Clinical Outcomes of Conversion Surgery after FOLFIRINOX in Patients with Unresectable Advanced Pancreatic Cancer: A Retrospective Cohort Study at a Single Center
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Patient Selection
2.2. Assessment of Resectability
2.3. First-Line Chemotherapy and Assessment of Clinical Outcomes
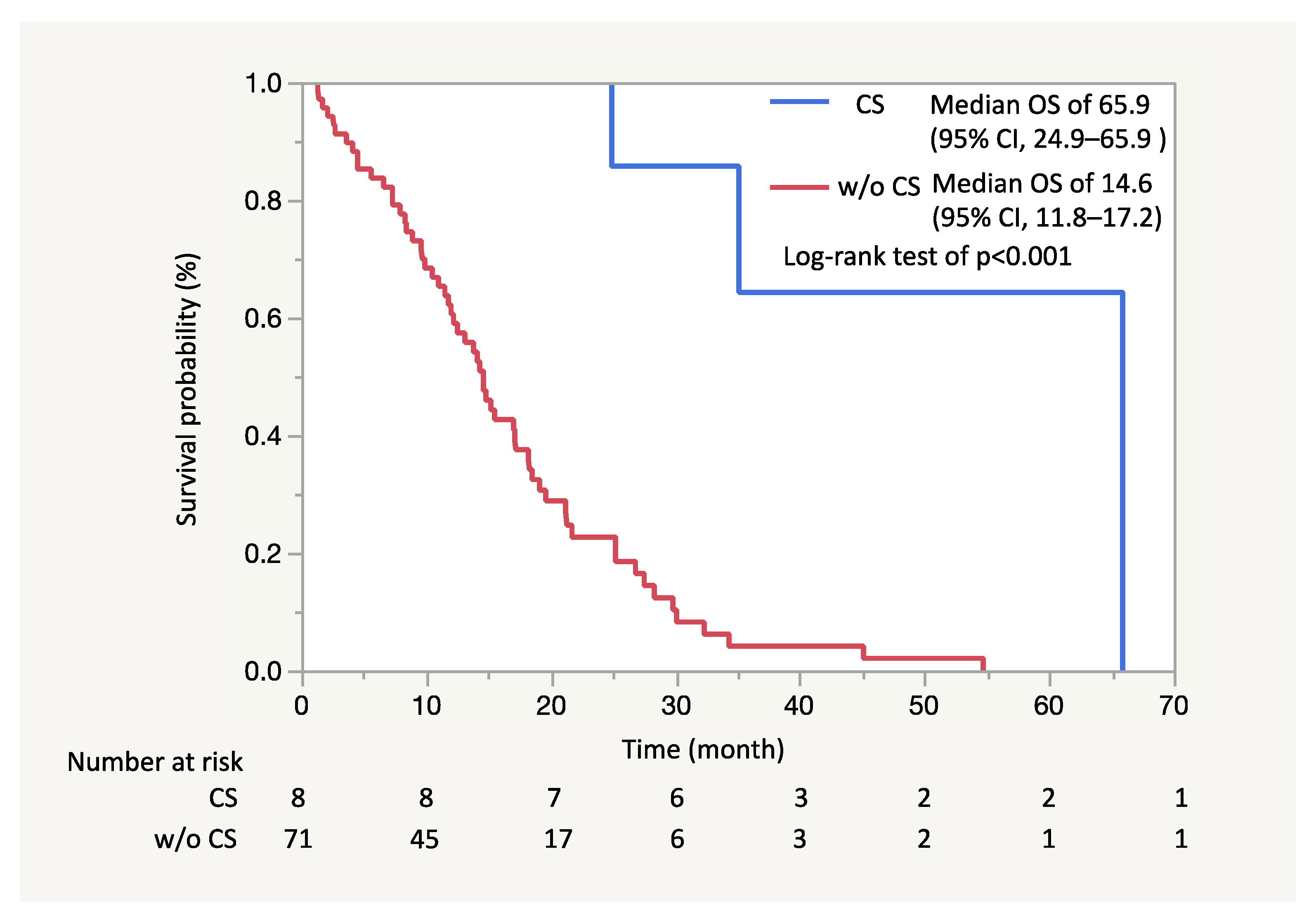
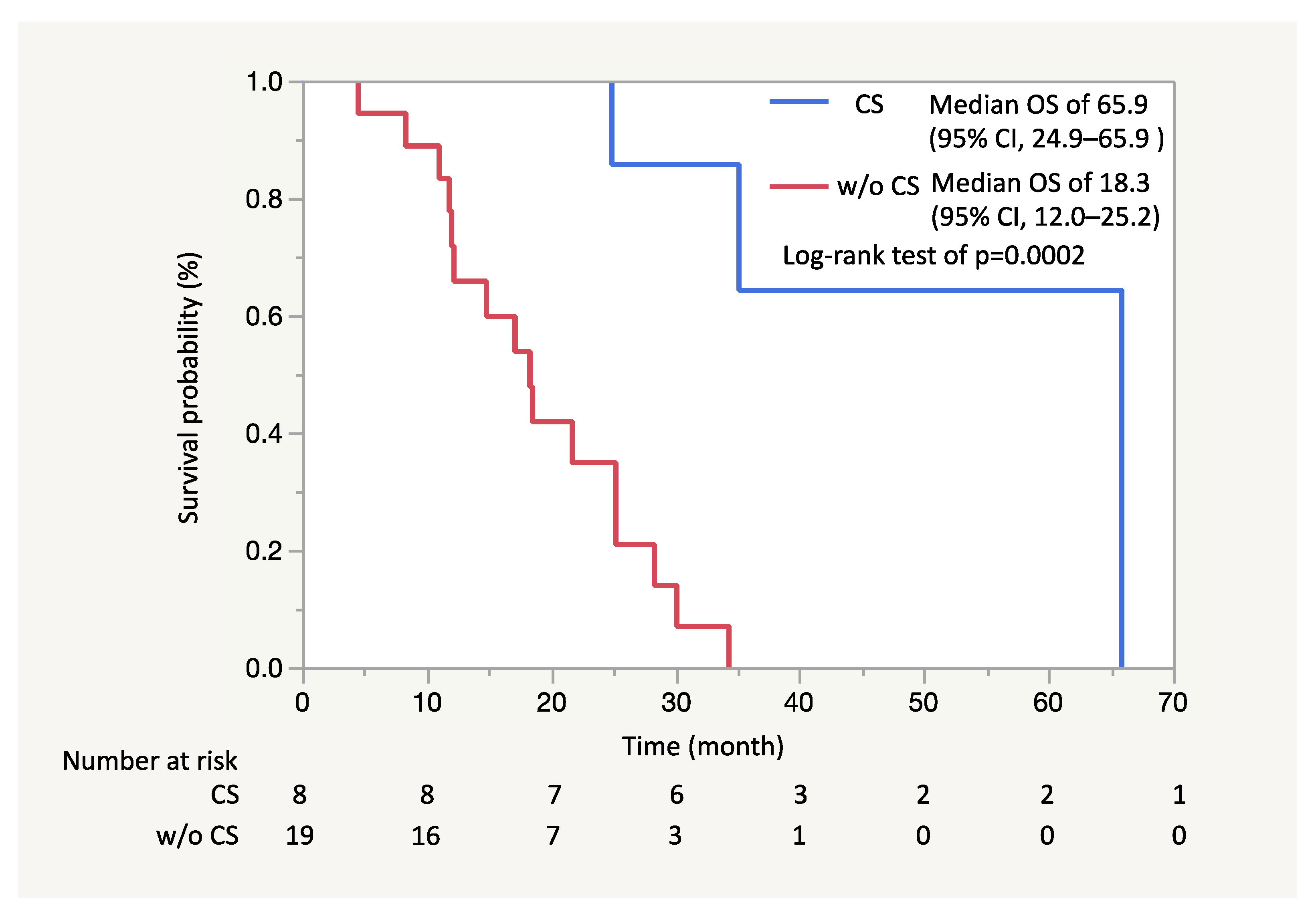
2.4. Study Outcomes and Statistical Analysis
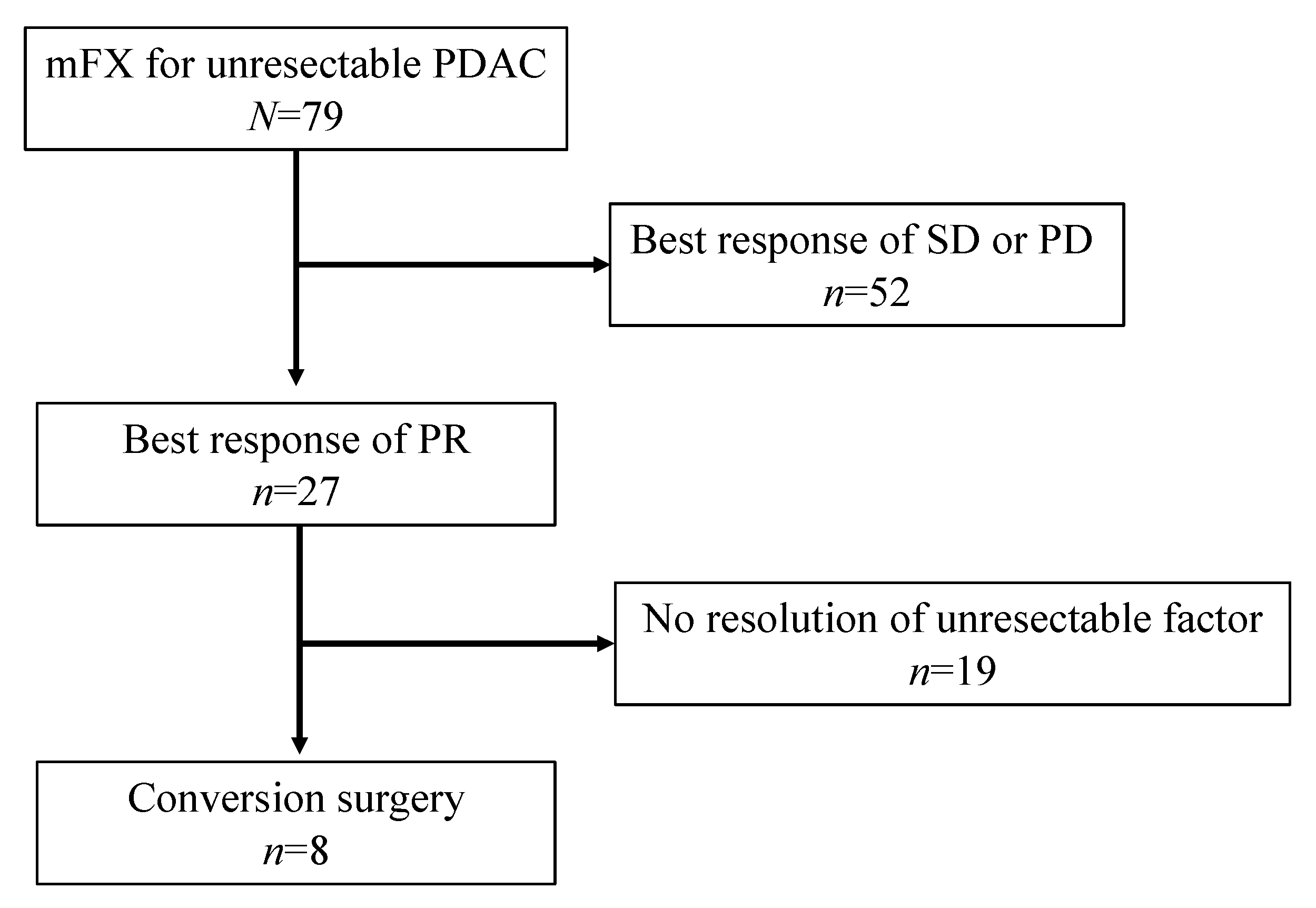
