Computed Tomography in Adults with Bronchiectasis and Nontuberculous Mycobacterial Pulmonary Disease: Typical Imaging Findings
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. CT Data Acquisition
2.3. CT Features and Semiquantitative Scoring
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. CT Features of NTM-PD and Non-NTM Bronchiectasis
3.3. Difference of CT Findings before and after Microbiological Cure Completion
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, M.M.; Odell, J.A. Nontuberculous mycobacterial pulmonary infections. J. Thorac. Dis. 2014, 6, 210–220. [Google Scholar] [PubMed]
- Winthrop, K.L.; Marras, T.K.; Adjemian, J.; Zhang, H.; Wang, P.; Zhang, Q. Incidence and Prevalence of Nontuberculous Mycobacterial Lung Disease in a Large U.S. Managed Care Health Plan, 2008–2015. Ann. Am. Thorac. Soc. 2020, 17, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Ringshausen, F.C.; Ewen, R.; Multmeier, J.; Monga, B.; Obradovic, M.; van der Laan, R.; Diel, R. Predictive modeling of nontuberculous mycobacterial pulmonary disease epidemiology using German health claims data. Int. J. Infect. Dis. 2021, 104, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Diel, R.; Chalmers, J.D.; Rabe, K.F.; Nienhaus, A.; Loddenkemper, R.; Ringshausen, F.C. Economic burden of bronchiectasis in Germany. Eur. Respir. J. 2019, 53, 1802033. [Google Scholar] [CrossRef] [PubMed]
- Poppelwell, L.; Chalmers, J.D. Defining severity in non-cystic fibrosis bronchiectasis. Expert Rev. Respir. Med. 2014, 8, 249–262. [Google Scholar] [CrossRef]
- Chalmers, J.D.; Aliberti, S.; Polverino, E.; Vendrell, M.; Crichton, M.; Loebinger, M.; Dimakou, K.; Clifton, I.; Van Der Eerden, M.; Rohde, G.; et al. The EMBARC European Bronchiectasis Registry: Protocol for an international observational study. ERJ Open Res. 2016, 2. [Google Scholar] [CrossRef]
- Rademacher, J.; de Roux, A.; Ringshausen, F.C. PROGNOSIS-The PROspective German NOn-CF BronchiectaSIS Patient Registry. Pneumologie 2015, 69, 391–393. [Google Scholar]
- Faverio, P.; Stainer, A.; Bonaiti, G.; Zucchetti, S.C.; Simonetta, E.; Lapadula, G.; Marruchella, A.; Gori, A.; Blasi, F.; Codecasa, L.; et al. Characterizing Non-Tuberculous Mycobacteria Infection in Bronchiectasis. Int. J. Mol. Sci. 2016, 17, 1913. [Google Scholar] [CrossRef]
- Máiz, L.; Girón, R.; Olveira, C.; Vendrell, M.; Nieto, R.; Martínez-García, M.A. Prevalence and factors associated with nontuberculous mycobacteria in non-cystic fibrosis bronchiectasis: A multicenter observational study. BMC Infect. Dis. 2016, 16, 1–7. [Google Scholar] [CrossRef]
- Aksamit, T.R.; O’Donnell, A.E.; Barker, A.; Olivier, K.N.; Winthrop, K.L.; Daniels, M.L.A.; Johnson, M.; Eden, E.; Griffith, D.; Knowles, M.; et al. Adult Patients With Bronchiectasis: A First Look at the US Bronchiectasis Research Registry. Chest 2017, 151, 982–992. [Google Scholar] [CrossRef]
- Ku, J.H.; Henkle, E.M.; Carlson, K.F.; Marino, M.; Winthrop, K.L. Validity of Diagnosis Code-Based Claims to Identify Pulmonary NTM Disease in Bronchiectasis Patients. Infect. Dis. 2021, 27, 982–985. [Google Scholar] [CrossRef]
- Wagner, D.; Van Ingen, J.; Van Der Laan, R.; Obradovic, M. Non-tuberculous mycobacterial lung disease in patients with bronchiectasis: Perceived risk, severity and guideline adherence in a European physician survey. BMJ Open Respir. Res. 2020, 7, e000498. [Google Scholar] [CrossRef] [PubMed]
