Advanced Neuroimaging Preceding Intravenous Thrombolysis in Acute Ischemic Stroke Patients Is Safe and Effective
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
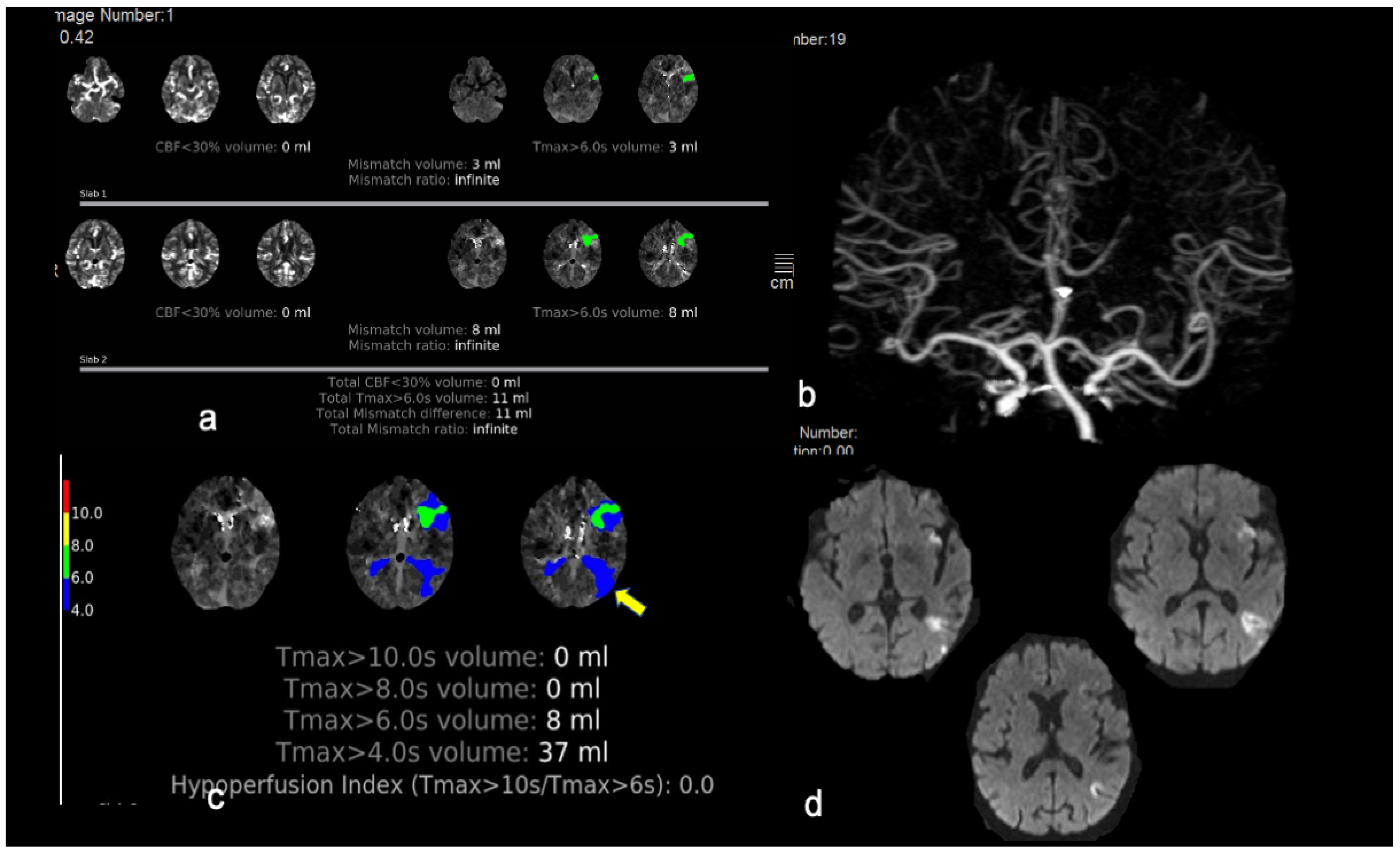
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berge, E.; Whiteley, W.; Audebert, H.; De Marchis, G.M.; Fonseca, A.C.; Padiglioni, C.; de la Ossa, N.P.; Strbian, D.; Tsivgoulis, G.; Turc, G. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur. Stroke J. 2021, 6, 1–62. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef] [PubMed]
- Tsivgoulis, G.; Kargiotis, O.; Alexandrov, A.V. Intravenous thrombolysis for acute ischemic stroke: A bridge between two centuries. Expert Rev. Neurother. 2017, 17, 819–837. [Google Scholar] [CrossRef]
- Eissa, A.; Krass, I.; Levi, C.; Sturm, J.; Ibrahim, R.; Bajorek, B. Understanding the reasons behind the low utilisation of thrombolysis in stroke. Australas. Med. J. 2013, 6, 152–167. [Google Scholar] [CrossRef]
- De Sousa, D.A.; Von Martial, R.; Abilleira, S.; Gattringer, T.; Kobayashi, A.; Gallofré, M.; Fazekas, F.; Szikora, I.; Feigin, V.; Caso, V.; et al. Access to and delivery of acute ischaemic stroke treatments: A survey of national scientific societies and stroke experts in 44 European countries. Eur. Stroke J. 2019, 4, 13–28. [Google Scholar] [CrossRef] [Green Version]
- Soto-Cámara, R.; González-Santos, J.; González-Berna, J.; Trejo-Gabriel-Galán, J.M. Factors associated with a rapid call for assistance for patients with ischemic stroke. Emergencias 2020, 32, 33–39. [Google Scholar]
- Tsivgoulis, G.; Kargiotis, O.; De Marchis, G.; Kohrmann, M.; Sandset, E.C.; Karapanayiotides, T.; de Sousa, D.A.; Sarraj, A.; Safouris, A.; Psychogios, K.; et al. Off-label use of intravenous thrombolysis for acute ischemic stroke: A critical appraisal of randomized and real-world evidence. Ther. Adv. Neurol. Disord. 2021, 14, 1756286421997368. [Google Scholar] [CrossRef]
- Tsivgoulis, G.; Safouris, A.; Alexandrov, A.V. Safety of intravenous thrombolysis for acute ischemic stroke in specific conditions. Expert Opin. Drug Saf. 2015, 14, 845–864. [Google Scholar] [CrossRef]
- Tsivgoulis, G.; Katsanos, A.H.; Schellinger, P.D.; Köhrmann, M.; Caso, V.; Palaiodimou, L.; Magoufis, G.; Arthur, A.; Fischer, U.; Alexandrov, A.V. Advanced Neuroimaging in Stroke Patient Selection for Mechanical Thrombectomy. Stroke 2018, 49, 3067–3070. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, R.G.; Jadhav, A.P.; Haussen, D.C.; Bonafe, A.; Budzik, R.F.; Bhuva, P.; Yavagal, D.R.; Ribo, M.; Cognard, C.; Hanel, R.A.; et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N. Engl. J. Med. 2018, 378, 11–21. [Google Scholar] [CrossRef]