3. Results
3.1. Patient and Tumor Characteristics
3.2. Preoperative Therapy
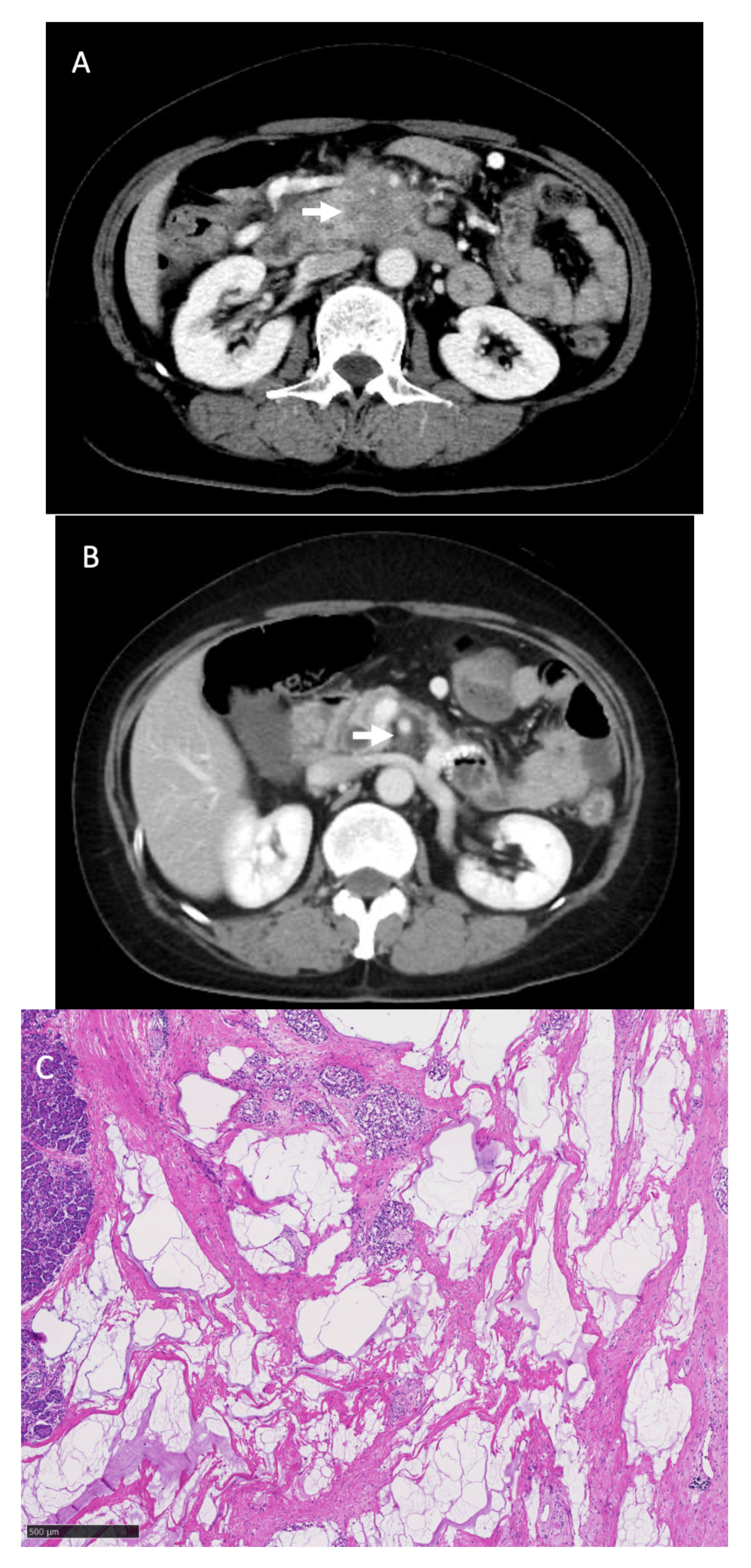
3.3. Surgical Outcomes and Pathological Findings
3.4. Adjuvant Chemotherapy and Postoperative Outcomes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Foundation for Promotion of Cancer Research. Cancer Statistics in Japan, 2019. Available online: https://ganjoho.jp/en/professional/statistics/brochure/2019_en.html (accessed on 20 May 2021).
