Phenotypic Features and Genetic Findings in a Cohort of Italian Pseudoxanthoma Elasticum Patients and Update of the Ophthalmologic Evaluation Score
Abstract
1. Introduction
2. Materials and Methods
2.1. PXE Patients and Genetic Analyses
2.2. In Silico Analysis for Pathogenisity Predictions of Rare Sequence Variants
2.3. Ophthalmologic Examinations
2.4. Statistical Analyses
3. Results and Discussion
3.1. ABCC6 Rare Sequence Variants
3.2. Phenotype Features of PXE Patients
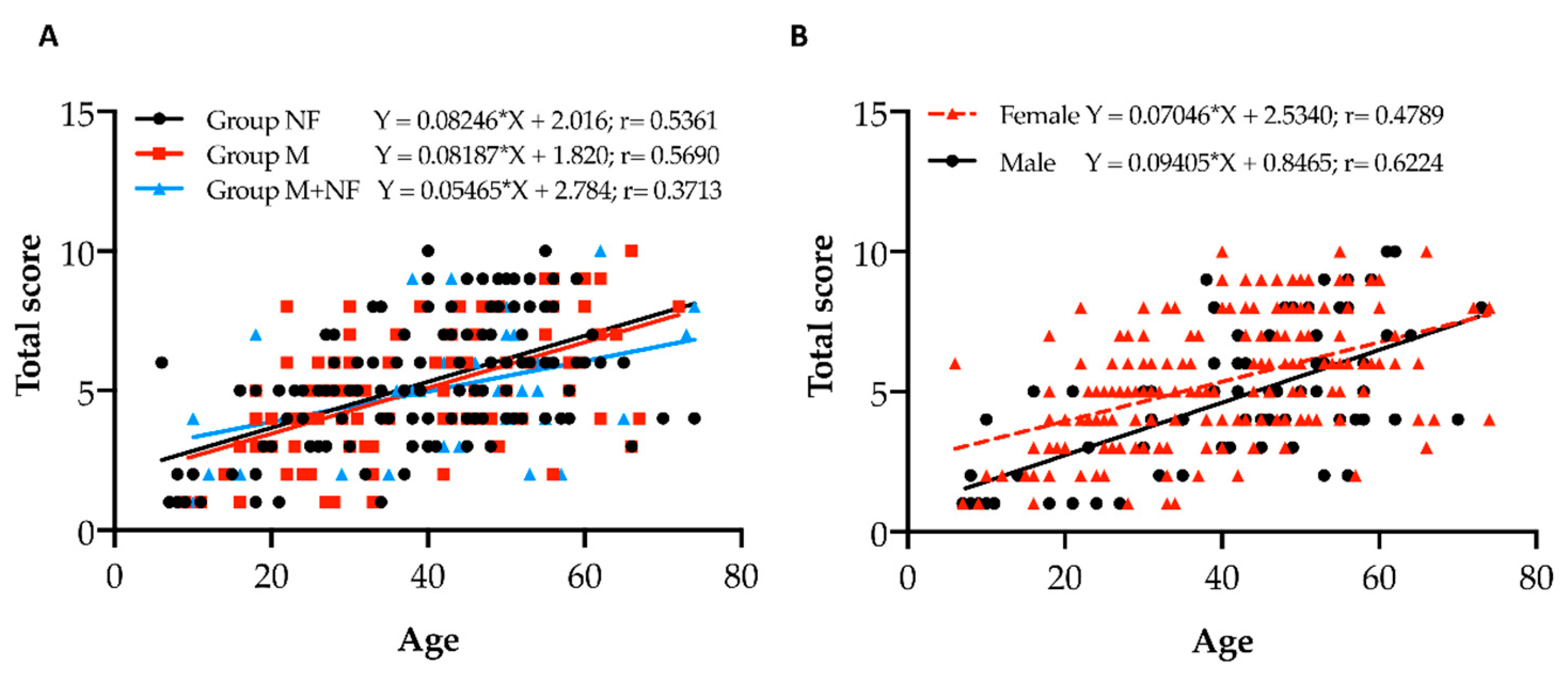
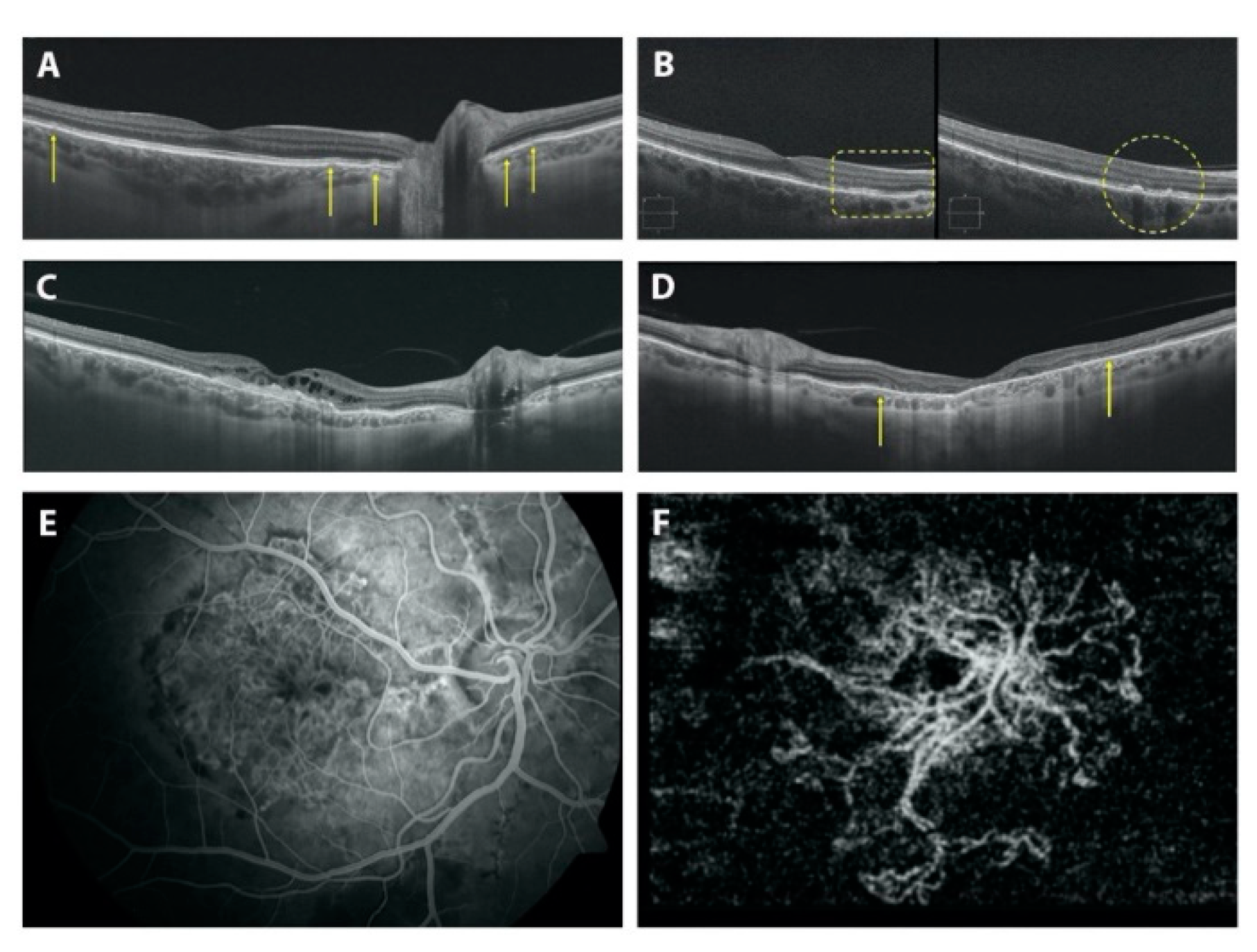
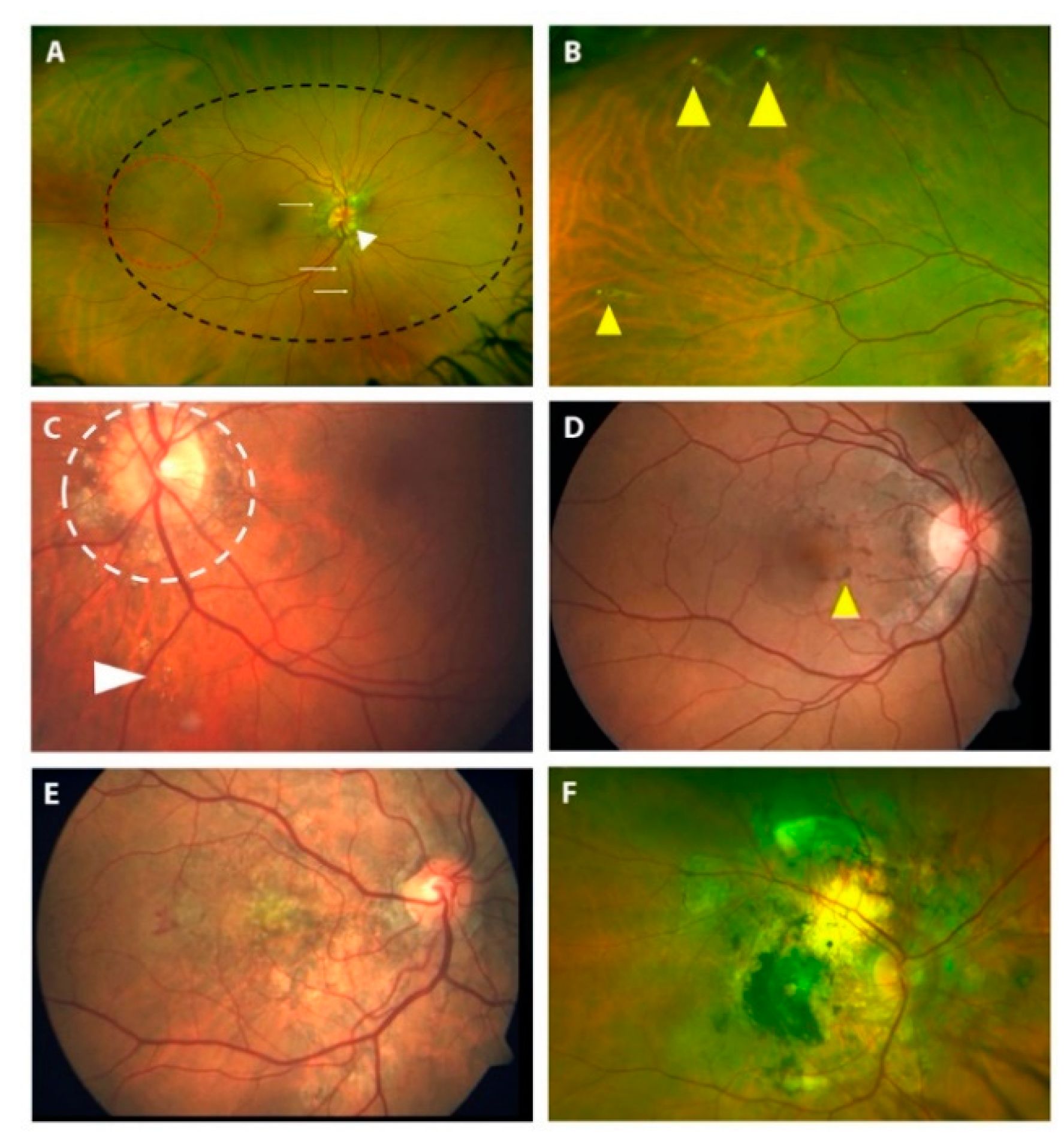
3.3. Fundoscopic Findings in PXE Patients
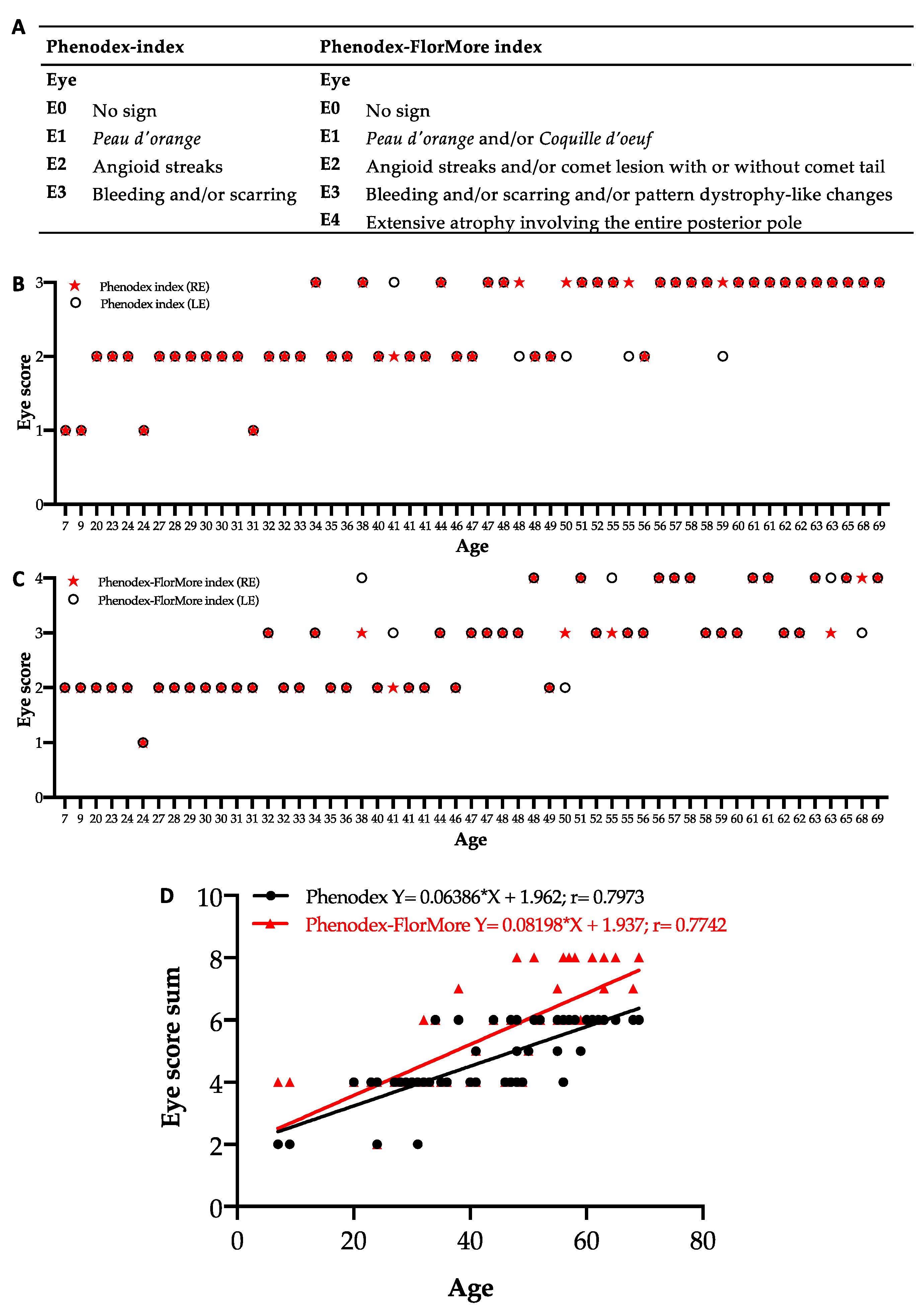
3.4. Update of Phenodex Index: Focus on Ocular Manifestations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quaglino, D.; Boraldi, F.; Annovi, G.; Ronchetti, I. The Multifaceted Complexity of Genetic Diseases: A Lesson from Pseudoxanthoma elasticum. In Advances in the Study of Genetic Disorders; Ikehara, K., Ed.; InTech: Rijeka, Croatia, 2011; ISBN 978-953-307-305-7. [Google Scholar]
- Gheduzzi, D.; Sammarco, R.; Quaglino, D.; Bercovitch, L.; Terry, S.; Taylor, W.; Ronchetti, I.P. Extracutaneous Ultrastructural Alterations in Pseudoxanthoma elasticum. Ultrastruct. Pathol. 2003, 27, 375–384. [Google Scholar] [CrossRef]
- Neldner, K.H.; Struk, B. Pseudoxanthoma elasticum. In Connective Tissue and Its Heritable Disorders; Royce, P.M., Steinmann, B., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2002; pp. 561–583. ISBN 978-0-471-22192-0. [Google Scholar]
- Mendelsohn, G.; Bulkley, B.H.; Hutchins, G.M. Cardiovascular Manifestations of Pseudoxanthoma elasticum. Arch. Pathol. Lab. Med. 1978, 102, 298–302. [Google Scholar] [PubMed]
- Vanakker, O.M.; Leroy, B.P.; Coucke, P.; Bercovitch, L.G.; Uitto, J.; Viljoen, D.; Terry, S.F.; Van Acker, P.; Matthys, D.; Loeys, B.; et al. Novel Clinico-Molecular Insights in Pseudoxanthoma elasticum Provide an Efficient Molecular Screening Method and a Comprehensive Diagnostic Flowchart. Hum. Mutat. 2008, 29, 205. [Google Scholar] [CrossRef]
