Rectus Femoris Mimicking Ultrasound Phantom for Muscle Mass Assessment: Design, Research, and Training Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Settings
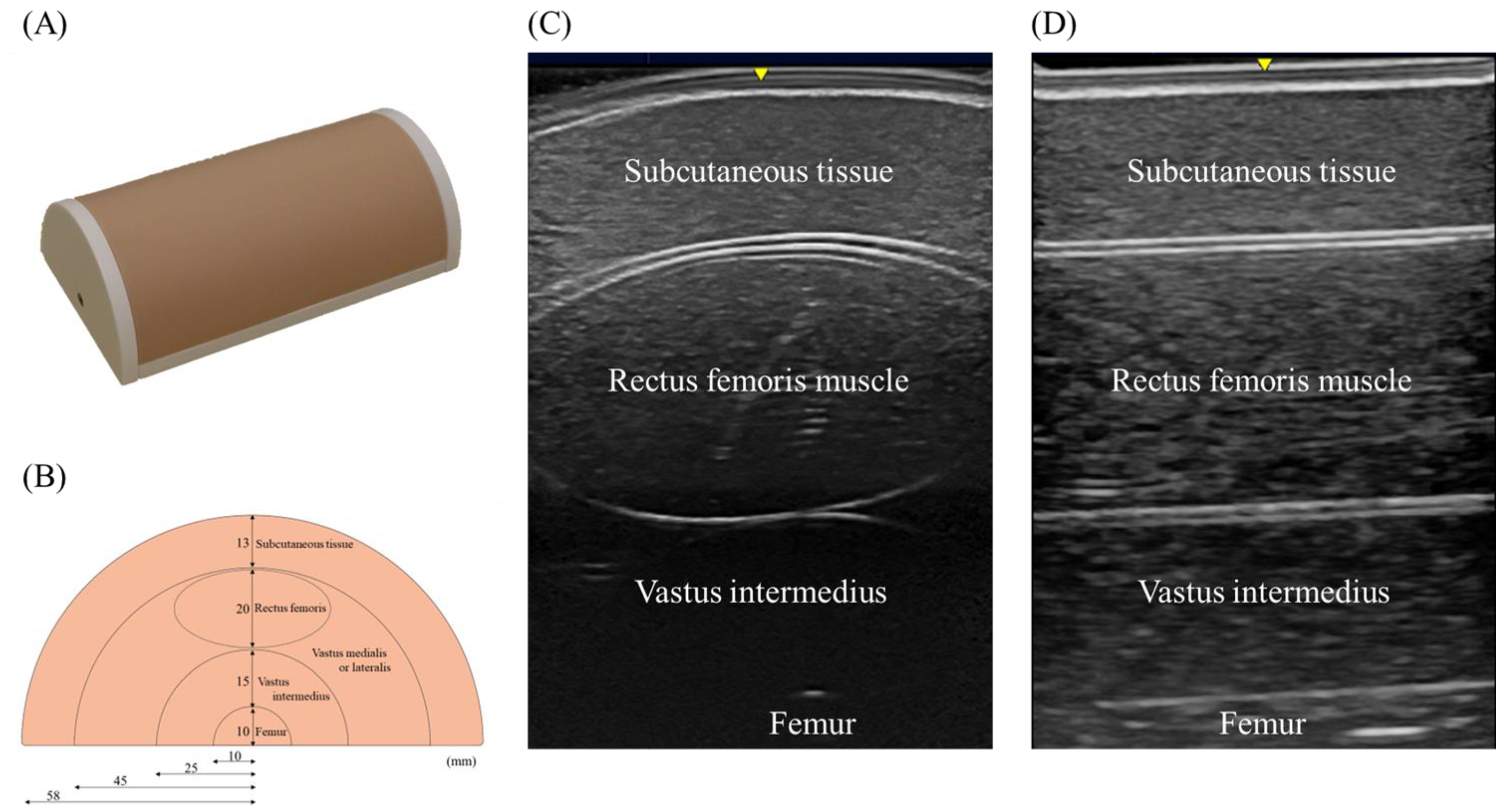
2.2. Phantom Model
2.3. Study 1: Research Application
2.4. Study 2-1: Training Purpose In Vitro
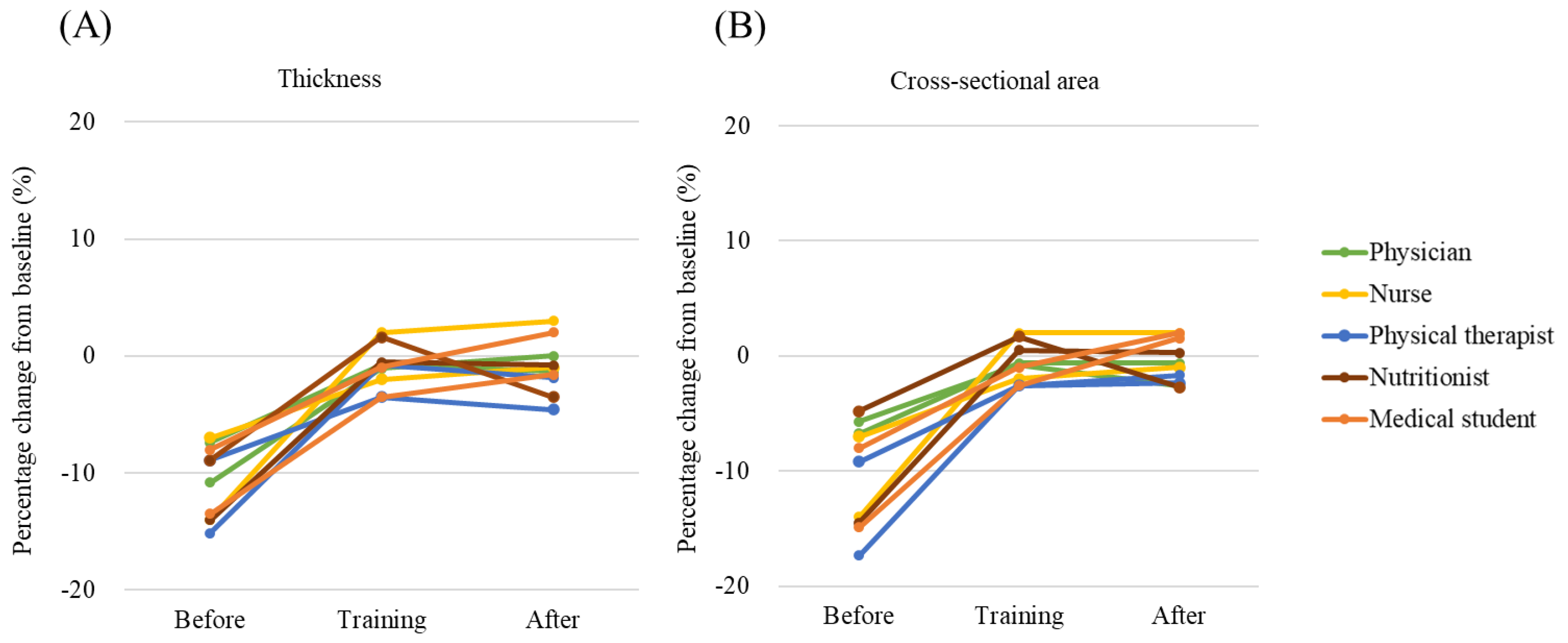
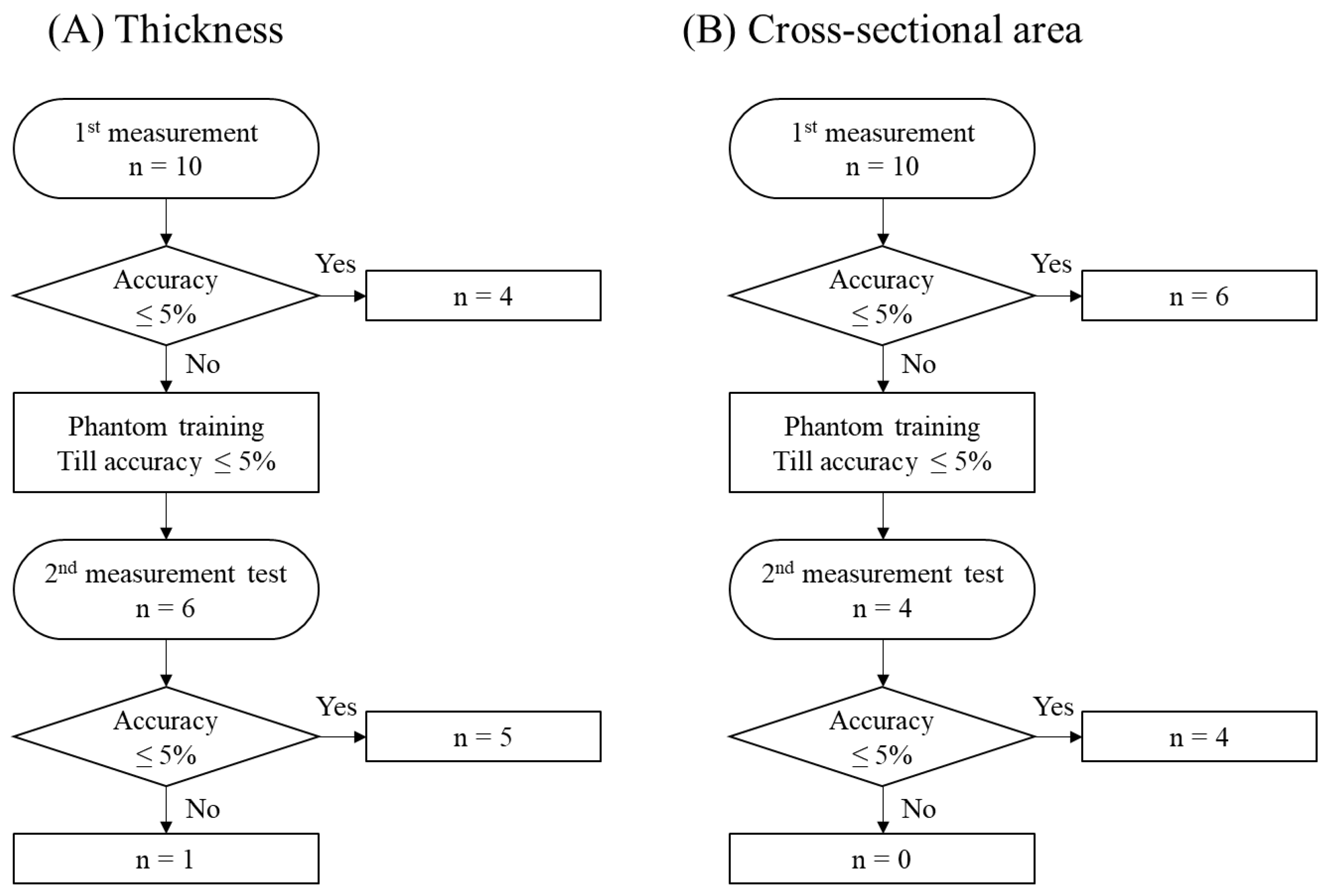
2.5. Study 2-2: Training Purpose In Vivo
2.6. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, P.; Hao, Q.; Hai, S.; Wang, H.; Cao, L.; Dong, B. Sarcopenia as a predictor of all-cause mortality among community-dwelling older people: A systematic review and meta-analysis. Maturitas 2017, 103, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Jaitovich, A.; Dumas, C.L.; Itty, R.; Chieng, H.C.; Khan, M.M.H.S.; Naqvi, A.; Fantauzzi, J.; Hall, J.B.; Feustel, P.J.; Judson, M.A. ICU admission body composition: Skeletal muscle, bone, and fat effects on mortality and disability at hospital discharge—A prospective, cohort study. Crit. Care 2020, 24, 566. [Google Scholar] [CrossRef]
- Nakanishi, N.; Oto, J.; Tsutsumi, R.; Akimoto, Y.; Nakano, Y.; Nishimura, M. Upper limb muscle atrophy associated with in-hospital mortality and physical function impairments in mechanically ventilated critically ill adults: A two-center prospective observational study. J. Intensive Care 2020, 8, 87. [Google Scholar] [CrossRef]
- Lee, Z.Y.; Ong, S.P.; Ng, C.C.; Yap, C.S.L.; Engkasan, J.P.; Barakatun-Nisak, M.Y.; Heyland, D.K.; Hasan, M.S. Association between ultrasound quadriceps muscle status with premorbid functional status and 60-day mortality in mechanically ventilated critically ill patient: A single-center prospective observational study. Clin. Nutr. 2020, 40, 1338–1347. [Google Scholar] [CrossRef]