- Polverino, E.; Goeminne, P.C.; McDonnell, M.J.; Aliberti, S.; Marshall, S.E.; Loebinger, M.R.; Murris-Espin, M.; Cantón, R.; Torres, A.; Dimakou, K.; et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur. Respir. J. 2017, 50, 1700629. [Google Scholar] [CrossRef] [PubMed]
- Griffith, D.E.; Aksamit, T.; Brown-Elliott, B.A.; Catanzaro, A.; Daley, C.; Gordin, F.; Holland, S.M.; Horsburgh, R.; Huitt, G.; Iademarco, M.F.; et al. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 2007, 175, 367–416. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.-J.; Moon, S.M.; Kim, S.-Y.; Woo, M.-A.; Kim, S.; Jhun, B.W.; Park, H.Y.; Jeon, K.; Huh, H.J.; Ki, C.-S.; et al. Outcomes of Mycobacterium avium complex lung disease based on clinical phenotype. Eur. Respir. J. 2017, 50, 1602503. [Google Scholar] [CrossRef]
- Miura, K.; Nakamura, M.; Taooka, Y.; Hotta, T.; Hamaguchi, M.; Okimoto, T.; Tsubata, Y.; Hamaguchi, S.; Kuraki, T.; Isobe, T. Comparison of the chest computed tomography findings between patients with pulmonary tuberculosis and those with Mycobacterium avium complex lung disease. Respir. Investig. 2020, 58, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.R.; Lee, J.; Choi, S.M.; Seong, M.W.; Kim, S.A.; Kim, M.; Chae, K.O.; Lee, J.S.; Yim, J.-J. Phenotypic, immunologic, and clinical characteristics of patients with nontuberculous mycobacterial lung disease in Korea. BMC Infect. Dis. 2013, 13, 1–7. [Google Scholar] [CrossRef]
- Naidich, D.P.; McCauley, D.I.; Khouri, N.F.; Stitik, F.P.; Siegelman, S.S. Computed tomography of bronchiectasis. J. Comput. Assist. Tomogr. 1982, 6, 437–444. [Google Scholar] [CrossRef]
- Reiff, D.B.; Wells, A.U.; Carr, D.H.; Cole, P.J.; Hansell, D.M. CT findings in bronchiectasis: Limited value in distinguishing between idiopathic and specific types. Am. J. Roentgenol. 1995, 165, 261–267. [Google Scholar] [CrossRef]
- Reid, L.M. Reduction in bronchial subdivision in bronchiectasis. Thorax 1950, 5, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, K.S.; Koh, W.J.; Jeon, K.; Lee, E.J.; Kang, H.; Ahn, J. Serial CT findings of Mycobacterium massiliense pulmonary disease compared with Mycobacterium abscessus disease after treatment with antibiotic therapy. Radiology 2012, 263, 260–270. [Google Scholar] [CrossRef]
- Hansell, D.M.; Bankier, A.A.; MacMahon, H.; McLoud, T.C.; Müller, N.L.; Remy, J. Fleischner Society: Glossary of terms for thoracic imaging. Radiology 2008, 246, 697–722. [Google Scholar] [CrossRef] [PubMed]
- Lonni, S.; Chalmers, J.D.; Goeminne, P.C.; McDonnell, M.J.; Dimakou, K.; De Soyza, A.; Polverino, E.; van de Kerkhove, C.; Rutherford, R.; Davison, J.; et al. Etiology of Non-Cystic Fibrosis Bronchiectasis in Adults and Its Correlation to Disease Severity. Ann. Am. Thorac. Soc. 2015, 12, 1764–1770. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, J.; Dettmer, S.; Fuge, J.; Vogel-Claussen, J.; Shin, H.-O.; Shah, A.; Pedro, P.I.; Wilson, R.; Welte, T.; Wacker, F.; et al. The Primary Ciliary Dyskinesia Computed Tomography Score in Adults with Bronchiectasis: A Derivation und Validation Study. Respiration 2021, 100, 499–509. [Google Scholar] [CrossRef]
- Lee, P.H.; Carr, D.H.; Rubens, M.B.; Cole, P.; Hansell, D.M. Accuracy of CT in predicting the cause of bronchiectasis. Clin. Radiol. 1995, 50, 839–841. [Google Scholar] [CrossRef]