- Albers, G.W.; Marks, M.P.; Kemp, S.; Christensen, S.; Tsai, J.P.; Ortega-Gutierrez, S.; McTaggart, R.A.; Torbey, M.T.; Kim-Tenser, M.; Leslie-Mazwi, T.; et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N. Engl. J. Med. 2018, 378, 708–718. [Google Scholar] [CrossRef]
- Ma, H.; Campbell, B.C.V.; Parsons, M.W.; Churilov, L.; Levi, C.R.; Hsu, C.; Kleinig, T.J.; Wijeratne, T.; Curtze, S.; Dewey, H.M.; et al. Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N. Engl. J. Med. 2019, 380, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.V.; Ma, H.; Ringleb, P.A.; Parsons, M.W.; Churilov, L.; Bendszus, M.; Levi, C.R.; Hsu, C.; Kleinig, T.J.; Fatar, M.; et al. Extending thrombolysis to 4·5–9 h and wake-up stroke using perfusion imaging: A systematic review and meta-analysis of individual patient data. Lancet 2019, 394, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Thomalla, G.; Boutitie, F.; Ma, H.; Koga, M.; Ringleb, P.; Schwamm, L.H.; Wu, O.; Bendszus, M.; Bladin, C.F.; Campbell, B.C.V.; et al. Intravenous alteplase for stroke with unknown time of onset guided by advanced imaging: Systematic review and meta-analysis of individual patient data. Lancet 2020, 396, 1574–1584. [Google Scholar] [CrossRef]
- Hill, M.D.; Goyal, M.; Demchuk, A.M.; Fisher, M. Ischemic Stroke Tissue-Window in the New Era of Endovascular Treatment. Stroke 2015, 46, 2332–2334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsivgoulis, G.; Kargiotis, O.; Rudolf, J.; Komnos, A.; Tavernarakis, A.; Karapanayiotides, T.; Ellul, J.; Katsanos, A.H.; Giannopoulos, S.; Gryllia, M.; et al. Intravenous thrombolysis for acute ischemic stroke in Greece: The Safe Implementation of Thrombolysis in Stroke registry 15-year experience. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418783578. [Google Scholar] [CrossRef] [PubMed]
- Tsivgoulis, G.; Goyal, N.; Mikulik, R.; Sharma, V.K.; Katsanos, A.H.; Zand, R.; Paliwal, P.R.; Roussopoulou, A.; Volny, O.; Pandhi, A.; et al. Eligibility for mechanical thrombectomy in acute ischemic stroke: A phase IV multi-center screening log registry. J. Neurol. Sci. 2016, 371, 96–99. [Google Scholar] [CrossRef]
- Thomalla, G.; Simonsen, C.Z.; Boutitie, F.; Andersen, G.; Berthezene, Y.; Cheng, B.; Cheripelli, B.; Cho, T.-H.; Fazekas, F.; Fiehler, J.; et al. MRI-Guided Thrombolysis for Stroke with Unknown Time of Onset. New Engl. J. Med. 2018, 379, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Psychogios, K.; Magoufis, G.; Safouris, A.; Kargiotis, O.; Katsanos, A.H.; Spiliopoulos, S.; Papageorgiou, E.; Palaiodimou, L.; Brountzos, E.; Stamboulis, E.; et al. Eligibility for intravenous thrombolysis in acute ischemic stroke patients presenting in the 4.5–9 h window. Neuroradiology 2020, 62, 733–739. [Google Scholar] [CrossRef]