- Vincent, A.; Herman, J.; Schulick, R.; Hruban, R.H.; Goggins, M. Pancreatic Cancer. Lancet 2011, 378, 607–620. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased Survival in Pancreatic Cancer with Nab-Paclitaxel plus Gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapam, S.; Sohei, O.; Yanagimoto, H.; Yamamoto, T.; Toyokawa, H.; Hirooka, S.; Yamaki, S. Role of Adjuvant Surgery in Initially Unresectable Pancreatic Cancer after Long-Term Chemotherapy or Chemoradiation Therapy: Survival Benefit? J. Hepato Biliary Pancreat. Sci. 2014, 21, 695–702. [Google Scholar]
- Natsume, S.; Shimizu, Y.; Senda, Y.; Hijioka, S.; Matsuo, K.; Ito, S.; Komori, K. Conversion Surgery Only for Highly Selected Patients with Unresectable Pancreatic Cancer: A Satisfactory Outcome in Exchange for a Lower Resection Rate. Surg. Today 2019, 49, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Yoshitomi, H.; Takano, S.; Furukawa, K.; Takayashiki, T.; Kuboki, S. Conversion Surgery for Initially Unresectable Pancreatic Cancer: Current Status and Unresolved Issues. Surg. Today 2019, 49, 894–906. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Kondo, S.; Hirano, S.; Tanaka, E.; Shichinohe, T.; Tsuchikawa, T.; Matsumoto, J. Adjuvant Surgical Therapy for Patients with Initially-Unresectable Pancreatic Cancer with Long-Term Favorable Responses to Chemotherapy. J. Hepato Biliary Pancratic Sci. 2011, 18, 712–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitsche, U.; Wenzel, P.; Siveke, J.T.; Braren, R.; Holzapfel, K.; Schlitter, A.M.; Stöß, C.; Kong, B.; Esposito, I.; Erkan, M.; et al. Resectability After First-Line FOLFIRINOX in Initially Unresectable Locally Advanced Pancreatic Cancer: A Single-Center Experience. Ann. Surg. Oncol. 2015, 22, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Bickenbach, K.A.; Gonen, M.; Tang, L.H.; O’Reilly, E.; Goodman, K.; Brennan, M.F.; D’Angelica, M.I.; DeMatteo, R.P.; Fong, Y.; Jarnagin, W.R.; et al. Downstaging in Pancreatic Cancer: A Matched Analysis of Patients Resected Following Systemic Treatment of Initially Locally Unresectable Disease. Ann. Surg. Oncol. 2012, 19, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Furuse, J.; Shibahara, J.; Sugiyama, M. Development of Chemotherapy and Significance of Conversion Surgery after Chemotherapy in Unresectable Pancreatic Cancer. J. Hepato Biliary Pancreatic Sci. 2018, 25, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Iwashita, T.; Uemura, S.; Maruta, A. A Multicenter Prospective Phase II Study of First-Line Modified FOLFIRINOX for Unresectable Advanced Pancreatic Cancer. Oncotarget 2017, 8, 111346–111355. [Google Scholar] [CrossRef] [PubMed]