- Cartier, Y.; Kavanagh, P.V.; Johkoh, T.; Mason, A.C.; Müller, N.L. Bronchiectasis: Accuracy of high-resolution CT in the differentiation of specific diseases. AJR Am. J. Roentgenol. 1999, 173, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Dos Anjos, L.R.B.; Parreira, P.L.; Torres, P.P.T.S.; Kipnis, A.; Junqueira-Kipnis, A.P.; Rabahi, M.F. Non-tuberculous mycobacterial lung disease: A brief review focusing on radiological findings. Rev. Soc. Bras. Med. Trop. 2020, 53, e20200241. [Google Scholar] [CrossRef]
- Eisenberg, I.; Yasin, A.; Fuks, L.; Stein, N.; Saliba, W.; Kramer, M.R.; Adir, Y.; Shteinberg, M. Radiologic Characteristics of Non-tuberculous Mycobacteria Infection in Patients with Bronchiectasis. Lung 2020, 198, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Levy, H.; Kalish, L.A.; Cannon, C.L.; EdM, K.C.G.; Gerard, C.; Goldmann, D.; Pier, G.; Weiss, S.T.; Colin, A. Predictors of mucoid Pseudomonas colonization in cystic fibrosis patients. Pediatr. Pulmonol. 2008, 43, 463–471. [Google Scholar] [CrossRef]
- Mehta, M.; Marras, T.K. Impaired health-related quality of life in pulmonary nontuberculous mycobacterial disease. Respir. Med. 2011, 105, 1718–1725. [Google Scholar] [CrossRef]
- Khan, Z.; Miller, A.; Bachan, M.; Donath, J. Mycobacterium Avium Complex (MAC) Lung Disease in Two Inner City Community Hospitals: Recognition, Prevalence, Co-Infection with Mycobacterium Tuberculosis (MTB) and Pulmonary Function (PF) Improvements After Treatment. Open Respir. Med. J. 2010, 4, 76. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Daley, C.L.; Iaccarino, J.M.; Lange, C.; Cambau, E.; Wallace, R.J., Jr.; Andrejak, C.; Böttger, E.C.; Brozek, J.; Griffith, D.E.; Guglielmetti, L.; et al. Treatment of Nontuberculous Mycobacterial Pulmonary Disease: An Official ATS/ERS/ESCMID/IDSA Clinical Practice Guideline. Clin. Infect. Dis. 2020, 71, e1–e36. [Google Scholar] [CrossRef] [PubMed]
- Van Ingen, J.; Aksamit, T.; Andrejak, C.; Böttger, E.C.; Cambau, E.; Daley, C.L.; Griffith, D.E.; Guglielmetti, L.; Holland, S.M.; Huitt, G.A.; et al. Treatment outcome definitions in nontuberculous mycobacterial pulmonary disease: An NTM-NET consensus statement. Eur. Respir. J. 2018, 51, 1800170. [Google Scholar] [CrossRef]
- Kitada, S.; Uenami, T.; Yoshimura, K.; Tateishi, Y.; Miki, K.; Miki, M.; Hashimoto, H.; Fujikawa, T.; Mori, M.; Matsuura, K.; et al. Long-term radiographic outcome of nodular bronchiectatic Mycobacterium avium complex pulmonary disease. Int. J. Tuberc. Lung Dis. 2012, 16, 660–664. [Google Scholar] [CrossRef]
- Choi, H.; Cha, M.J.; Kim, Y.S.; Choi, J.C. High-Resolution CT Findings as Predictive Factors for Recurrent Nontuberculous Mycobacterial Pulmonary Disease after Successful Treatment. J. Clin. Med. 2021, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.S.; Koh, W.-J.; Han, J.; Chung, M.J.; Lee, J.H.; Lee, K.S.; Kwon, O.J. Hypothesis on the evolution of cavitary lesions in nontuberculous mycobacterial pulmonary infection: Thin-section CT and histopathologic correlation. Am. J. Roentgenol. 2005, 184, 1247–1252. [Google Scholar] [CrossRef]
- American Association of Physicists in Medicine. Adult Routine Chest CT Protocols Version 2.0. 2016. Available online: http://www.aapm.org/pubs/CTProtocols/documents/AdultRoutineChestCT.pdf (accessed on 22 May 2016).