- Dos Santos, C.; Hussain, S.N.; Mathur, S.; Picard, M.; Herridge, M.; Correa, J.; Bain, A.; Guo, Y.; Advani, A.; Advani, S.L.; et al. Mechanisms of chronic muscle wasting and dysfunction after an intensive care unit stay. a pilot study. Am. J. Respir. Crit. Care Med. 2016, 194, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.S.; Mourtzakis, M.; Aronson Friedman, L.; Dinglas, V.D.; Hough, C.L.; Ely, E.W.; Morris, P.E.; Hopkins, R.O.; Needham, D.M. Evaluating muscle mass in survivors of acute respiratory distress syndrome: A 1-year multicenter longitudinal study. Crit. Care Med. 2018, 46, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Dong, V.; Karvellas, C.J. Using technology to assess nutritional status and optimize nutrition therapy in critically ill patients. Curr. Opin. Clin. Nutr. Metab. Care 2020, 24, 189–194. [Google Scholar] [CrossRef]
- Nakanishi, N.; Tsutsumi, R.; Okayama, Y.; Takashima, T.; Ueno, Y.; Itagaki, T.; Tsutsumi, Y.; Sakaue, H.; Oto, J. Monitoring of muscle mass in critically ill patients: Comparison of ultrasound and two bioelectrical impedance analysis devices. J. Intensive Care 2019, 7, 61. [Google Scholar] [CrossRef]
- Mayer, K.P.; Thompson Bastin, M.L.; Montgomery-Yates, A.A.; Pastva, A.M.; Dupont-Versteegden, E.E.; Parry, S.M.; Morris, P.E. Acute skeletal muscle wasting and dysfunction predict physical disability at hospital discharge in patients with critical illness. Crit. Care 2020, 24, 637. [Google Scholar] [CrossRef]