- Mita, N.; Iwashita, T.; Uemura, S.; Yoshida, K.; Iwasa, Y.; Ando, N.; Iwata, K.; Okuno, M.; Mukai, T.; Shimizu, M. Second-Line Gemcitabine Plus Nab-Paclitaxel for Patients with Unresectable Advanced Pancreatic Cancer after First-Line FOLFIRINOX Failure. J. Clin. Med. 2019, 8, 761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Japan Pancreas Society. Classification of Pancreatic Carcinoma (4th English Edition); Kanehara Co.: Tokyo, Japan, 2017. [Google Scholar]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of Surgical Complications: A New Proposal with Evaluation in a Cohort of 6336 Patients and Results of a Surgery. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.B.; Rich, T.A.; Byrd, D.R.; Cleary, K.R.; Connelly, J.H.; Levin, B.; Charnsangavej, C.; Fenoglio, C.J.; Ames, F.C. Preoperative Chemoradiation and Pancreaticoduodenectomy for Adenocarcinoma of the Pancreas. Arch. Surg. 1992, 127, 1335–1339. [Google Scholar] [CrossRef] [PubMed]
- Sadot, E.; Doussot, A.; Reilly, E.M.O.; Lowery, M.A.; Karyn, A.; Kinh, R.; Do, G.; Tang, L.H.; Gönen, M.; Angelica, M.I.D.; et al. FOLFIRINOX Induction Therapy for Stage 3 Pancreatic Adenocarcinoma. Ann. Surg. Oncol. 2015, 22, 3512–3521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Lee, J.C.; Gromski, M.A.; Kim, H.W.; Kim, J.; Kim, J.; Hwang, J.H. Clinical outcomes of FOLFIRINOX in locally advanced pancreatic cancer: A single center experience. Medicine 2018. [Google Scholar] [CrossRef] [PubMed]
- Satoi, S.; Yamamoto, T.; Yamaki, S.; Sakaguchi, T.; Sekimoto, M. Surgical Indication for and Desirable Outcomes of Conversion Surgery in Patients with Initially Unresectable Pancreatic Ductal Adenocarcinoma. Ann. Gastroenterol. Surg. 2020, 4, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Heckler, M.; Mihaljevic, A.L.; Sun, H.; Klaiber, U.; Heger, U.; Büchler, M.W.; Hackert, T. CT Response of Primary Tumor and CA19-9 Predict Resectability of Metastasized Pancreatic Cancer after FOLFIRINOX. Eur. J. Surg. Oncol. 2019, 45, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Satoi, S.; Yamaue, H.; Kato, K.; Takahashi, S.; Hirono, S.; Takeda, S.; Eguchi, H.; Sho, M.; Wada, K.; Shinchi, H.; et al. Role of Adjuvant Surgery for Patients with Initially Unresectable Pancreatic Cancer with a Long-Term Favorable Response to Non-Surgical Anti-Cancer Treatments: Results of a Project Study for Pancreatic Surgery by the Japanese Society of Hepato-Biliary-Pan. J. Hepato Biliary Pancreatic Sci. 2013, 20, 590–600. [Google Scholar] [CrossRef] [PubMed]