- Kauw, F.; Kranenburg, G.; Kappelle, L.J.; Hendrikse, J.; Koek, H.L.; Visseren, F.L.J.; Mali, W.P.T.; de Jong, P.A.; Spiering, W. Cerebral Disease in a Nationwide Dutch Pseudoxanthoma elasticum Cohort with a Systematic Review of the Literature. J. Neurol. Sci. 2017, 373, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Golliet-Mercier, N.; Allaouchiche, B.; Monneuse, O. Pseudoxanthoma elasticum with severe gastrointestinal bleeding. Ann. Fr. Anesth. Reanim. 2005, 24, 833–834. [Google Scholar] [CrossRef] [PubMed]
- Booij, J.C.; Baas, D.C.; Beisekeeva, J.; Gorgels, T.G.M.F.; Bergen, A.A.B. The Dynamic Nature of Bruch’s Membrane. Prog. Retin. Eye Res. 2010, 29, 1–18. [Google Scholar] [CrossRef]
- Risseeuw, S.; van Leeuwen, R.; Imhof, S.M.; Spiering, W.; Norel, J.O. The Natural History of Bruch’s Membrane Calcification in Pseudoxanthoma elasticum. Ophthalmol. Sci. 2021, 1, 100001. [Google Scholar] [CrossRef]
- Georgalas, I.; Tservakis, I.; Papaconstaninou, D.; Kardara, M.; Koutsandrea, C.; Ladas, I. Pseudoxanthoma elasticum, Ocular Manifestations, Complications and Treatment. Clin. Exp. Optom. 2011, 94, 169–180. [Google Scholar] [CrossRef]
- Risseeuw, S.; Ossewaarde-van Norel, J.; van Buchem, C.; Spiering, W.; Imhof, S.M.; van Leeuwen, R. The Extent of Angioid Streaks Correlates with Macular Degeneration in Pseudoxanthoma elasticum. Am. J. Ophthalmol. 2020, 220, 82–90. [Google Scholar] [CrossRef]
- Le Saux, O.; Urban, Z.; Tschuch, C.; Csiszar, K.; Bacchelli, B.; Quaglino, D.; Pasquali-Ronchetti, I.; Pope, F.M.; Richards, A.; Terry, S.; et al. Mutations in a Gene Encoding an ABC Transporter Cause Pseudoxanthoma elasticum. Nat. Genet. 2000, 25, 223–227. [Google Scholar] [CrossRef]
- Hu, X.; Plomp, A.S.; van Soest, S.; Wijnholds, J.; de Jong, P.T.V.M.; Bergen, A.A.B. Pseudoxanthoma elasticum: A Clinical, Histopathological, and Molecular Update. Surv. Ophthalmol. 2003, 48, 424–438. [Google Scholar] [CrossRef]