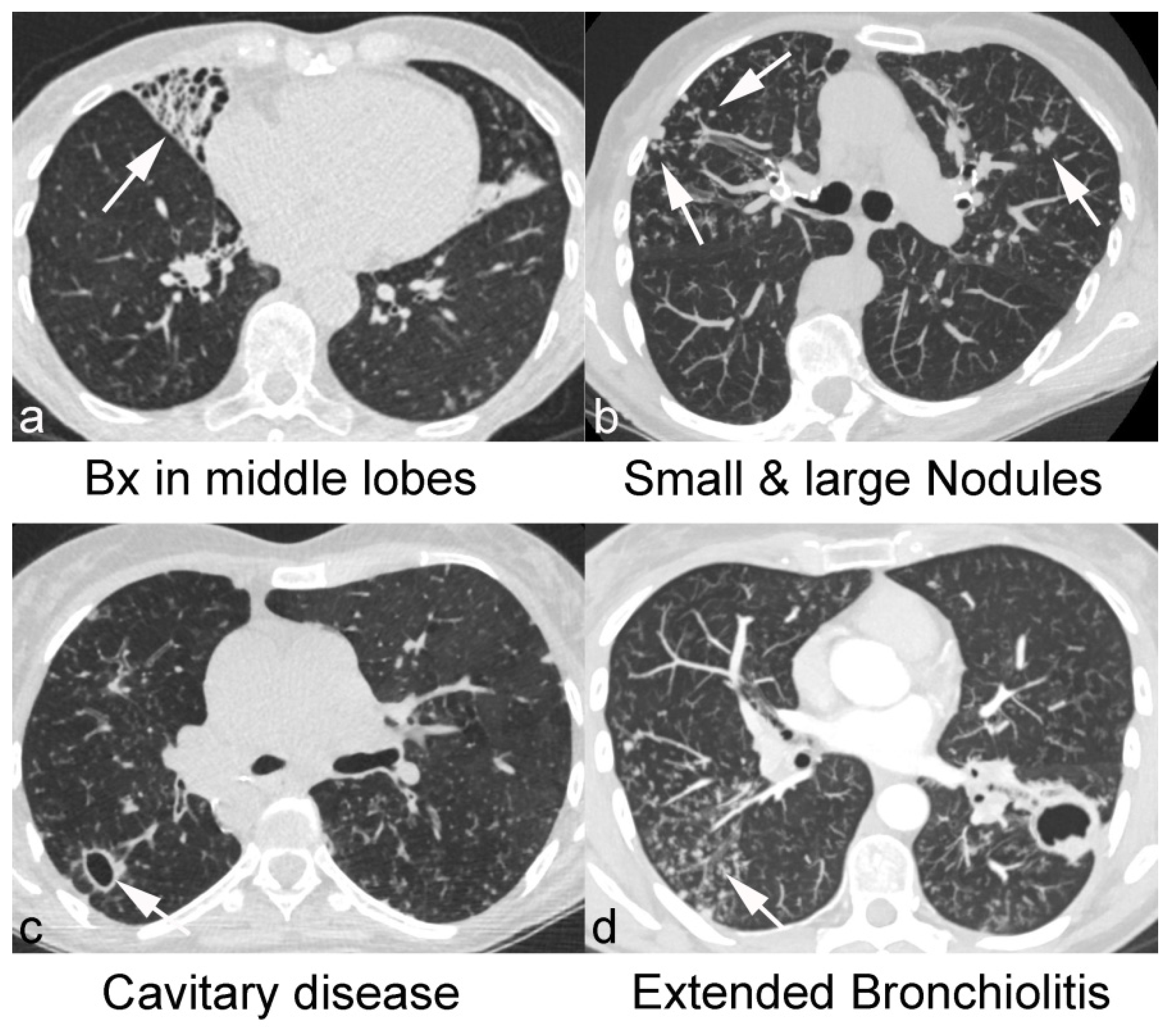
| Characteristics | Non-NTM (n = 92) | NTM (n = 36) | |
|---|---|---|---|
| Sex-n (%) | Male | 40 (43) | 10 (28) |
| Female | 52 (57) | 26 (72) | |
| Age at CT–Median (IQR) | 50 (33–64) | 65 (61–75) | |
| Aetiology-n (%) | Idiopathic | 26 (28.3) | 8 (22.2) |
| PCD/Kartagener | 11 (12) | 0 | |
| Asthma/ABPA | 10 (10.8) | 0 | |
| Immunodeficiency | 8 (8.7) | 1 (2.8) | |
| COPD/A1AT | 8 (8.7) | 0 | |
| Postinfectious | 4 (4.3) | 3 (8.3) | |
| CFTR-related disorder | 4 (4.3) | 1 (2.8) | |
| GvHD | 3 (3.3) | 0 | |
| Others | 7 (7.6) | 0 | |
| NTM-PD | 23 (63.9) | ||
| Sputum microbiology-(%) | MAC | 32 (88.9) | |
| M. abscessus subsp. abscessus | 2 (5.6) | ||
| M. kansasii | 2 (5.6) | ||
| Pseudomonas aeruginosa | 26 (28.2) | 6 (16.7) | |
| Aspergillus sp. | 17 (18.5) | 4 (11.1) | |
| Feature | OVERALL | NTM | Non NTM | Group Comparison * | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | Median | n | Mean | Median | n | Mean | Median | p-Value | ||
| Involvement | right upper lobe | 128 | 1.07 | 1.00 | 36 | 1.25 | 1.00 | 92 | 1.00 | 1.00 | 0.172 |
| middle lobe | 120 | 1.44 | 2.00 | 35 | 1.54 | 2.00 | 85 | 1.40 | 1.00 | 0.336 | |
| right lower lobe | 126 | 1.44 | 2.00 | 36 | 1.25 | 1.50 | 90 | 1.51 | 2.00 | 0.108 | |
| left upper lobe | 127 | 0.85 | 1.00 | 36 | 0.97 | 1.00 | 91 | 0.80 | 0.00 | 0.347 | |
| lingula | 126 | 1.17 | 1.00 | 36 | 1.36 | 2.00 | 90 | 1.10 | 1.00 | 0.119 | |
| left lower lobe | 122 | 1.36 | 2.00 | 36 | 1.17 | 1.50 | 86 | 1.44 | 2.00 | 0.142 | |
| Bronchial dilatation | right upper lobe | 128 | 1.09 | 1.00 | 36 | 1.19 | 1.00 | 92 | 1.05 | 1.00 | 0.298 |
| middle lobe | 119 | 1.54 | 1.00 | 35 | 1.66 | 2.00 | 84 | 1.49 | 1.00 | 0.395 | |