- Lambell, K.J.; Tierney, A.C.; Wang, J.C.; Nanjayya, V.; Forsyth, A.; Goh, G.S.; Vicendese, D.; Ridley, E.J.; Parry, S.M.; Mourtzakis, M.; et al. Comparison of ultrasound-derived muscle thickness with computed tomography muscle cross-sectional area on admission to the intensive care unit: A pilot cross-sectional study. JPEN J. Parenter. Enteral Nutr. 2020, 45, 136–145. [Google Scholar] [CrossRef]
- Parry, S.M.; El-Ansary, D.; Cartwright, M.S.; Sarwal, A.; Berney, S.; Koopman, R.; Annoni, R.; Puthucheary, Z.; Gordon, I.R.; Morris, P.E.; et al. Ultrasonography in the intensive care setting can be used to detect changes in the quality and quantity of muscle and is related to muscle strength and function. J. Crit. Care 2015, 30, 1151. [Google Scholar] [CrossRef]
- Patel, S.; Bear, D.; Patel, B.; Puthucheary, Z. Clinical application of ultrasound in intensive care unit-acquired weakness. Ultraschall Med. 2020, 41, 244–266. [Google Scholar] [CrossRef]
- Wu, M.; Peng, L.; Donroe, J.H.; Kohler, M.J.; Wang, L.; Zeng, X.; Li, M.; Hsieh, E. Musculoskeletal ultrasound imaging training, use, and knowledge among rheumatologists in China. Clin. Rheumatol. 2021, 40, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Alves, N.; Kim, A.; Tan, J.; Hwang, G.; Javed, T.; Neagu, B.; Courtney, B.K. Cardiac tissue-mimicking ballistic gel phantom for ultrasound imaging in clinical and research applications. Ultrasound Med. Biol. 2020, 46, 2057–2069. [Google Scholar] [CrossRef]
- Uetake, C.; Nakamoto, A.; Suda, T.; Tamano, M. Abdominal ultrasound examination training using an ultrasound phantom and volume navigation system. J. Med. Ultrason. 2016, 43, 381–386. [Google Scholar] [CrossRef]