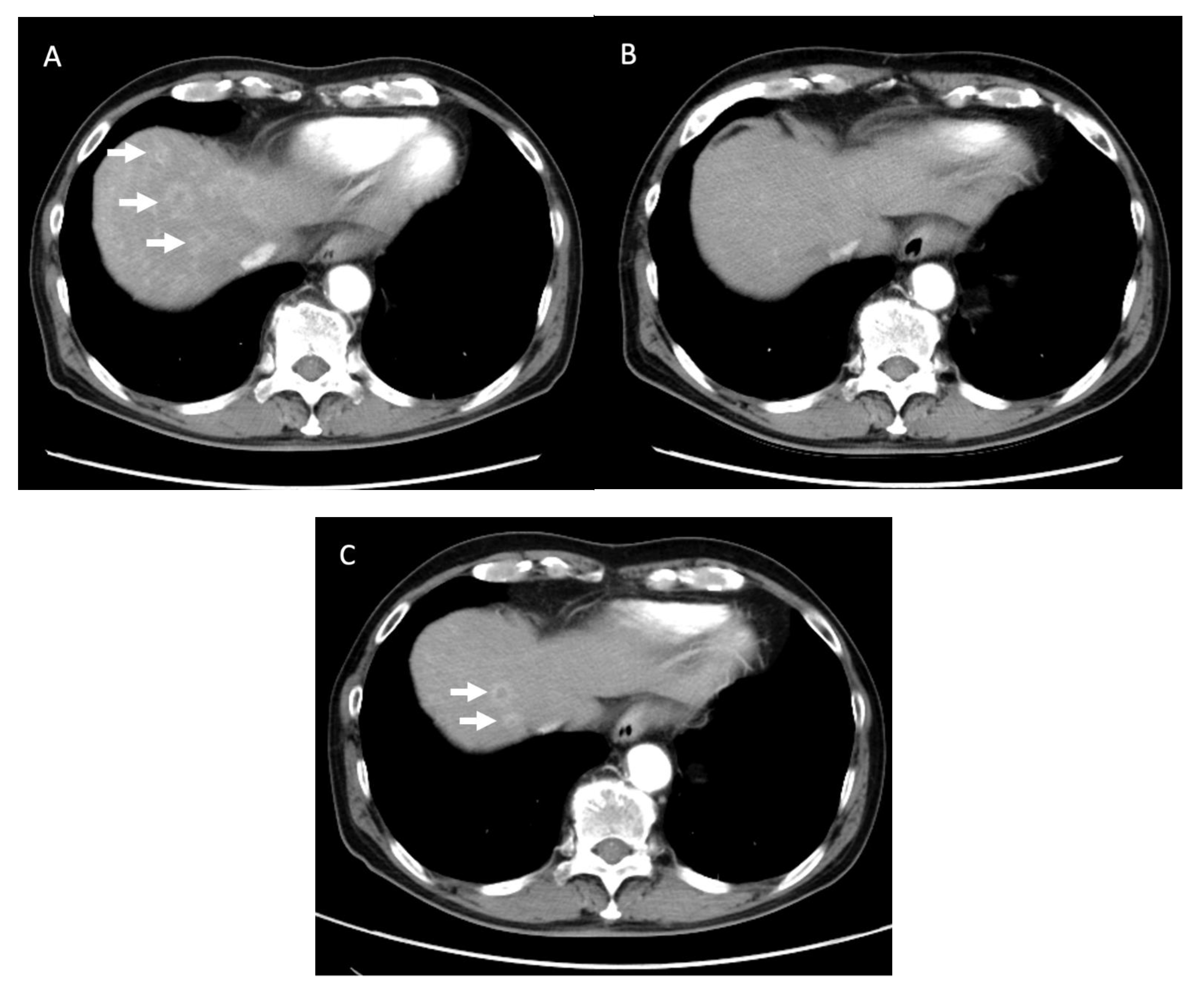
| Age, Years | Median (Range) | 64 (38–74) | |
| Sex | n (%) | men | 44 (55.7) |
| Metastasis | n (%) | yes | 59 (74.7) |
| Best response based on imaging studies | n (%) | CR | 0 |
| PR | 27 (34.2) | ||
| SD | 32 (40.5) | ||
| PD | 20 (25.3) | ||
| Conversion surgery | n (%) | 8 (10.1) | |
| Patient No. | Age (years) | Gender | Site of Primary Tumor | Unresectable Factor |
|---|---|---|---|---|
| 1 | 63 | Male | Tail | M (HEP) |
| 2 | 72 | Male | Head | LA (SMV) |
| 3 | 59 | Male | Body | M (PER) |
| 4 | 63 | Female | Body | M (HEP) |
| 5 | 58 | Male | Head | LA (Ao) |
| 6 | 57 | Female | Head | LA (SMA, SMV) |
| 7 | 63 | Male | Body | M (PER) |
| 8 | 63 | Female | Head | M (HEP) |
| Patient No. | Duration (Months) (Cycles) | Treatment Effect (RECIST) | Adverse Events (≥Grade 3) | CA19–9(U/mL) Peak/after Chemotherapy | Relative Dose Intensity (%) | Findings of Unresectable Factor | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| L-OHP | CPT-11 | 5-FU | LV | |||||||
| 1 | 7.1 (10) | PR | Neutropenia G4 | 6529 / 32 | 42.9 | 56.2 | 68.1 | 68.1 | M (HEP/Multi) | disappeared |
| 2 | 8.0 (13) | PR | Peripheral sensory neuropathy G3 | 1462 / 55 | 59.6 | 74.5 | 85.0 | 85.0 | LA (SMV) | disappeared |
| 3 | 23.9 (47) | PR | Neutropenia G3 | 250 / 13 | 65.1 | 79.8 | 86.8 | 87.7 | M (PER/Multi) | disappeared |
| 4 | 23.3 (31) | PR | Neutropenia G4 | 4379 / 81 | 42.8 | 56.4 | 69.9 | 68 | M (HEP/Multi) | disappeared |
| 5 | 6.8 (11) | PR | Neutropenia G4 | 171 / 12 | 71.8 | 81.4 | 83.4 | 83.0 | LA (Ao) | disappeared |
| 6 | 13.3 (27) | PR | Neutropenia G4 | 5431 / 20 | 60.9 | 89.7 | 92.7 | 92.2 | LA (SMA, SMV) | improved |
| 7 | 4.5 (6) | PR | Neutropenia G3 | 370 / 177 | 50.2 | 53.3 | 65 | 66 | M (PER/Multi) | disappeared |
| 8 | 24.0 (36) | PR | Neutropenia G4 | 10424 / 27 | 41.7 | 56.5 | 65.7 | 66.4 | M (HEP/Multi) | disappeared |
| Patient No. | Operative Procedure | Operative Time (min) | Blood Loss (ml) | Major Complications | Hospital Stay (Days) | pTNM | Residual Tumor | Tumor Viability (Evans) |
|---|---|---|---|---|---|---|---|---|
| 1 | DP | 220 | 100 | - | 20 | pT2N0M0, stageIB | R0 | IIb |
| 2 | PD | 520 | 710 | - | 50 | pT2N1M0, stageIIB | R0 | IIb |
| 3 | DP | 445 | 1840 | - | 22 | pT3N0M0, stageIIA | R0 | IIa |
| 4 | DP | 350 | 290 | - | 19 | pT3N0M0, stageIIA | R0 | IIb |
| 5 | PD | 500 | 685 | - | 31 | pT3N0M0, stageIIA | R0 | IIb |
| 6 | PD | 880 | 1790 | GDA pseudoaneurysm rupture Pancreatic fistula | 47 | pCR | R0 | IV |
| 7 | DP-CAR | 360 | 330 | - | 15 | pT3N1M0, stageIIB | R0 | IIb |
| 8 | PD | 430 | 610 | Chylous ascites | 41 | pCR | R0 | IV |
| Patient No. | Adjuvant Chemotherapy | Recurrence Site/Time to Recurrence from CS (Month) | Final Outcome | OS (Months) from Initial Treatment [from Surgery] |
|---|---|---|---|---|
| 1 | mFX | Liver/8.5 | Death | 24.9 [17.7] |
| 2 | S1 | Remnant pancreas/20.1 | Death | 65.9 [57.8] |
| 3 | mFX | No | Alive | 40.9 [16.7] |
| 4 | mFX | No | Alive | 35.2 [12.0] |
| 5 | mFX | No | Alive | 26.1 [19.3] |
| 6 | No | No | Alive | 27.3 [13.9] |
| 7 | S1 | Lung/24.1 | Death | 35.1 [30.6] |
| 8 | S1 | No | Alive | 26.7 [12.6] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mita, N.; Iwashita, T.; Ichikawa, H.; Iwasa, Y.; Uemura, S.; Murase, K.; Shimizu, M. Clinical Outcomes of Conversion Surgery after FOLFIRINOX in Patients with Unresectable Advanced Pancreatic Cancer: A Retrospective Cohort Study at a Single Center. J. Clin. Med. 2021, 10, 2848. https://doi.org/10.3390/jcm10132848
Mita N, Iwashita T, Ichikawa H, Iwasa Y, Uemura S, Murase K, Shimizu M. Clinical Outcomes of Conversion Surgery after FOLFIRINOX in Patients with Unresectable Advanced Pancreatic Cancer: A Retrospective Cohort Study at a Single Center. Journal of Clinical Medicine. 2021; 10(13):2848. https://doi.org/10.3390/jcm10132848
Chicago/Turabian StyleMita, Naoki, Takuji Iwashita, Hironao Ichikawa, Yuhei Iwasa, Shinya Uemura, Katsutoshi Murase, and Masahito Shimizu. 2021. "Clinical Outcomes of Conversion Surgery after FOLFIRINOX in Patients with Unresectable Advanced Pancreatic Cancer: A Retrospective Cohort Study at a Single Center" Journal of Clinical Medicine 10, no. 13: 2848. https://doi.org/10.3390/jcm10132848
APA StyleMita, N., Iwashita, T., Ichikawa, H., Iwasa, Y., Uemura, S., Murase, K., & Shimizu, M. (2021). Clinical Outcomes of Conversion Surgery after FOLFIRINOX in Patients with Unresectable Advanced Pancreatic Cancer: A Retrospective Cohort Study at a Single Center. Journal of Clinical Medicine, 10(13), 2848. https://doi.org/10.3390/jcm10132848







