Retrospective Monocentric Clinical Study on Male Infertility: Comparison between Two Different Therapeutic Schemes Using Follicle-Stimulating Hormone
Abstract
1. Introduction
2. Patients and Methods
2.1. Patient Selection
2.2. Semen Analysis
2.3. Sperm DNA Fragmentation
2.4. Scrotal Ultrasound Evaluation
2.5. Hormonal Measurements
2.6. Statistical Analysis
2.7. Ethical Approval
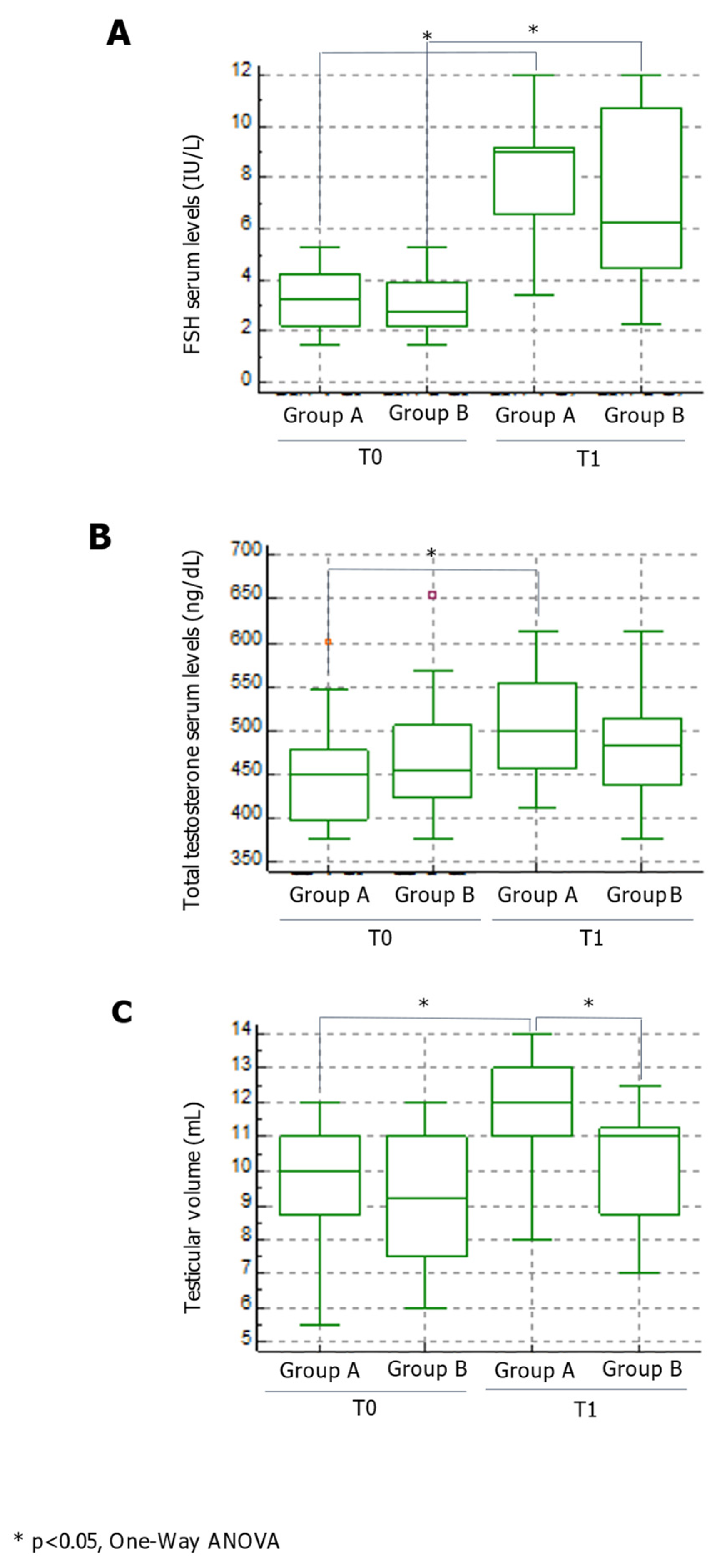
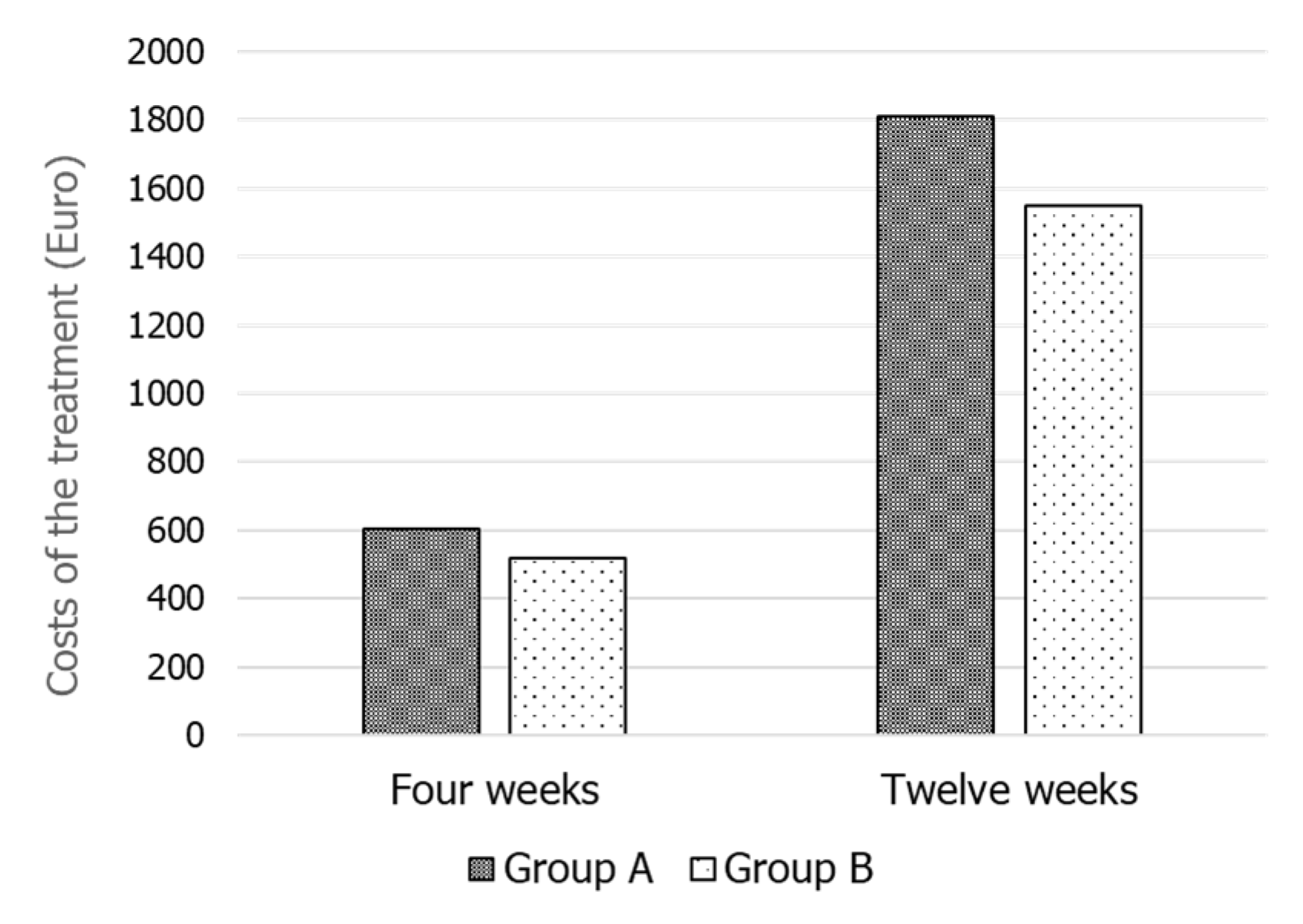
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Limits of the Study
References
- McKenna, S.P.; Whalley, D. Assessment of biotechnology drugs: What are the issues? Health Policy 1999, 49, 195–197. [Google Scholar] [CrossRef]
- Zwart-van Rijkom, J.E.; Broekmans, F.J.; Leufkens, H.G. From HMG through purified urinary FSH preparations to recombinant FSH: A substitution study. Hum. Reprod. 2002, 17, 857–865. [Google Scholar] [CrossRef]
- Cannarella, R.; La Vignera, S.; Condorelli, R.A.; Mongioì, L.M.; Calogero, A.E. FSH dosage effect on conventional sperm parameters: A meta-analysis of randomized controlled studies. Asian J. Androl. 2020, 22, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Arato, I.; Grande, G.; Barrachina, F.; Bellucci, C.; Lilli, C.; Jodar, M.; Aglietti, M.C.; Mancini, F.; Vincenzoni, F.; Pontecorvi, A.; et al. In vitro Effect of Different Follicle-Stimulating Hormone Preparations on Sertoli Cells: Toward a Personalized Treatment for Male Infertility. Front. Endocrinol. 2020, 11, 401. [Google Scholar] [CrossRef]
- Foresta, C.; Bettella, A.; Merico, M.; Garolla, A.; Ferlin, A.; Rossato, M. Use of recombinant human follicle-stimulating hormone in the treatment of male factor infertility. Fertil. Steril. 2002, 77, 238–244. [Google Scholar] [CrossRef]
- Foresta, C.; Bettella, A.; Garolla, A.; Ambrosini, G.; Ferlin, A. Treatment of male idiopathic infertility with recombinant human follicle-stimulating hormone: A prospective, controlled, randomized clinical study. Fertil. Steril. 2005, 84, 654–661. [Google Scholar] [CrossRef]