- Gheduzzi, D.; Guidetti, R.; Anzivino, C.; Tarugi, P.; Di Leo, E.; Quaglino, D.; Ronchetti, I.P. ABCC6 Mutations in Italian Families Affected by Pseudoxanthoma elasticum (PXE). Hum. Mutat. 2004, 24, 438–439. [Google Scholar] [CrossRef]
- Charbel Issa, P.; Tysoe, C.; Caswell, R. Late-Onset Pseudoxanthoma elasticum Associated with a Hypomorphic ABCC6 Variant. Am. J. Ophthalmol. 2020, 218, 255–260. [Google Scholar] [CrossRef]
- Li, Q.; Arányi, T.; Váradi, A.; Terry, S.F.; Uitto, J. Research Progress in Pseudoxanthoma elasticum and Related Ectopic Mineralization Disorders. J. Investig. Dermatol. 2016, 136, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Le Saux, O.; Beck, K.; Sachsinger, C.; Silvestri, C.; Treiber, C.; Göring, H.H.; Johnson, E.W.; De Paepe, A.; Pope, F.M.; Pasquali-Ronchetti, I.; et al. A Spectrum of ABCC6 Mutations Is Responsible for Pseudoxanthoma elasticum. Am. J. Hum. Genet. 2001, 69, 749–764. [Google Scholar] [CrossRef] [PubMed]
- Costrop, L.M.F.; Vanakker, O.O.M.; Van Laer, L.; Le Saux, O.; Martin, L.; Chassaing, N.; Guerra, D.; Pasquali-Ronchetti, I.; Coucke, P.J.; De Paepe, A. Novel Deletions Causing Pseudoxanthoma elasticum Underscore the Genomic Instability of the ABCC6 Region. J. Hum. Genet. 2010, 55, 112–117. [Google Scholar] [CrossRef]
- Legrand, A.; Cornez, L.; Samkari, W.; Mazzella, J.-M.; Venisse, A.; Boccio, V.; Auribault, K.; Keren, B.; Benistan, K.; Germain, D.P.; et al. Mutation Spectrum in the ABCC6 Gene and Genotype–Phenotype Correlations in a French Cohort with Pseudoxanthoma elasticum. Genet. Med. 2017, 19, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Pfendner, E.G.; Vanakker, O.M.; Terry, S.F.; Vourthis, S.; McAndrew, P.E.; McClain, M.R.; Fratta, S.; Marais, A.-S.; Hariri, S.; Coucke, P.J.; et al. Mutation Detection in the ABCC6 Gene and Genotype-Phenotype Analysis in a Large International Case Series Affected by Pseudoxanthoma elasticum. J. Med. Genet. 2007, 44, 621–628. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and Guidelines for the Interpretation of Sequence Variants: A Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Kopanos, C.; Tsiolkas, V.; Kouris, A.; Chapple, C.E.; Albarca Aguilera, M.; Meyer, R.; Massouras, A. VarSome: The Human Genomic Variant Search Engine. Bioinformatics 2019, 35, 1978–1980. [Google Scholar] [CrossRef]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A Method and Server for Predicting Damaging Missense Mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Ebert, D.; Huang, X.; Thomas, P.D. PANTHER Version 14: More Genomes, a New PANTHER GO-Slim and Improvements in Enrichment Analysis Tools. Nucleic Acids Res. 2019, 47, D419–D426. [Google Scholar] [CrossRef]
- Tavtigian, S.V.; Deffenbaugh, A.M.; Yin, L.; Judkins, T.; Scholl, T.; Samollow, P.B.; de Silva, D.; Zharkikh, A.; Thomas, A. Comprehensive Statistical Study of 452 BRCA1 Missense Substitutions with Classification of Eight Recurrent Substitutions as Neutral. J. Med. Genet. 2006, 43, 295–305. [Google Scholar] [CrossRef]
- Gass, J. Stereoscopic Atlas of Macular Diseases: Diagnosis and Treatment, 4th ed.; CV Mosby: St Louis, MO, USA, 1997; Volume 1. [Google Scholar]
- Boraldi, F.; Costa, S.; Rabacchi, C.; Ciani, M.; Vanakker, O.; Quaglino, D. Can APOE and MTHFR Polymorphisms Have an Influence on the Severity of Cardiovascular Manifestations in Italian Pseudoxanthoma elasticum Affected Patients? Mol. Genet. Metab. Rep. 2014, 1, 477–482. [Google Scholar] [CrossRef]
- Guerra, D.; Roggiani, J.; Panico, F.; De Santis, G.; Gheduzzi, D.; Quaglino, D.; Pasquali-Ronchetti, I. ABCC6 Mutations in Italian PXE Patients: An Update Describing 22 Novel Mutations. Connect. Tissue Res. 2009, 50, 80–81. [Google Scholar] [CrossRef]