| right lower lobe | 126 | 1.38 | 1.00 | 36 | 1.00 | 1.00 | 90 | 1.53 | 1.00 | 0.007 | |
| left upper lobe | 127 | 0.76 | 0.00 | 36 | 0.78 | 1.00 | 91 | 0.75 | 0.00 | 0.507 | |
| lingula | 125 | 1.27 | 1.00 | 36 | 1.33 | 1.00 | 89 | 1.25 | 1.00 | 0.662 | |
| left lower lobe | 122 | 1.37 | 1.00 | 36 | 0.89 | 1.00 | 86 | 1.57 | 2.00 | 0.001 | |
| Bronchial wall thickening | right upper lobe | 127 | 0.54 | 0.00 | 36 | 0.56 | 0.00 | 91 | 0.54 | 0.00 | 0.810 |
| middle lobe | 115 | 0.80 | 1.00 | 32 | 0.81 | 1.00 | 83 | 0.80 | 1.00 | 0.802 | |
| right lower lobe | 123 | 0.97 | 1.00 | 36 | 0.56 | 0.00 | 87 | 1.14 | 1.00 | <0.001 | |
| left upper lobe | 127 | 0.33 | 0.00 | 36 | 0.42 | 0.00 | 91 | 0.30 | 0.00 | 0.369 | |
| lingula | 122 | 0.66 | 1.00 | 33 | 0.67 | 1.00 | 89 | 0.65 | 1.00 | 0.924 | |
| left lower lobe | 121 | 0.90 | 1.00 | 36 | 0.42 | 0.00 | 85 | 1.11 | 1.00 | <0.001 | |
| Overall (N = 128) | NTM (N = 36) | Non-NTM (N = 92) | p-Value * | ||
|---|---|---|---|---|---|
| Lobar distribution | widespread | 25 (20) | 4 (11) | 21 (23) | 0.214 |
| predominantly upper | 10 (8) | 3 (8) | 7 (8) | 1.000 | |
| predominantly middle | 32 (25) | 20 (56) | 12 (13) | <0.001 | |
| predominantly lower | 60 (47) | 9 (25) | 51 (55) | 0.002 | |
| NE | 1 (1) | 0 (0) | 1 (1) | ||
| Symmetry | symmetric | 108 (84) | 28 (78) | 80 (87) | 0.008 |
| asymmetric | 20 (16) | 8 (22) | 12 (13) | 0.013 | |
| Site | central | 69 (54) | 1 (3) | 68 (74) | <0.001 |
| peripheral | 36 (28) | 26 (72) | 10 (11) | <0.001 | |
| mixed | 21 (16) | 7 (19) | 14 (15) | 0.599 | |
| NE | 2 (2) | 2 (6) | 0 (0) | ||