- Chatelin, S.; Bernal, M.; Deffieux, T.; Papadacci, C.; Flaud, P.; Nahas, A.; Boccara, C.; Gennisson, J.L.; Tanter, M.; Pernot, M. Anisotropic polyvinyl alcohol hydrogel phantom for shear wave elastography in fibrous biological soft tissue: A multimodality characterization. Phys. Med. Biol. 2014, 59, 6923–6940. [Google Scholar] [CrossRef] [PubMed]
- Aristizabal, S.; Amador, C.; Qiang, B.; Kinnick, R.R.; Nenadic, I.Z.; Greenleaf, J.F.; Urban, M.W. Shear wave vibrometry evaluation in transverse isotropic tissue mimicking phantoms and skeletal muscle. Phys. Med. Biol. 2014, 59, 7735–7752. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.C.; Villegas, L.; Jones, D.B. Basic ultrasound curriculum for medical students: Validation of content and phantom. J. Laparoendosc. Adv. Surg. Tech. A 2004, 14, 374–379. [Google Scholar] [CrossRef]
- Hadda, V.; Kumar, R.; Hussain, T.; Khan, M.A.; Madan, K.; Mohan, A.; Khilnani, G.C.; Guleria, R. Reliability of ultrasonographic arm muscle thickness measurement by various levels of health care providers in ICU. Clin. Nutr. ESPEN 2018, 24, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Palakshappa, J.A.; Bakhru, R.N. Bedside ultrasonography can and should be used in the ICU to evaluate muscle atrophy. Ann. Am. Thorac. Soc. 2019, 16, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Puthucheary, Z.A.; McNelly, A.S.; Rawal, J.; Connolly, B.; Sidhu, P.S.; Rowlerson, A.; Moxham, J.; Harridge, S.D.; Hart, N.; Montgomery, H.E. Rectus femoris cross-sectional area and muscle layer thickness: Comparative markers of muscle wasting and weakness. Am. J. Respir. Crit. Care Med. 2017, 195, 136–138. [Google Scholar] [CrossRef]