- Condorelli, R.A.; Calogero, A.E.; Vicari, E.; Mongioi’, L.; Burgio, G.; Cannarella, R.; Giacone, F.; Iacoviello, L.; Morgia, G.; Favilla, V.; et al. Reduced Seminal Concentration of CD45pos Cells after Follicle-Stimulating Hormone Treatment in Selected Patients with Idiopathic Oligoasthenoteratozoospermia. Int. J. Endocrinol. 2014, 2014, 372060. [Google Scholar] [CrossRef] [PubMed]
- Colacurci, N.; Monti, M.G.; Fornaro, F.; Izzo, G.; Izzo, P.; Trotta, C.; Mele, D.; De Franciscis, P. Recombinant human FSH reduces sperm DNA fragmentation in men with idiopathic oligoasthenoteratozoospermia. J. Androl. 2012, 33, 588–593. [Google Scholar] [CrossRef]
- Ruvolo, G.; Roccheri, M.C.; Brucculeri, A.M.; Longobardi, S.; Cittadini, E.; Bosco, L. Lower sperm DNA fragmentation after r-FSH administration in functional hypogonadotropic hypogonadism. J. Assist. Reprod. Genet. 2013, 30, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Santi, D.; Granata, A.R.; Simoni, M. FSH treatment of male idiopathic infertility improves pregnancy rate: A meta-analysis. Endocr. Connect. 2015, 4, R46–R58. [Google Scholar] [CrossRef]
- De Rocco Ponce, M.; Foresta, C.; Rago, R.; Dal Lago, A.; Balercia, G.; Calogero, A.E.; La Vignera, S.; Cosci, I.; Di Nisio, A.; Garolla, A. Use of Biosimilar Follicle-Stimulating Hormone in Asthenozoospermic Infertile Patients: A Multicentric Study. J. Clin. Med. 2020, 9, 1478. [Google Scholar] [CrossRef]
- La Vignera, S.; Condorelli, R.A.; Cannarella, R.; Cimino, L.; Mongioi’, L.; Duca, Y.; Giacone, F.; Calogero, A.E. Testosterone levels after treatment with urofollitropin in infertile patients with idiopathic mild reduction of testicular volume. Endocrine 2019, 66, 381–385. [Google Scholar] [CrossRef]
- Sakamoto, H.; Ogawa, Y.; Yoshida, H. Relationship between testicular volume and testicular function: Comparison of the Prader orchidometric and ultrasonographic measurements in patients with infertility. Asian J. Androl. 