- Schulz, V.; Hendig, D.; Henjakovic, M.; Szliska, C.; Kleesiek, K.; Götting, C. Mutational Analysis of the ABCC6 Gene and the Proximal ABCC6 Gene Promoter in German Patients with Pseudoxanthoma elasticum (PXE). Hum. Mutat. 2006, 27, 831. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, Y.; Baujat, G.; Botschen, U.; Wittkampf, T.; du Moulin, M.; Stella, J.; Le Merrer, M.; Guest, G.; Lambot, K.; Tazarourte-Pinturier, M.-F.; et al. Generalized Arterial Calcification of Infancy and Pseudoxanthoma elasticum Can Be Caused by Mutations in Either ENPP1 or ABCC6. Am. J. Hum. Genet. 2012, 90, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Pulkkinen, L.; Nakano, A.; Ringpfeil, F.; Uitto, J. Identification of ABCC6 Pseudogenes on Human Chromosome 16p: Implications for Mutation Detection in Pseudoxanthoma elasticum. Hum. Genet. 2001, 109, 356–365. [Google Scholar] [CrossRef]
- Miksch, S.; Lumsden, A.; Guenther, U.P.; Foernzler, D.; Christen-Zäch, S.; Daugherty, C.; Ramesar, R.K.S.; Lebwohl, M.; Hohl, D.; Neldner, K.H.; et al. Molecular Genetics of Pseudoxanthoma elasticum: Type and Frequency of Mutations in ABCC6. Hum. Mutat. 2005, 26, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Murro, V.; Mucciolo, D.P.; Giorgio, D.; Sodi, A.; Boraldi, F.; Quaglino, D.; Virgili, G.; Giansanti, F. Pattern Dystrophy-like Changes and Coquille d’oeuf Atrophy in Elderly Patients Affected by Pseudoxanthoma elasticum. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 1881–1892. [Google Scholar] [CrossRef]
- Meloni, I.; Rubegni, P.; De Aloe, G.; Bruttini, M.; Pianigiani, E.; Cusano, R.; Seri, M.; Mondillo, S.; Federico, A.; Bardelli, A.M.; et al. Pseudoxanthoma elasticum: Point Mutations in the ABCC6 Gene and a Large Deletion Including Also ABCC1 and MYH11. Hum. Mutat. 2001, 18, 85. [Google Scholar] [CrossRef] [PubMed]
- Götting, C.; Schulz, V.; Hendig, D.; Grundt, A.; Dreier, J.; Szliska, C.; Brinkmann, T.; Kleesiek, K. Assessment of a Rapid-Cycle PCR Assay for the Identification of the Recurrent c.3421C>T Mutation in the ABCC6 Gene in Pseudoxanthoma elasticum Patients. Lab. Investig. 2004, 84, 122–130. [Google Scholar] [CrossRef]
- Ringpfeil, F.; Lebwohl, M.G.; Christiano, A.M.; Uitto, J. Pseudoxanthoma elasticum: Mutations in the MRP6 Gene Encoding a Transmembrane ATP-Binding Cassette (ABC) Transporter. Proc. Natl. Acad. Sci. USA 2000, 97, 6001–6006. [Google Scholar] [CrossRef]