- Mair, G.; Boyd, E.V.; Chappell, F.M.; Von Kummer, R.; Lindley, R.I.; Sandercock, P.; Wardlaw, J.M.; IST-3 Collaborative Group. Sensitivity and Specificity of the Hyperdense Artery Sign for Arterial Obstruction in Acute Ischemic Stroke. Stroke 2015, 46, 102–107. [Google Scholar] [CrossRef] [Green Version]
- Riedel, C.H.; Jensen, U.; Rohr, A.; Ulmer, S.; Tietke, M.; Alfke, K.; Jansen, O. Assessment of thrombus in acute stroke using ultra-thin slice nonenhanced CT reconstructions. Stroke 2010, 41, 1659–1664. [Google Scholar] [CrossRef]
- Psychogios, K.; Palaiodimou, L.; Katsanos, A.H.; Magoufis, G.; Safouris, A.; Kargiotis, O.; Spiliopoulos, S.; Papageorgiou, E.; Theodorou, A.; Voumvourakis, K.; et al. Real-world comparative safety and efficacy of tenecteplase versus alteplase in acute ischemic stroke patients with large vessel occlusion. Ther. Adv. Neurol. Disord. 2021, 14, 1756286420986727. [Google Scholar] [CrossRef]
- Fiorelli, M.; Bastianello, S.; Von Kummer, R.; Del Zoppo, G.J.; Larrue, V.; Lesaffre, E.; Ringleb, A.P.; Lorenzano, S.; Manelfe, C.; Bozzao, L. Hemorrhagic Transformation Within 36 Hours of a Cerebral Infarct: Relationships with early clinical deterioration and 3-month outcome in the European Cooperative Acute Stroke Study I (ECASS I) cohort. Stroke 1999, 30, 2280–2284. [Google Scholar] [CrossRef] [PubMed]
- Goyal, N.; Tsivgoulis, G.; Pandhi, A.; Dillard, K.; Katsanos, A.H.; Magoufis, G.; Chang, J.J.; Zand, R.; Hoit, D.; Safouris, A.; et al. Admission hyperglycemia and outcomes in large vessel occlusion strokes treated with mechanical thrombectomy. J. NeuroInterv. Surg. 2018, 10, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Tsivgoulis, G.; Goyal, N.; Katsanos, A.H.; Malhotra, K.; Ishfaq, M.F.; Pandhi, A.; Frohler, M.T.; Spiotta, A.M.; Anadani, M.; Psychogios, M.; et al. Intravenous thrombolysis for large vessel or distal occlusions presenting with mild stroke severity. Eur. J. Neurol. 2020, 27, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Psychogios, K.; Kargiotis, O.; Safouris, A.; Magoufis, G.; Gelagoti, M.; Bonakis, A.; Stamboulis, E.; Tsivgoulis, G. Perfusion imaging averting intravenous thrombolysis in stroke mimics. Neurol. Sci. 2021, 10, 2591–2594. [Google Scholar] [CrossRef] [PubMed]
- Turk, A.S.; Nyberg, E.M.; Chaudry, M.I.; Turner, R.D.; Magarik, J.A.; Nicholas, J.S.; Holmstedt, C.A.; Chalela, J.A.; Hays, A.; Lazaridis, C.; et al. Utilization of CT perfusion patient selection for mechanical thrombectomy irrespective of time: A comparison of functional outcomes and complications. J. NeuroInterv. Surg. 2012, 5, 518–522. [Google Scholar] [CrossRef]