2008, 10, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Pilatz, A.; Rusz, A.; Wagenlehner, F.; Weidner, W.; Altinkilic, B. Reference values for testicular volume, epididymal head size and peak systolic velocity of the testicular artery in adult males measured by ultrasonography. Ultraschall Med. 2013, 34, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Barbonetti, A.; Calogero, A.E.; Balercia, G.; Garolla, A.; Krausz, C.; La Vignera, S.; Lombardo, L.; Jannini, E.A.; Maggi, M.; Lenzi, A.; et al. The use of follicle stimulating hormone (FSH) for the treatment of the infertile man: Position statement from the Italian Society of Andrology and Sexual Medicine (SIAMS). J. Endocrinol. Investig. 2018, 41, 1107–1122. [Google Scholar] [CrossRef]
- Foresta, C.; Bettella, A.; Ferlin, A.; Garolla, A.; Rossato, M. Evidence for a stimulatory role of follicle stimulating hormone on the spermatogonial population in adult males. Fertil. Steril. 1998, 69, 636–642. [Google Scholar] [CrossRef]
- Arnaldi, G.; Balercia, G.; Barbatelli, G.; Mantero, F. Effects of long-term treatment with human pure follicle stimulationg hormone on semen parameters and sperm cell ultrastructure in idiopathic oligoasthenozoospermia. Andrologia 2000, 32, 155–161. [Google Scholar] [CrossRef]
- Ben-Rafael, Z.; Farhi, J.; Feldberg, D.; Bartoov, B.; Kovo, M.; Eltes, F.; Ashkenazi, J. Follicle-stimulating hormone treatment for men with idiopathic oligoteratoasthenozoospermia before in-vitro fertilization: The impact on sperm microstructure and fertilization potential. Fertil. Steril. 2000, 73, 24–30. [Google Scholar] [CrossRef]
- Baccetti, B.; Piomboni, P.; Bruni, E.; Capitani, E.; Gambera, L.; Moretti, E.; Sterzik, K.; Strehler, E. Effect of follicle-stimulating hormone on sperm quality and pregnancy rate. Asian J. Androl. 2004, 6, 133–137. [Google Scholar] [PubMed]
- Mancuso, F.; Calvitti, M.; Milardi, D.; Grande, G.; Falabella, G.; Arato, I.; Giovagnoli, S.; Vincenzoni, F.; Mancini, F.; Nastruzzi, C.; et al. Testosterone and FSH modulate Sertoli cell extracellular secretion: Proteomic analysis. Mol. Cell. Endocrinol. 2018, 476, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, D.; Alvarez, J.G. Sperm DNA fragmentation: Mechanisms of origin, impact on reproductive outcome and analysis. Fertil. Steril. 2010, 93, 1027–1036. [Google Scholar] [CrossRef]