- Murro, V.; Mucciolo, D.P.; Giorgio, D.; Pavese, L.; Boraldi, F.; Quaglino, D.; Finocchio, L.; Sodi, A.; Virgili, G.; Giansanti, F. Adaptive Optics Imaging in Patients Affected by Pseudoxanthoma elasticum. Am. J. Ophthalmol. 2020, 224, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, N.; Martin, L.; Mazereeuw, J.; Barrié, L.; Nizard, S.; Bonafé, J.-L.; Calvas, P.; Hovnanian, A. Novel ABCC6 Mutations in Pseudoxanthoma elasticum. J. Investig. Dermatol. 2004, 122, 608–613. [Google Scholar] [CrossRef]
- Struk, B.; Cai, L.; Zäch, S.; Ji, W.; Chung, J.; Lumsden, A.; Stumm, M.; Huber, M.; Schaen, L.; Kim, C.A.; et al. Mutations of the Gene Encoding the Transmembrane Transporter Protein ABC-C6 Cause Pseudoxanthoma elasticum. J. Mol. Med. 2000, 78, 282–286. [Google Scholar] [CrossRef]
- Germain, D.P.; Perdu, J.; Remones, V.; Jeunemaitre, X. Homozygosity for the R1268Q Mutation in MRP6, the Pseudoxanthoma elasticum Gene, Is Not Disease-Causing. Biochem. Biophys. Res. Commun. 2000, 274, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fernandez, M.I.; Gheduzzi, D.; Boraldi, F.; Paolinelli, C.D.; Sanchez, P.; Valdivielso, P.; Morilla, M.J.; Quaglino, D.; Guerra, D.; Casolari, S.; et al. Parameters of Oxidative Stress Are Present in the Circulation of PXE Patients. Biochim. Biophys. Acta 2008, 1782, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Jiang, Q.; Wu, Z.; Shao, C.; Zhou, Y.; Yang, L.; Uitto, J.; Wang, G. Genetic Heterogeneity of Pseudoxanthoma elasticum: The Chinese Signature Profile of ABCC6 and ENPP1 Mutations. J. Investig. Dermatol. 2015, 135, 1294–1302. [Google Scholar] [CrossRef]