- Palakshappa, J.A.; Reilly, J.P.; Schweickert, W.D.; Anderson, B.J.; Khoury, V.; Shashaty, M.G.; Fitzgerald, D.; Forker, C.; Butler, K.; Ittner, C.A.; et al. Quantitative peripheral muscle ultrasound in sepsis: Muscle area superior to thickness. J. Crit. Care 2018, 47, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Harris-Love, M.O.; Monfaredi, R.; Ismail, C.; Blackman, M.R.; Cleary, K. Quantitative ultrasound: Measurement considerations for the assessment of muscular dystrophy and sarcopenia. Front. Aging Neurosci. 2014, 6, 172. [Google Scholar] [CrossRef] [PubMed]
- Ishida, H.; Watanabe, S. Influence of inward pressure of the transducer on lateral abdominal muscle thickness during ultrasound imaging. J. Orthop. Sports Phys. Ther. 2012, 42, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, J.L.; Warner, M.B.; Stokes, M.J. Induced transducer orientation during ultrasound imaging: Effects on abdominal muscle thickness and bladder position. Ultrasound Med. Biol. 2009, 35, 1803–1811. [Google Scholar] [CrossRef]
- Hacker, E.D.; Peters, T.; Garkova, M. Ultrasound assessment of the rectus femoris cross-sectional area: Subject position implications. West J. Nurs. Res. 2016, 38, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, N.; Takashima, T.; Oto, J. Muscle atrophy in critically ill patients: A review of its cause, evaluation, and prevention. J. Med. Investig. 2020, 67, 1–10. [Google Scholar] [CrossRef]
- Taniguchi, M.; Yamada, Y.; Fukumoto, Y.; Sawano, S.; Minami, S.; Ikezoe, T.; Watanabe, Y.; Kimura, M.; Ichihashi, N. Increase in echo intensity and extracellular-to-intracellular water ratio is independently associated with muscle weakness in elderly women. Eur. J. Appl. Physiol 2017, 117, 2001–2007. [Google Scholar] [CrossRef]