- Turk, A.; Magarik, J.A.; Chaudry, I.; Turner, R.D.; Nicholas, J.; Holmstedt, C.A.; Chalela, J.; Hays, A.; Lazaridis, C.; Jauch, E.; et al. CT perfusion-guided patient selection for endovascular treatment of acute ischemic stroke is safe and effective. J. NeuroInterv. Surg. 2011, 4, 261–265. [Google Scholar] [CrossRef] [Green Version]
- Campbell, B.; Mitchell, P.J.; Kleinig, T.; Dewey, H.M.; Churilov, L.; Yassi, N.; Yan, B.; Dowling, R.J.; Parsons, M.W.; Oxley, T.; et al. Endovascular Therapy for Ischemic Stroke with Perfusion-Imaging Selection. New Engl. J. Med. 2015, 372, 1009–1018. [Google Scholar] [CrossRef] [Green Version]
- Saver, J.L.; Goyal, M.; Bonafe, A.; Diener, H.-C.; Levy, E.I.; Pereira, V.M.; Albers, G.W.; Cognard, C.; Cohen, D.J.; Hacke, W.; et al. Stent-Retriever Thrombectomy after Intravenous t-PA vs. t-PA Alone in Stroke. New Engl. J. Med. 2015, 372, 2285–2295. [Google Scholar] [CrossRef] [Green Version]
- Rudilosso, S.; Urra, X.; Román, L.S.; Laredo, C.; López-Rueda, A.; Amaro, S.; Oleaga, L.; Chamorro, Á. Perfusion Deficits and Mismatch in Patients with Acute Lacunar Infarcts Studied with Whole-Brain CT Perfusion. AJNR Am. J. Neuroradiol. 2015, 36, 1407–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostman, C.; Garcia-Esperon, C.; Lillicrap, T.; Tomari, S.; Holliday, E.; Levi, C.; Bivard, A.; Parsons, M.W.; Spratt, N.J. Multimodal Computed Tomography Increases the Detection of Posterior Fossa Strokes Compared to Brain Non-contrast Computed Tomography. Front. Neurol. 2020, 11, 588064. [Google Scholar] [CrossRef]
- Olivot, J.-M.; Mlynash, M.; Thijs, V.N.; Kemp, S.; Lansberg, M.G.; Wechsler, L.; Bammer, R.; Marks, M.P.; Albers, G.W. Optimal Tmax Threshold for Predicting Penumbral Tissue in Acute Stroke. Stroke 2009, 40, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Bivard, A.; Levi, C.; Spratt, N.; Parsons, M. Perfusion CT in Acute Stroke: A Comprehensive Analysis of Infarct and Penumbra. Radiology 2013, 267, 543–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheeler, H.M.; Mlynash, M.; Inoue, M.; Tipirneni, A.; Liggins, J.; Zaharchuk, G.; Straka, M.; Kemp, S.; Bammer, R.; Lansberg, M.G.; et al. Early Diffusion-Weighted Imaging and Perfusion-Weighted Imaging Lesion Volumes Forecast Final Infarct Size in DEFUSE 2. Stroke 2013, 44, 681–685. [Google Scholar] [CrossRef]