- Noji, Y.; Inazu, A.; Higashikata, T.; Nohara, A.; Kawashiri, M.; Yu, W.; Todo, Y.; Nozue, T.; Uno, Y.; Hifumi, S.; et al. Identification of Two Novel Missense Mutations (p.R1221C and p.R1357W) in the ABCC6 (MRP6) Gene in a Japanese Patient with Pseudoxanthoma elasticum (PXE). Intern. Med. 2004, 43, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Atzori, L.; Zucca, M.; Vivanet, C.; Sanna, C.; Pilloni, L.; Ferreli, C. Aesthetic Complaints as Clue to Pseudoxanthoma elasticum. Glob. Dermatol. 2015, 2, 103–106. [Google Scholar] [CrossRef][Green Version]
- Uitto, J.; Pulkkinen, L.; Ringpfeil, F. Molecular Genetics of Pseudoxanthoma elasticum: A Metabolic Disorder at the Environment-Genome Interface? Trends Mol. Med. 2001, 7, 13–17. [Google Scholar] [CrossRef]
- Iwanaga, A.; Okubo, Y.; Yozaki, M.; Koike, Y.; Kuwatsuka, Y.; Tomimura, S.; Yamamoto, Y.; Tamura, H.; Ikeda, S.; Maemura, K.; et al. Analysis of Clinical Symptoms and ABCC6 Mutations in 76 Japanese Patients with Pseudoxanthoma elasticum. J. Dermatol. 2017, 44, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Zuily, S.; Angioi, K.; Fauret, A.-L.; Golmard, L.; Saadi, L.; Huttin, O.; Anxionnat, R.; Evon, P.; Marie, P.-Y.; Jeunemaitre, X.; et al. Severe and Diffuse Arterial Lesions in a Patient with Pseudoxanthoma elasticum. J. Am. Coll. Cardiol. 2012, 59, 1991. [Google Scholar] [CrossRef]
- Boraldi, F.; Lofaro, F.D.; Costa, S.; Moscarelli, P.; Quaglino, D. Rare Co-Occurrence of Beta-Thalassemia and Pseudoxanthoma elasticum: Novel Biomolecular Findings. Front. Med. 2020, 6, 322. [Google Scholar] [CrossRef]
- Murro, V.; Mucciolo, D.P.; Sodi, A.; Boraldi, F.; Quaglino, D.; Virgili, G.; Rizzo, S. Peripapillary Comet Lesions and Comet Rain in PXE-Related Retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- Hendig, D.; Schulz, V.; Eichgrün, J.; Szliska, C.; Götting, C.; Kleesiek, K. New ABCC6 Gene Mutations in German Pseudoxanthoma elasticum Patients. J. Mol. Med. 2005, 83, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Boraldi, F.; Lofaro, F.D.; Losi, L.; Quaglino, D. Dermal Alterations in Clinically Unaffected Skin of Pseudoxanthoma elasticum Patients. J. Clin. Med. 2021, 10, 500. [Google Scholar] [CrossRef]