- Pillen, S.; Tak, R.O.; Zwarts, M.J.; Lammens, M.M.; Verrijp, K.N.; Arts, I.M.; van der Laak, J.A.; Hoogerbrugge, P.M.; van Engelen, B.G.; Verrips, A. Skeletal muscle ultrasound: Correlation between fibrous tissue and echo intensity. Ultrasound Med. Biol. 2009, 35, 443–446. [Google Scholar] [CrossRef]
- Puthucheary, Z.A.; Phadke, R.; Rawal, J.; McPhail, M.J.; Sidhu, P.S.; Rowlerson, A.; Moxham, J.; Harridge, S.; Hart, N.; Montgomery, H.E. Qualitative ultrasound in acute critical illness muscle wasting. Crit. Care Med. 2015, 43, 1603–1611. [Google Scholar] [CrossRef]
- Harris-Love, M.O.; Ismail, C.; Monfaredi, R.; Hernandez, H.J.; Pennington, D.; Woletz, P.; McIntosh, V.; Adams, B.; Blackman, M.R. Interrater reliability of quantitative ultrasound using force feedback among examiners with varied levels of experience. PeerJ 2016, 4, e2146. [Google Scholar] [CrossRef] [PubMed]
- Pillen, S.; van Dijk, J.P.; Weijers, G.; Raijmann, W.; de Korte, C.L.; Zwarts, M.J. Quantitative gray-scale analysis in skeletal muscle ultrasound: A comparison study of two ultrasound devices. Muscle Nerve 2009, 39, 781–786. [Google Scholar] [CrossRef] [PubMed]
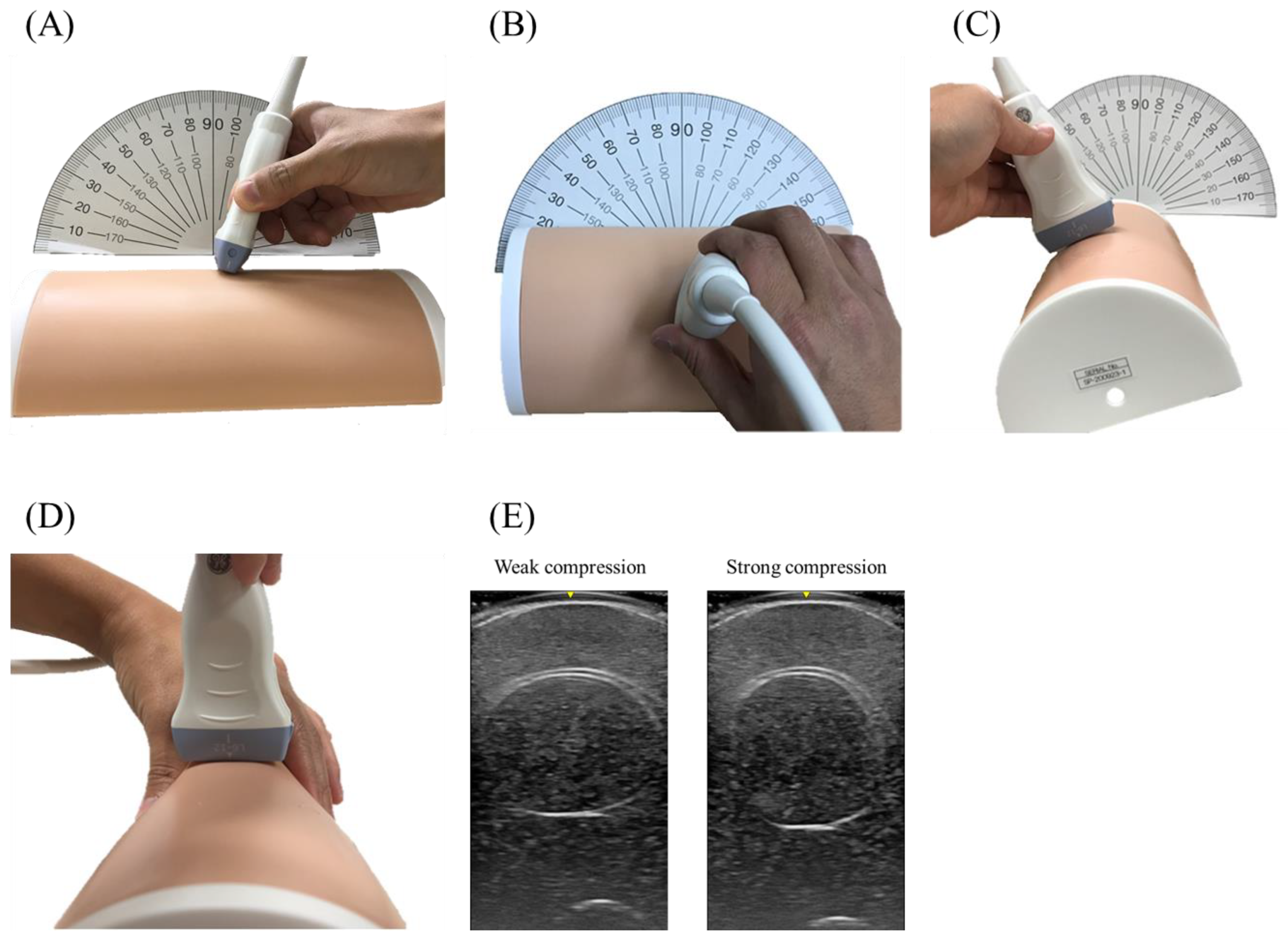
| Point | Description |
|---|---|
| 1 | Generous amount of gel to avoid compression |
| 2 | No probe compression |
| 3 | Perpendicular vertical and horizontal probe position |
| 4 | No knee flexion (No side compression of phantom) |
| 5 | No leg rotation (No rotation of phantom) |
| 6 | Stabilized position till image capture |
| Thickness | Cross-Sectional Area | ||||||
|---|---|---|---|---|---|---|---|
| cm | % | p | cm2 | % | p | ||
| Baseline | 3.70 ± 0.01 | 7.74 ± 0.06 | |||||
| (1) Probe compression strength | 0.5 cm | 3.59 ± 0.03 | −3.0 ± 1.0 | <0.01 | 7.58 ± 0.12 | −2.0 ± 1.4 | 0.03 |
| 1.0 cm | 3.45 ± 0.03 | −6.9 ± 0.7 † | <0.01 | 7.43 ± 0.12 | −4.0 ± 1.8 † | <0.01 | |
| (2) Pennation angle change | 10° | 3.76 ± 0.03 | 1.6 ± 0.9 * | <0.01 | 8.00 ± 0.11 | 3.4 ± 1.0 * | <0.01 |
| 20° | 3.81 ± 0.03 | 2.8 ± 0.9 † | <0.01 | 8.21 ± 0.10 | 6.0 ± 1.5 † | <0.01 | |
| (3) Horizontal angle change | 10° | 3.70 ± 0.02 | −0.1 ± 0.5 * | 0.86 | 7.95 ± 0.14 | 2.7 ± 1.9 * | 0.02 |
| 20° | 3.69 ± 0.02 | −0.4 ± 0.8 † | 0.15 | 8.13 ± 0.15 | 5.0 ± 1.8 † | <0.01 | |
| (4) Rotation of phantom | 20° | 3.69 ± 0.02 | −0.4 ± 0.7 | 0.28 | 7.67 ± 0.13 | −0.9 ± 1.7 | 0.30 |
| 40° | 3.70 ± 0.03 | −0.1 ± 1.2 | 0.90 | 7.76 ± 0.25 | 0.2 ± 3.1 | 0.89 | |
| (5) Compression from side | weak | 4.23 ± 0.01 | 14.3 ± 0.5 † | <0.01 | 7.44 ± 0.10 | −3.9 ± 1.1 † | <0.01 |
| strong | 4.55 ± 0.06 | 22.9 ± 1.7 † | <0.01 | 6.94 ± 0.09 | −10.3 ± 1.5 † | <0.01 | |
| (6) Different equipment | Convex probe | 3.66 ± 0.02 | −1.1 ± 0.7 † | <0.01 | 7.87 ± 0.09 | 1.6 ± 0.8 † | 0.04 |
| Different device | 3.70 ± 0.02 | −0.1 ± 1.0 | 0.75 | 7.68 ± 0.10 | −0.8 ± 2.2 | 0.29 | |
| Echogenicity | ||||
|---|---|---|---|---|
| Pixel | % | p | ||
| Baseline | 52.2 ± 0.44 | |||
| (1) Probe compression strength | 0.5 cm | 47.1 ± 2.31 | −9.7 ± 4.6 | <0.01 |
| 1.0 cm | 47.9 ± 1.24 | −8.4 ± 2.4 | <0.01 | |
| (2) Pennation angle change | 10° | 40.1 ± 1.41 | −23.1 ± 2.6 | <0.01 |
| 20° | 37.7 ± 3.00 | −27.7 ± 5.6 | <0.01 | |
| (3) Horizontal angle change | 10° | 51.4 ± 3.42 | −1.6 ± 6.8 | 0.60 |
| 20° | 49.9 ± 1.97 | −4.3 ± 4.1 | 0.04 | |
| (4) Rotation of phantom | 20° | 48.6 ± 0.94 | −6.8 ± 1.8 | <0.01 |
| 40° | 50.0 ± 1.19 | −4.2 ± 1.8 | <0.01 | |
| (5) Compression from side | weak | 37.0 ± 2.21 | −29.1 ± 4.2 | <0.01 |
| strong | 36.9 ± 2.33 | −29.3 ± 4.2 | <0.01 | |
| (6) Different equipment | Convex probe | 57.1 ± 1.74 | 9.3 ± 3.8 | <0.01 |
| Different device | 40.0 ± 3.81 | −23.4 ± 7.4 | <0.01 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakanishi, N.; Inoue, S.; Tsutsumi, R.; Akimoto, Y.; Ono, Y.; Kotani, J.; Sakaue, H.; Oto, J. Rectus Femoris Mimicking Ultrasound Phantom for Muscle Mass Assessment: Design, Research, and Training Application. J. Clin. Med. 2021, 10, 2721. https://doi.org/10.3390/jcm10122721
Nakanishi N, Inoue S, Tsutsumi R, Akimoto Y, Ono Y, Kotani J, Sakaue H, Oto J. Rectus Femoris Mimicking Ultrasound Phantom for Muscle Mass Assessment: Design, Research, and Training Application. Journal of Clinical Medicine. 2021; 10(12):2721. https://doi.org/10.3390/jcm10122721
Chicago/Turabian StyleNakanishi, Nobuto, Shigeaki Inoue, Rie Tsutsumi, Yusuke Akimoto, Yuko Ono, Joji Kotani, Hiroshi Sakaue, and Jun Oto. 2021. "Rectus Femoris Mimicking Ultrasound Phantom for Muscle Mass Assessment: Design, Research, and Training Application" Journal of Clinical Medicine 10, no. 12: 2721. https://doi.org/10.3390/jcm10122721
APA StyleNakanishi, N., Inoue, S., Tsutsumi, R., Akimoto, Y., Ono, Y., Kotani, J., Sakaue, H., & Oto, J. (2021). Rectus Femoris Mimicking Ultrasound Phantom for Muscle Mass Assessment: Design, Research, and Training Application. Journal of Clinical Medicine, 10(12), 2721. https://doi.org/10.3390/jcm10122721