- Bivard, A.; Lou, M.; Levi, C.R.; Krishnamurthy, V.; Cheng, X.; Aviv, R.I.; McElduff, P.; Lin, L.; Kleinig, T.; O’Brien, B.; et al. Too good to treat? ischemic stroke patients with small computed tomography perfusion lesions may not benefit from thrombolysis. Ann. Neurol. 2016, 80, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Tikkinen, K.A.O.; Guyatt, G.H. Understanding of research results, evidence summaries and their applicability—not critical appraisal—are core skills of medical curriculum. BMJ Evid. Based Med. 2021. [Google Scholar] [CrossRef]

| Baseline Characteristics | AN− (n = 29) | AN+ (n = 47) | p-Value |
|---|---|---|---|
| Age, years (mean, SD) | 63 ± 16 | 73 ± 13 | 0.003 |
| Weight, kg (mean, SD) | 82 ± 18 | 80 ± 21 | 0.631 |
| Smoking (%) | 27.6% | 25.5% | 0.850 |
| Hypertension (%) | 72.4% | 57.4% | 0.189 |
| Diabetes (%) | 31.0% | 17.0% | 0.154 |
| Hypercholesterolemia (%) | 27.6% | 42.6% | 0.189 |
| Prior stroke (%) | 3.4% | 4.3% | 0.861 |
| Prior TIA (%) | 0.0% | 6.4% | 0.165 |
| Congestive heart failure (%) | 3.0% | 0.0% | 0.200 |
| Valvular disease (%) | 6.9% | 0.0% | 0.068 |
| Coronary artery disease (%) | 10.3% | 4.3% | 0.298 |
| Peripheral Arterial Disease (%) | 3.4% | 6.4% | 0.578 |
| Extended window 4.5–9 h (%) | 0.0% | 14.9% | 0.029 |
| Wake up stroke (%) | 3.4% | 8.5% | 0.387 |
| Extended window or wake up | 3.4% | 23.4% | 0.020 |
| NIHSS-score on admission, points (median, IQR) | 4 (2–7) | 5 (4–9) | 0.047 |
| Systolic BP on admission, mmHg (mean ± SD) | 152 ± 34 | 153 ± 21 | 0.837 |
| Diastolic BP on admission, mmHg (mean ± SD) | 80 ± 15 | 82 ± 14 | 0.549 |
| Platelet count on admission, ×109/L (mean ± SD) | 267 ± 152 | 228 ± 83 | 0.477 |
| LDL on admission, mg/dL (mean ± SD) | 137.5 ± 49 | 129.5 ± 34 | 0.554 |
| Glucose on admission, mg/dL (mean ± SD) | 129 ± 39 | 129 ± 39 | 0.174 |
| Onset-to-imaging time, min (median, IQR) | 105 (87.5–161) | 160 (120–202.5) | 0.011 |
| Door-to-needle time, min (median, IQR) | 43.5 (36–60) | 45 (30–61) | 0.956 |
| Onset-to-treatment time (median, IQR) | 121 (110–153) | 197.5 (151–240) | <0.001 |
| ASPECTS (median, IQR) | 10 (9–10) | 10 (9–10) | 0.278 |
| Duration of Hospitalization (median, IQR) | 10 (8–18) | 9.5 (5–16.5) | 0.725 |
| Location of stroke in the left hemisphere (%) | 43.2% | 56.8% | 0.564 |
| Location of stroke in posterior circulation (%) | 34.5% | 19.1% | 0.199 |
| Hyperdense vessel sign in CT (%) | 3.6% | 4.3% | 0.870 |
| MR imaging (%) | 6.9% | 10.6% | 0.584 |
| Thrombus length, mm (median, IQR) | 8.5 (5.75–14) | 12 (9–20) | 0.053 |
| Large Vessel occlusion (%) | 17.2% | 19.1% | 0.835 |
| Medium Vessel Occlusion (%) | 44.8% | 40.4% | 0.706 |
| Mean ischemic core volume (rCBF < 30%) (mean ± SD) (mL) | 2.1 ± 1.2 |
| Mean volume of critical hypo perfusion (Tmax > 6 s) (mean ± SD) (mL) | 16.3 ± 4 |
| Mean mismatch volume (mean ± SD) (mL) | 13.5 ± 3.3 |
| Outcomes | AN− (n = 29) | AN+ (n = 47) | p-Value |