- Moustafa, M.H.; Sharma, R.K.; Thornton, J.; Mascha, E.; Abdel Hafez, M.A. Relationship between ROS production, apoptosis and DNA denaturation in spermatozoa from patients examined for infertility. Hum. Reprod. 2004, 19, 129–138. [Google Scholar] [CrossRef]
- Ramos, L.; De Boer, P.; Meuleman, E.J.; Braat, D.D.; Wetzels, A.M. Chromatin condensation and DNA damage of human epididymal spermatozoa in obstructive azoospermia. Reprod. Biomed. Online 2004, 8, 392–397. [Google Scholar] [CrossRef]
- Santi, D.; Spaggiari, G.; Simoni, M. Sperm DNA fragmentation index as a promising predictive tool for male infertility diagnosis and treatment management-meta-analyses. Reprod. Biomed. Online 2018, 37, 315–326. [Google Scholar] [CrossRef]
- Attia, A.M.; Abou-Setta, A.M.; Al-Inany, H.G. Gonadotrophins for idiopathic male factor subfertility. Cochrane Database Syst. Rev. 2013, CD005071. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Q.; Wang, Y.; Li, Y. Whether sperm deoxyribonucleic acid fragmentation has an effect on pregnancy and miscarriage after in vitro fertilization/intracytoplasmic sperm injection: A systematic review and meta-analysis. Fertil. Steril. 2014, 102, 998–1005.e8. [Google Scholar] [CrossRef]
- Condorelli, R.A.; La Vignera, S.; Barbagallo, F.; Alamo, A.; Mongioì, L.M.; Cannarella, R.; Aversa, A.; Calogero, A.E. Bio-functional sperm parameters: Does age matter? Front. Endocrinol. 2020, 11, 558374. [Google Scholar] [CrossRef] [PubMed]
- Iliadou, P.K.; Tsametis, C.; Kaprara, A.; Papadimas, I.; Goulis, D.G. The Sertoli cell: Novel clinical potentiality. Hormones 2015, 14, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Saez, J.M.; Perrard-Sapori, M.H.; Chatelain, P.G.; Tabone, E.; Rivarola, M.A. Paracrine regulation of testicular function. J. Steroid. Biochem. 1987, 27, 317–329. [Google Scholar] [CrossRef]

| Group A (n = 24) | Group B (n = 24) | |
|---|---|---|
| Age (years) | 33.8 ± 6.1 | 34.8 ± 5.2 |