| Overall (N = 128) | NTM-PD (N = 36) | Non-NTM (N = 92) | Group Comparison p-Value * | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | Median | Mean | Median | Mean | Median | |||
| Cavity | diameter | 0.13 | 0.00 | 0.28 | 0.00 | 0.08 | 0.00 | 0.038 |
| wall thickness | 0.19 | 0.00 | 0.42 | 0.00 | 0.10 | 0.00 | 0.019 | |
| extent | 0.13 | 0.00 | 0.25 | 0.00 | 0.09 | 0.00 | 0.016 | |
| Mucus plugging extent | 1.16 | 1.00 | 1.03 | 1.00 | 1.22 | 1.00 | 0.117 | |
| Bronchiolitis | severity | 1.33 | 2.00 | 1.58 | 2.00 | 1.23 | 2.00 | 0.084 |
| extent | 0.85 | 1.00 | 1.08 | 1.00 | 0.76 | 1.00 | 0.032 | |
| Nodules | small | 0.54 | 0.00 | 1.47 | 2.00 | 0.17 | 0.00 | <0.001 |
| large | 0.17 | 0.00 | 0.53 | 0.50 | 0.03 | 0.00 | <0.001 | |
| Atelectasis extent | 0.78 | 1.00 | 0.89 | 1.00 | 0.74 | 1.00 | 0.241 | |
| Consolidations peripheral extent | 0.53 | 0.00 | 0.53 | 0.00 | 0.53 | 0.00 | 0.916 | |
| Consolidations central extent | 0.05 | 0.00 | 0.03 | 0.00 | 0.05 | 0.00 | 0.524 | |
| Ground-glass peripheral extent | 0.70 | 1.00 | 0.42 | 0.00 | 0.80 | 1.00 | 0.003 | |
| Ground-glass central extent | 0.17 | 0.00 | 0.08 | 0.00 | 0.21 | 0.00 | 0.116 | |
| Interlobular thickening extent | 0.57 | 0.00 | 0.28 | 0.00 | 0.68 | 1.00 | 0.001 | |
| Intralobular lines extent | 0.52 | 0.00 | 0.11 | 0.00 | 0.67 | 1.00 | <0.001 | |
| Baseline Mean (Median) | Follow-Up Mean (Median) | p-Value * | ||
|---|---|---|---|---|
| Sum | 10.92 (10.50) | 8.67 (10.00) | 0.073 | |
| Bronchiectasis | sum | 4.92 (5.00) | 4.92 (5.50) | 1.000 |
| severity | 2.25 (3.00) | 2.25 (3.00) | 1.000 | |
| extent | 1.75 (1.50) | 1.83 (2.00) | 0.317 | |
| Mucus plugging | 0.92 (1.00) | 0.83 (1.00) | 0.317 | |
| Cavity | sum | 1.33 (0.00) | 0.75 (0.00) | 0.102 |
| diameter | 0.42 (0.00) | 0.25 (0.00) | 0.317 | |