- Bergen, A.A.; Plomp, A.S.; Schuurman, E.J.; Terry, S.; Breuning, M.; Dauwerse, H.; Swart, J.; Kool, M.; van Soest, S.; Baas, F.; et al. Mutations in ABCC6 Cause Pseudoxanthoma elasticum. Nat. Genet. 2000, 25, 228–231. [Google Scholar] [CrossRef]
- Plomp, A.S.; Florijn, R.J.; Ten Brink, J.; Castle, B.; Kingston, H.; Martín-Santiago, A.; Gorgels, T.G.M.F.; de Jong, P.T.V.M.; Bergen, A.A.B. ABCC6 Mutations in Pseudoxanthoma elasticum: An Update Including Eight Novel Ones. Mol. Vis. 2008, 14, 118–124. [Google Scholar]
- Theal, M.; Sleik, K.; Anand, S.; Yi, Q.; Yusuf, S.; Lonn, E. Prevalence of Mitral Valve Prolapse in Ethnic Groups. Can. J. Cardiol. 2004, 20, 511–515. [Google Scholar] [PubMed]
- Thrift, A.G.; Cadilhac, D.A.; Thayabaranathan, T.; Howard, G.; Howard, V.J.; Rothwell, P.M.; Donnan, G.A. Global Stroke Statistics. Int. J. Stroke 2014, 9, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Townsend, N.; Wilson, L.; Bhatnagar, P.; Wickramasinghe, K.; Rayner, M.; Nichols, M. Cardiovascular Disease in Europe: Epidemiological Update 2016. Eur. Heart J. 2016, 37, 3232–3245. [Google Scholar] [CrossRef]
- SPREAD—Stroke Prevention and Educational Awareness Diffusion: Ictus Cerebrale: Linee Guida Italiane Di Prevenzione e Trattamento, 8th ed.; Pub Health: Italy, 2016.
- Romero, V.; Akpinar, H.; Assimos, D.G. Kidney Stones: A Global Picture of Prevalence, Incidence, and Associated Risk Factors. Rev. Urol. 2010, 12, e86–e96. [Google Scholar]
- LaRusso, J.; Ringpfeil, F.; Uitto, J. Pseudoxanthoma elasticum: A Streamlined, Ethnicity-based Mutation Detection Strategy. Clin. Transl. Sci. 2010, 3, 295–298. [Google Scholar] [CrossRef]
- Verschuere, S.; Navassiolava, N.; Martin, L.; Nevalainen, P.I.; Coucke, P.J.; Vanakker, O.M. Reassessment of Causality of ABCC6 Missense Variants Associated with Pseudoxanthoma elasticum Based on Sherloc. Genet. Med. 2021, 23, 131–139. [Google Scholar] [CrossRef]
- Li, Q.; Grange, D.K.; Armstrong, N.L.; Whelan, A.J.; Hurley, M.Y.; Rishavy, M.A.; Hallgren, K.W.; Berkner, K.L.; Schurgers, L.J.; Jiang, Q.; et al. