism during pregnancy. Am. J. Surg. 1976, 131, 328–337. [Google Scholar] [CrossRef]
- Rigg, J.; Gilbertson, E.; Barrett, H.L.; Britten, F.L.; Lust, K. Primary Hyperparathyroidism in Pregnancy: Maternofetal Outcomes at a Quaternary Referral Obstetric Hospital, 2000 Through 2015. J. Clin. Endocrinol. Metab. 2019, 104, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, I.-L.; Adner, N.; Reihnér, E.; Palme-Kilander, C.; Edstrom, G.; Degerblad, M. Primary Hyperparathyroidism in Pregnancy: A Diagnostic and Therapeutic Challenge. J. Womens Health 2010, 19, 1117–1121. [Google Scholar] [CrossRef]
- Matthias, G.S.H.; Helliwell, T.R.; Williams, A. Postpartum hyperparathyroid crisis. Case report. BJOG Int. J. Obstet. Gynaecol. 1987, 94, 807–810. [Google Scholar] [CrossRef]
- Ali, D.; Dandurand, K.; Khan, A. Hypoparathyroidism in Pregnancy and Lactation: Current Approach to Diagnosis and Management. J. Clin. Med. 2021, 10, 1378. [Google Scholar] [CrossRef] [PubMed]
- Edling, K.L.; Korenman, S.G.; Janzen, C.; Sohsman, M.Y.; Apple, S.K.; Bhuta, S.; Yeh, M.W. A Pregnant Dilemma: Primary Hyperparathyroidism Due to Parathyromatosis in Pregnancy. Endocr. Pract. 2014, 20, e14–e17. [Google Scholar] [CrossRef]
- Lee, C.-C.; Chao, A.-S.; Chang, Y.-L.; Peng, H.-H.; Wang, T.-H.; Chao, A. Acute pancreatitis secondary to primary hyperparathyroidism in a postpartum patient: A case report and literature review. Taiwan. J. Obstet. Gynecol. 2014, 53, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, R.; Wilk, M.; Okopien, B. Recurrent pancreatitis induced by hyperparathyroidism in pregnancy. Arch. Gynecol. Obstet. 2011, 284, 531–534. [Google Scholar] [CrossRef]
- Diaz-Soto, G.; Linglart, A.; Sénat, M.-V.; Kamenický, P.; Chanson, P. Primary hyperparathyroidism in pregnancy. Endocrine 2013, 44, 591–597. [Google Scholar] [CrossRef]
- Kondo, Y.; Nagai, H.; Kasahara, K.; Kanazawa, K. Primary hyperparathyroidism and acute pancreatitis during pregnancy. Report of a case and a review of the English and Japanese literature. Int. J. Pancreatol. 1998, 24, 43–47. [Google Scholar] [CrossRef]
- Warneke, G.; Henning, H.V.; Isemer, F.E.; Mueller, H.J.; Scheler, F. Primary hyperparathyroidism with acute pancreatitis during pregnancy. Dtsch. Med. Wochenschr. 1988, 113, 641–643. [Google Scholar] [CrossRef]
- Hong, M.K.; Hsieh, C.C.; Chen, B.H.; Tu, S.T.; Chou, P.H. Primary hyperparathyroidism and acute pancreatitis during the third trimester of pregnancy. J. Matern. Fetal Med. 2001, 10, 214–218. [Google Scholar] [CrossRef]
- Schnatz, F.P.; Thaxton, S. Parathyroidectomy in the Third Trimester of Pregnancy. Obstet. Gynecol. Surv. 2005, 60, 10. [Google Scholar] [CrossRef]
- García, M.A.; Feria, M.A.; Moreno, A.S.; Fuentes, E.D.; González, E.N.; Thong, D.Q.; Del Valle, A.; Delgado, D.A.; Jiménez, R.A. Primary hyperparathyroidism in pregnancy. Gynecol. Endocrinol. 2004, 19, 111–114. [Google Scholar] [CrossRef]
- Gokkaya, N.; Gungor, A.; Bilen, A.; Bilen, H.; Gviniashvili, D.; Karadeniz, Y. Primary hyperparathyroidism in pregnancy: A case series and literature review. Gynecol. Endocrinol. 2016, 32, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Haenel, C.L.; Mayfield, R.K. Primary Hyperparathyroidism in a Twin Pregnancy and Review of Fetal/Maternal Calcium Homeostasis. Am. J. Med. Sci. 2000, 319, 191–194. [Google Scholar] [CrossRef]
- Alharbi, B.A.; Alqahtani, M.A.; Hmoud, M.; Alhejaili, E.A.; Badros, R. Preeclampsia: A Possible Complication of Primary Hyperparathyroidism. Case Rep. Obstet. Gynecol. 2016, 2016, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, I.-L.; Rastad, J.; Johansson, K.; Lind, L. Endothelial vasodilatory function and blood pressure response to local and systemic hypercalcemia. Surgery 2001, 130, 986–990. [Google Scholar] [CrossRef]
- Vlachakis, N.D.; Frederics, R.; Valasquez, M.; Alexander, N.; Singer, F.; Maronde, R.F. Sympathetic system function and vascular reactivity in hypercalcemic patients. Hypertension 1982, 4, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.-K.; Lin, Y.-C.; Wei, Y.-C.; Chu, T.-Y. Parathyroid Adenoma With Hypertensive Crisis and Intracerebral Hemorrhage Mimicking Hemolysis, Elevated Liver Enzymes, Low Platelets Syndrome. Obstet. Gynecol. 2011, 117, 498–500. [Google Scholar] [CrossRef]
- Yılmaz, B.A.; Altay, M.; Değertekin, C.K.; Çimen, A.R.; Iyidir, O.T.; Biri, A.; Yuksel, O.; Toruner, F.B.; Arslan, M. Hyperparathyroid crisis presenting with hyperemesis gravidarum. Arch. Gynecol. Obstet. 2014, 290, 811–814. [Google Scholar] [CrossRef]
- Gennari, C.; Nami, R.; Gonnelli, S. Hypertension and primary hyperparathyroidism: The role of adrenergic and renin-angiotensin-aldosterone systems. Miner. Electrolyte Metab. 1995, 21, 77–81. [Google Scholar]
- Kovacs, L.; Góth, M.I.; Szabolcs, I.; Dohan, O.; Ferencz, A.; Szilagyi, G. The effect of surgical treatment on secondary hyperaldosteronism and relative hyperinsulinemia in primary hyperparathyroidism. Eur. J. Endocrinol. 1998, 138, 543–547. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miller, P.D.; Bilezikian, J.P. Bone densitometry in asymptomatic primary hyperparathyroidism. J. Bone Miner. Res. 2002, 17, 98–102. [Google Scholar]
- Syed, Z.; Khan, A. Skeletal Effects of Primary Hyperparathyroidism. Endocr. Pract. 2000, 6, 385–388. [Google Scholar] [CrossRef]
- Mokrysheva, N.G.; Eremkina, A.K.; Mirnaya, S.; Rozhinskaya, L.Y.; Kuznetsov, N.S.; Yesayan, R.M.; Kan, N.E.; Dudinskaya, E.N. A Case of Pregnancy Complicated by Primary Hyperparathyroidism Due to a Parathyroid Adenoma. Am. J. Case Rep. 2019, 20, 53–59. [Google Scholar] [CrossRef]
- Negishi, H.; Kobayashi, M.; Nishida, R.; Yamada, H.; Ariga, S.; Sasaki, F.; Fujimoto, S. Primary Hyperparathyroidism and Simultaneous Bilateral Fracture of the Femoral Neck during Pregnancy. J. Trauma Inj. Infect. Crit. Care 2002, 52, 367–369. [Google Scholar] [CrossRef]
- García, I.G.; Fradejas, M.R.; Macías, M.D.L.A.M.; Ciganda, A.B.; Beaskoetxea, Z.B.; Pérez, E.R.; Matia, G.F.; Guisasola, J.M. Primary hyperparathyroidism in pregnancy treated with cinacalcet: A case report. J. Obstet. Gynaecol. 2017, 38, 132–134. [Google Scholar] [CrossRef]
- Çakır, U.; Alan, S.; Erdeve, Ö.; Atasay, B.; Şıklar, Z.; Berberoglu, M.; Arslan, S. Late neonatal hypocalcemic tetany as a manifestation of unrecognized maternal primary hyperparathyroidism. Turk. J. Pediatr. 2013, 55, 438–440. [Google Scholar]
- Razavi, C.R.; Charitou, M.; Marzouk, M. Maternal Atypical Parathyroid Adenoma as a Cause of Newborn Hypocalcemic Tetany. Otolaryngol. Neck Surg. 2014, 151, 1084–1085. [Google Scholar] [CrossRef]
- Dinçer, S.I.; Demir, A.; Kara, H.V.; Günlüoglu, M.Z. Thoracoscopic removal of a maternal mediastinal ectopic parathyroid adenoma causing neonatal hypocalcemia: A case report. Ann. Thorac. Cardiovasc. Surg. 2008, 14, 325–328. [Google Scholar]
- Shani, H.; Sivan, E.; Cassif, E.; Simchen, M. Maternal hypercalcemia as a possible cause of unexplained fetal polyhydramnion: A case series. Am. J. Obstet. Gynecol. 2008, 199, 410.e1–410.e5. [Google Scholar] [CrossRef]
- Jibhkate, S.; Valand, A.; Ansari, S.; Bharambe, B. Hyperparathyroidism complicating pregnancy: A diagnostic challenge? J. Postgrad. Med. 2014, 60, 329. [Google Scholar] [CrossRef]
- McDonnell, C.M.; Zacharin, M.R. Maternal primary hyperparathyroidism: Discordant outcomes in a twin pregnancy. J. Paediatr. Child Health 2006, 42, 70–71. [Google Scholar] [CrossRef]
- Schnatz, P.F.; Curry, S.L. Primary Hyperparathyroidism in Pregnancy:Evidence-Based Management. Obstet. Gynecol. Surv. 2002, 57, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Abood, A.; Vestergaard, P. Pregnancy outcomes in women with primary hyperparathyroidism. Eur. J. Endocrinol. 2014, 171, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Ardawi, M.S.; Nasrat, H.A.; Ba’Aqueel, H.S. Calcium-regulating hormones and parathyroid hormone-related peptide in normal human pregnancy and postpartum: A longitudinal study. Eur. J. Endocrinol. 1997, 137, 402–409. [Google Scholar] [CrossRef]
- Bertelloni, S.; Baroncelli, G.I.; Pelletti, A.; Battini, R.; Saggese, G. Parathyroid hormone-related protein in healthy pregnant women. Calcif. Tissue Int. 1994, 54, 195–197. [Google Scholar] [CrossRef]
- Gallacher, S.J.; Fraser, W.D.; Owens, O.J.; Dryburgh, F.J.; Logue, F.C.; Jenkins, A.; Kennedy, J.; Boyle, I.T. Changes in calciotrophic hormones and biochemical markers of bone turnover in normal human pregnancy. Eur. J. Endocrinol. 1994, 131, 369–374. [Google Scholar] [CrossRef]
- Yadav, S.; Yadav, Y.S.; Goel, M.M.; Singh, U.; Natu, S.M.; Negi, M.P.S. Calcitonin gene- and parathyroid hormone-related peptides in normotensive and preeclamptic pregnancies: A nested case–control study. Arch. Gynecol. Obstet. 2014, 290, 897–903. [Google Scholar] [CrossRef]
- Khan, A.A.; Clarke, B.; Rejnmark, L.; Brandi, M.L. Management of Endocrine Disease: Hypoparathyroidism in pregnancy: Review and evidence-based recommendations for management. Eur. J. Endocrinol. 2019, 180, R37–R44. [Google Scholar] [CrossRef]
- Khan, A.A.; Kenshole, A.; Ezzat, S.; Goguen, J.; Gomez-Hernandez, K.; Hegele, R.A.; Houlden, R.; Joy, T.; Keely, E.; Killinger, D.; et al. Tools for Enhancement and Quality Improvement of Peer Assessment and Clinical Care in Endocrinology and Metabolism. J. Clin. Densitom. 2019, 22, 125–149. [Google Scholar] [CrossRef]
- Thakker, R.V.; Newey, P.J.; Walls, G.V.; Bilezikian, J.; Dralle, H.; Ebeling, P.R.; Melmed, S.; Sakurai, A.; Tonelli, F.; Brandi, M.L. Clinical Practice Guidelines for Multiple Endocrine Neoplasia Type 1 (MEN1). J. Clin. Endocrinol. Metab. 2012, 97, 2990–3011. [Google Scholar] [CrossRef]
- Carella, J.M.; Gossain, V.V. Hyperparathyroidism and pregnancy: Case report and review. J. Gen. Intern. Med. 1992, 7, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Kohlmeier, L.; Marcus, R. Calcium Disorders of Pregnancy. Endocrinol. Metab. Clin. N. Am. 1995, 24, 15–39. [Google Scholar] [CrossRef]
- Som, M.; Stroup, J.S. Primary hyperparathyroidism and pregnancy. Bayl. Univ. Med. Cent. Proc. 2011, 24, 220–223. [Google Scholar] [CrossRef]
- Dandurand, K.; Ali, D.; Khan, A. Primary Hyperparathyroidism: A Narrative Review of Diagnosis and Medical Management. J. Clin. Med. 2021, 10, 1604. [Google Scholar] [CrossRef] [PubMed]
- Boudou, P.; Ibrahim, F.; Cormier, C.; Sarfati, E.; Souberbielle, J.C. A very high incidence of low 25 hydroxy-vitamin D serum concentration in a French population of patients with primary hyperparathyroidism. J. Endocrinol. Investig. 2006, 29, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.; Binkley, N.C.; Bischoff-Ferrari, H.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Bilezikian, J.P.; Brandi, M.L.; Eastell, R.; Silverberg, S.J.; Udelsman, R.; Marcocci, C.; Potts, J.T. Guidelines for the Management of Asymptomatic Primary Hyperparathyroidism: Summary Statement from the Fourth International Workshop. J. Clin. Endocrinol. Metab. 2014, 99, 3561–3569. [Google Scholar] [CrossRef]
- Ross, A.C. The 2011 report on dietary reference intakes for calcium and vitamin D. Public Health Nutr. 2011, 14, 938–939. [Google Scholar] [CrossRef]
- Kovacs, C.S. Physiology of Calcium, Phosphorus, and Bone Metabolism during Pregnancy, Lactation, and Postweaning. In Maternal-Fetal and Neonatal Endocrinology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 61–73. [Google Scholar]
- Kovacs, C.S. Calcium and Bone Metabolism Disorders during Pregnancy and Lactation. Endocrinol. Metab. Clin. N. Am. 2011, 40, 795–826. [Google Scholar] [CrossRef]
- Gunn, I.R.; Gaffney, D. Clinical and laboratory features of calcium-sensing receptor disorders: A systematic review. Ann. Clin. Biochem. Int. J. Lab. Med. 2004, 41, 441–458. [Google Scholar] [CrossRef]
- Jones, A.R.; Hare, M.J.; Brown, J.; Yang, J.; Meyer, C.; Milat, F.; Allan, C.A. Familial Hypocalciuric Hypercalcemia in Pregnancy: Diagnostic Pitfalls. JBMR Plus 2020, 4, e10362. [Google Scholar] [CrossRef] [PubMed]
- Udelsman, R.; Åkerström, G.; Biagini, C.; Duh, Q.-Y.; Miccoli, P.; Niederle, B.; Tonelli, F. The Surgical Management of Asymptomatic Primary Hyperparathyroidism: Proceedings of the Fourth International Workshop. J. Clin. Endocrinol. Metab. 2014, 99, 3595–3606. [Google Scholar] [CrossRef]
- Kairys, J.C.; Daskalakis, C.; Weigel, R.J. Surgeon-Performed Ultrasound for Preoperative Localization of Abnormal Parathyroid Glands in Patients with Primary Hyperparathyroidism. World J. Surg. 2006, 30, 1658–1663. [Google Scholar] [CrossRef]
- Vitetta, G.M.; Neri, P.; Chiecchio, A.; Carriero, A.; Cirillo, S.; Mussetto, A.B.; Codegone, A. Role of ultrasonography in the management of patients with primary hyperparathyroidism: Retrospective comparison with technetium-99m sestamibi scintigraphy. J. Ultrasound 2014, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kuzminski, S.J.; Sosa, J.A.; Hoang, J.K. Update in Parathyroid Imaging. Magn. Reson. Imaging Clin. N. Am. 2018, 26, 151–166. [Google Scholar] [CrossRef]
- Sepahdari, A.; Bahl, M.; Harari, A.; Kim, H.; Yeh, M.; Hoang, J. Predictors of Multigland Disease in Primary Hyperparathyroidism: A Scoring System with 4D-CT Imaging and Biochemical Markers. Am. J. Neuroradiol. 2015, 36, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Grayev, A.M.; Gentry, L.R.; Hartman, M.J.; Chen, H.; Perlman, S.B.; Reeder, S.B. Presurgical Localization of Parathyroid Adenomas with Magnetic Resonance Imaging at 3.0 T: An Adjunct Method to Supplement Traditional Imaging. Ann. Surg. Oncol. 2011, 19, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Gotway, M.B.; Reddy, G.P.; Webb, W.R.; Morita, E.T.; Clark, O.H.; Higgins, C.B. Comparison between MR Imaging and99mTc MIBI Scintigraphy in the Evaluation of Recurrent or Persistent Hyperparathyroidism. Radiology 2001, 218, 783–790. [Google Scholar] [CrossRef]
- Gehlert, J.; Morton, A. Hypercalcaemia during pregnancy: Review of maternal and fetal complications, investigations, and management. Obstet. Med. 2019, 12, 175–179. [Google Scholar] [CrossRef]
- Jain, C. ACOG Committee Opinion No. 723: Guidelines for Diagnostic Imaging during Pregnancy and Lactation. Obstet. Gynecol. 2019, 133, 186. [Google Scholar] [CrossRef] [PubMed]
- Applegate, K. Pregnancy Screening of Adolescents and Women Before Radiologic Testing: Does Radiology Need a National Guideline? J. Am. Coll. Radiol. 2007, 4, 533–536. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice. Committee Opinion No. 656: Guidelines for Diagnostic Imaging during Pregnancy and Lactation. Obstet. Gynecol. 2016, 127, e75–e80. [Google Scholar] [CrossRef]
- Rey, E.; Jacob, C.E.; Koolian, M.; Morin, F. Hypercalcemia in pregnancy—A multifaceted challenge: Case reports and literature review. Clin. Case Rep. 2016, 4, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.F.; Pacheco, L.D.; Costantine, M.M. Management of Ectopic Parathyroid Adenoma in Pregnancy. Obstet. Gynecol. 2014, 124, 478–480. [Google Scholar] [CrossRef] [PubMed]
- Hoover, C.; Briggs, G.G.; Freeman, R.K.; Yaffe, S.J.; Williams, L. Wilkins Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk, 6th Edition. J. Midwifery Womens Health 2003, 48, 294. [Google Scholar] [CrossRef]
- Horjus, C.; Groot, I.; Telting, D.; Van Setten, P.; Van Sorge, A.; Kovacs, C.S.; Hermus, A.; De Boer, H. Cinacalcet for Hyperparathyroidism in Pregnancy and Puerperium. J. Pediatr. Endocrinol. Metab. 2009, 22, 741–749. [Google Scholar] [CrossRef]
- Harris, R.; Padhi, D. Clinical Pharmacokinetic and Pharmacodynamic Profile of Cinacalcet Hydrochloride. Clin. Pharmacokinet. 2009, 48, 303–311. [Google Scholar] [CrossRef]
- Horton, W.B.; Stumpf, M.M.; Coppock, J.D.; Lancaster, L.; Dalkin, A.C.; Liu, Z.; Chisholm, C.A.; Smith, P.W.; Kirk, S.E. Gestational Primary Hyperparathyroidism Due to Ectopic Parathyroid Adenoma: Case Report and Literature Review. J. Endocr. Soc. 2017, 1, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Vera, L.; Oddo, S.; Di Iorgi, N.; Bentivoglio, G.; Giusti, M. Primary hyperparathyroidism in pregnancy treated with cinacalcet: A case report and review of the literature. J. Med. Case Rep. 2016, 10, 1–5. [Google Scholar] [CrossRef]
- Djokanovic, N.; Klieger-Grossmann, C.; Koren, G. Does Treatment With Bisphosphonates Endanger the Human Pregnancy? J. Obstet. Gynaecol. Can. 2008, 30, 1146–1148. [Google Scholar] [CrossRef]
- Boyce, R.W.; Varela, A.; Chouinard, L.; Bussiere, J.L.; Chellman, G.J.; Ominsky, M.S.; Pyrah, I.T. Infant cynomolgus monkeys exposed to denosumab in utero exhibit an osteoclast-poor osteopetrotic-like skeletal phenotype at birth and in the early postnatal period. Bone 2014, 64, 314–325. [Google Scholar] [CrossRef]
- Okamatsu, N.; Sakai, N.; Karakawa, A.; Kouyama, N.; Sato, Y.; Inagaki, K.; Kiuchi, Y.; Oguchi, K.; Negishi-Koga, T.; Takami, M. Biological effects of anti-RANKL antibody administration in pregnant mice and their newborns. Biochem. Biophys. Res. Commun. 2017, 491, 614–621. [Google Scholar] [CrossRef]
- Levy, H.A.; Pierucci, L.; Stroup, P. Oral phosphates treatment of hypercalcemia in pregnancy. J. Med. Soc. New Jersey 1981, 78, 113–115. [Google Scholar] [PubMed]
- Montoro, M.N.; Collea, J.V.; Mestman, J.H. Management of hyperparathyroidism in pregnancy with oral phosphate therapy. Obstet. Gynecol. 1980, 55, 431–434. [Google Scholar] [PubMed]
- Vernava, A.M.; O’Neal, L.W.; Palermo, V. Lethal hyperparathyroid crisis: Hazards of phosphate administration. Surgery 1987, 102, 941–948. [Google Scholar]
- Pothiwala, P.; Levine, S.N. Parathyroid surgery in pregnancy: Review of the literature and localization by aspiration for parathyroid hormone levels. J. Perinatol. 2009, 29, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Refardt, J.; Farina, P.; Hoesli, I.; Meier, C. Hypercalcemic crisis in third trimenon: Evaluating the optimal treatment strategy. Gynecol. Endocrinol. 2018, 34, 833–836. [Google Scholar] [CrossRef] [PubMed]
- Hui, E.; Osakwe, O.; Teoh, T.G.; Tolley, N.; Robinson, S. Three case reports of maternal primary hyperparathyroidism in each trimester and a review of optimal management in pregnancy. Obstet. Med. 2010, 3, 33–37. [Google Scholar] [CrossRef]
- Higgins, R.V.; Hisley, J.C. Primary hyperparathyroidism in pregnancy. A report of two cases. J. Reprod. Med. 1988, 33, 726–730. [Google Scholar]
- Peschgens, T.; Stollbrink-Peschgens, C.; Merz, U.; Schneider, B.; Maurin, N.; Kutta, T.; Hörnchen, H. [Primary hyperparathyroidism and pregnancy. Aspects of neonatal morbidity]. Z. Fur Geburtshilfe Perinatol. 1994, 198, 96–99. [Google Scholar]
- Schild, R.; Schroers, B.; Schneider, B.; Maurin, N. [Primary hyperparathyroidism in the third trimester of pregnancy]. Z. Geburtshilfe Perinatol. 1993, 197, 188–190. [Google Scholar]
- Stringer, K.M.; Gough, J.; Gough, I.R. Primary hyperparathyroidism during pregnancy: Management by minimally invasive surgery based on ultrasound localization. ANZ J. Surg. 2015, 87, E134–E137. [Google Scholar] [CrossRef] [PubMed]
- Kokrdova, Z. Pregnancy and primary hyperparathyroidism. J. Obstet. Gynaecol. 2010, 30, 57–59. [Google Scholar] [CrossRef]
- Truong, M.T.; Lalakea, M.L.; Robbins, P.; Friduss, M. Primary Hyperparathyroidism in Pregnancy: A Case Series and Review. Laryngoscope 2008, 118, 1966–1969. [Google Scholar] [CrossRef]
- Jesudason, W.; Murphy, J.; England, R. Primary hyperparathyroidism in pregnancy. J. Laryngol. Otol. 2004, 118, 891–892. [Google Scholar] [CrossRef]
- Gidiri, M.; Lindow, S.; Masso, E.; Philips, K. Parathyroidectomy in pregnancy for primary hyperparathyroidism with successful pregnancy outcome: A report of two pregnancies. J. Obstet. Gynaecol. 2004, 24, 318–319. [Google Scholar] [CrossRef] [PubMed]
- Ali, D.; Divilly, P.; Prichard, R.; O’Toole, D.; O’Shea, D.; Crowley, R.K. Primary hyperparathyroidism and Zollinger Ellison syndrome during pregnancy: A case report. Endocrinol. Diabetes Metab. Case Rep. 2021, 2021, 200130. [Google Scholar]
- Han, E.S.; Fritton, K.; Bacon, P.; Slodzinski, M.K.; Argani, C. Preterm Parturient with Polyhydramnios and Pancreatitis: Primary Presentation of Hyperparathyroidism. Case Rep. Obstet. Gynecol. 2018, 2018, 1–4. [Google Scholar] [CrossRef]
- Baumann, K.; Weichert, J.; Krokowski, M.; Diedrich, K.; Banz-Jansen, C. Coexistent parathyroid adenoma and thyroid papillary carcinoma in pregnancy. Arch. Gynecol. Obstet. 2011, 284, 91–94. [Google Scholar] [CrossRef]
- Tachamo, N.; Timilsina, B.; Dhital, R.; Lynn, T.; Magaji, V.; Gabriely, I. Primary Hyperparathyroidism in Pregnancy: Successful Parathyroidectomy during First Trimester. Case Rep. Endocrinol. 2018, 2018, 1–4. [Google Scholar] [CrossRef] [PubMed]
| Genetic Conditions (Consider If Age Is Less than 40 Years) | MEN1 | MEN2A | MEN4 | HPT-JT | Exclude FHH |
|---|---|---|---|---|---|
| Associated conditions/Features | Parathyroid adenoma/hyperplasia Enteropancreatic cell tumors Anterior pituitary lesion carcinoid tumors, adrenocortical tumors, meningiomas, | Parathyroid adenoma/hyperplasia MTC Pheochromocytoma | Parathyroid Pituitary Adrenals Kidneys Gonadal tumors | Parathyroid, uterine, testicular, renal tumors ossifying jaw fibroma, pancreatic adenocarcinoma Thyroid (Hurthle cell adenoma) | Family history of hypercalcemia CCCR < 0.02 |
| Primary investigations | Primary panel: serum ionized calcium, calcium adjusted for albumin, intact PTH, phosphorus, magnesium, creatinine, eGFR, 25(OH2)D3, TSH, freeT4, freeT3, Full blood count, alkaline phosphatase. +/− LH, FSH, PRL, estradiol, IGF1 (performed outside of pregnancy) ACTH *, cortisol *, gastrin, glucagon, chromogranin A, vasointestinalpolypeptide, pancreatic polypeptide, insulin, fasting glucose level | Primary panel +/− Calcitonin, plasma metanephrines, 24 h urinary metanephrines | Primary panel | Primary panel | Primary panel Gene sequencing FHH type1–CaSR inactive mutation FHH type2–GNA11 mutation FHH type3–AP2S1 gene mutation |
| Confirmatory Test | MEN1 gene analysis | RET protooncogene analysis | CDNK1B gene sequencing | CDC73 gene sequencing (parafibromin) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, D.S.; Dandurand, K.; Khan, A.A. Primary Hyperparathyroidism in Pregnancy: Literature Review of the Diagnosis and Management. J. Clin. Med. 2021, 10, 2956. https://doi.org/10.3390/jcm10132956
Ali DS, Dandurand K, Khan AA. Primary Hyperparathyroidism in Pregnancy: Literature Review of the Diagnosis and Management. Journal of Clinical Medicine. 2021; 10(13):2956. https://doi.org/10.3390/jcm10132956
Chicago/Turabian StyleAli, Dalal S., Karel Dandurand, and Aliya A. Khan. 2021. "Primary Hyperparathyroidism in Pregnancy: Literature Review of the Diagnosis and Management" Journal of Clinical Medicine 10, no. 13: 2956. https://doi.org/10.3390/jcm10132956
APA StyleAli, D. S., Dandurand, K., & Khan, A. A. (2021). Primary Hyperparathyroidism in Pregnancy: Literature Review of the Diagnosis and Management. Journal of Clinical Medicine, 10(13), 2956. https://doi.org/10.3390/jcm10132956