| wall thickness | 0.67 (0.00) | 0.33 (0.00) | 0.102 | |
| extent | 0.25 (0.00) | 0.17 (0.00) | 0.317 | |
| Bronchiolitis | sum | 2.75 (2.50) | 1.33 (0.00) | 0.045 |
| severity | 1.58 (1.50) | 0.83 (0.00) | 0.071 | |
| extent | 1.17 (1.00) | 0.50 (0.00) | 0.038 | |
| Nodules | 1.42 (1.50) | 1.33 (1.50) | 0.564 | |
| Consolidation | 0.50 (0.50) | 0.33 (0.00) | 0.157 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dettmer, S.; Ringshausen, F.C.; Fuge, J.; Maske, H.L.; Welte, T.; Wacker, F.; Rademacher, J. Computed Tomography in Adults with Bronchiectasis and Nontuberculous Mycobacterial Pulmonary Disease: Typical Imaging Findings. J. Clin. Med. 2021, 10, 2736. https://doi.org/10.3390/jcm10122736
Dettmer S, Ringshausen FC, Fuge J, Maske HL, Welte T, Wacker F, Rademacher J. Computed Tomography in Adults with Bronchiectasis and Nontuberculous Mycobacterial Pulmonary Disease: Typical Imaging Findings. Journal of Clinical Medicine. 2021; 10(12):2736. https://doi.org/10.3390/jcm10122736
Chicago/Turabian StyleDettmer, Sabine, Felix C. Ringshausen, Jan Fuge, Hannah Louise Maske, Tobias Welte, Frank Wacker, and Jessica Rademacher. 2021. "Computed Tomography in Adults with Bronchiectasis and Nontuberculous Mycobacterial Pulmonary Disease: Typical Imaging Findings" Journal of Clinical Medicine 10, no. 12: 2736. https://doi.org/10.3390/jcm10122736
APA StyleDettmer, S., Ringshausen, F. C., Fuge, J., Maske, H. L., Welte, T., Wacker, F., & Rademacher, J. (2021). Computed Tomography in Adults with Bronchiectasis and Nontuberculous Mycobacterial Pulmonary Disease: Typical Imaging Findings. Journal of Clinical Medicine, 10(12), 2736. https://doi.org/10.3390/jcm10122736