| Body mass index (kg m2 −1) | 25.5 ± 2.3 | 24.3 ± 2.8 |
| Follicle-stimulating hormone (IU L−1) | 3.1 ± 1.2 | 3.0 ± 1.1 |
| Total testosterone (ng L−1) | 452.1 ± 58.4 | 466.2 ± 65.1 |
| Testicular volume (mL) | 9.8 ± 1.6 | 9.2 ± 2.1 |
| Sperm concentration (mil mL−1) | 8.8 ± 2.4 | 9.5 ± 3.2 |
| Total sperm count (mil ejaculate−1) | 20.6 ± 10.5 | 21.6 ± 9.0 |
| Sperm progressive motility (%) | 10.1 ± 5.8 | 11.6 ± 6.3 |
| Sperm morphology (%) | 4.2 ± 2.0 | 3.2 ± 2.1 |
| Sperm vitality (%) | 15.7 ± 8.7 | 16.6 ± 11.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Condorelli, R.A.; Cannarella, R.; Crafa, A.; Barbagallo, F.; Mongioì, L.M.; Aversa, A.; Greco, E.; Calogero, A.E.; La Vignera, S. Retrospective Monocentric Clinical Study on Male Infertility: Comparison between Two Different Therapeutic Schemes Using Follicle-Stimulating Hormone. J. Clin. Med. 2021, 10, 2665. https://doi.org/10.3390/jcm10122665
Condorelli RA, Cannarella R, Crafa A, Barbagallo F, Mongioì LM, Aversa A, Greco E, Calogero AE, La Vignera S. Retrospective Monocentric Clinical Study on Male Infertility: Comparison between Two Different Therapeutic Schemes Using Follicle-Stimulating Hormone. Journal of Clinical Medicine. 2021; 10(12):2665. https://doi.org/10.3390/jcm10122665
Chicago/Turabian StyleCondorelli, Rosita A., Rossella Cannarella, Andrea Crafa, Federica Barbagallo, Laura M. Mongioì, Antonio Aversa, Emanuela Greco, Aldo E. Calogero, and Sandro La Vignera. 2021. "Retrospective Monocentric Clinical Study on Male Infertility: Comparison between Two Different Therapeutic Schemes Using Follicle-Stimulating Hormone" Journal of Clinical Medicine 10, no. 12: 2665. https://doi.org/10.3390/jcm10122665
APA StyleCondorelli, R. A., Cannarella, R., Crafa, A., Barbagallo, F., Mongioì, L. M., Aversa, A., Greco, E., Calogero, A. E., & La Vignera, S. (2021). Retrospective Monocentric Clinical Study on Male Infertility: Comparison between Two Different Therapeutic Schemes Using Follicle-Stimulating Hormone. Journal of Clinical Medicine, 10(12), 2665. https://doi.org/10.3390/jcm10122665