|---|---|---|---|
| Any Hemorrhagic transformation (%) | 6.9% | 10.6% | 0.584 |
| Symptomatic Intracranial Hemorrhage (%) | 3.4% | 0.0% | 0.200 |
| NIHSS-score 2 h, points (median, IQR) | 2 (0.5–3.5) | 3 (1–5.25) | 0.230 |
| NIHSS 24 h, points (median, IQR) | 1 (0–4) | 1.5 (0–4) | 0.697 |
| Discharge NIHSS (median, IQR) | 0 (0–2.5) | 0 (0–3) | 0.977 |
| 3-month mRS-score, points (median, IQR) | 2 (1–4) | 3 (1–5) | 0.614 *** |
| 3-month Functional Independence (%) * | 82.1% | 89.1% | 0.394 |
| 3-month Favorable Functional Outcome (%) ** | 75.0% | 78.3% | 0.746 |
| 3-month Mortality (%) | 0.0% | 4.3% | 0.263 |
| Outcomes | Crude OR (95% CI) | p-Value | Adjusted * OR (95% CI) | p-Value |
|---|---|---|---|---|
| Any ICH | 1.60 (0.29, 8.88) | 0.586 | 1.30 (0.21, 8.01) | 0.840 |
| Functional Independence at 3 months | 1.78 (0.47, 6.80) | 0.398 | 12.89 (1.47, 113.00) | 0.021 |
| Favorable Functional Outcome at 3 months | 1.20 (0.40, 3.63) | 0.747 | 1.97 (0.54, 7.17) | 0.304 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Psychogios, K.; Safouris, A.; Kargiotis, O.; Magoufis, G.; Andrikopoulou, A.; Papageorgiou, E.; Chondrogianni, M.; Papadimitropoulos, G.; Polyzogopoulou, E.; Spiliopoulos, S.; et al. Advanced Neuroimaging Preceding Intravenous Thrombolysis in Acute Ischemic Stroke Patients Is Safe and Effective. J. Clin. Med. 2021, 10, 2819. https://doi.org/10.3390/jcm10132819
Psychogios K, Safouris A, Kargiotis O, Magoufis G, Andrikopoulou A, Papageorgiou E, Chondrogianni M, Papadimitropoulos G, Polyzogopoulou E, Spiliopoulos S, et al. Advanced Neuroimaging Preceding Intravenous Thrombolysis in Acute Ischemic Stroke Patients Is Safe and Effective. Journal of Clinical Medicine. 2021; 10(13):2819. https://doi.org/10.3390/jcm10132819
Chicago/Turabian StylePsychogios, Klearchos, Apostolos Safouris, Odysseas Kargiotis, Georgios Magoufis, Athina Andrikopoulou, Ermioni Papageorgiou, Maria Chondrogianni, Georgios Papadimitropoulos, Eftihia Polyzogopoulou, Stavros Spiliopoulos, and et al. 2021. "Advanced Neuroimaging Preceding Intravenous Thrombolysis in Acute Ischemic Stroke Patients Is Safe and Effective" Journal of Clinical Medicine 10, no. 13: 2819. https://doi.org/10.3390/jcm10132819
APA StylePsychogios, K., Safouris, A., Kargiotis, O., Magoufis, G., Andrikopoulou, A., Papageorgiou, E., Chondrogianni, M., Papadimitropoulos, G., Polyzogopoulou, E., Spiliopoulos, S., Brountzos, E., Stamboulis, E., Giannopoulos, S., & Tsivgoulis, G. (2021). Advanced Neuroimaging Preceding Intravenous Thrombolysis in Acute Ischemic Stroke Patients Is Safe and Effective. Journal of Clinical Medicine, 10(13), 2819. https://doi.org/10.3390/jcm10132819









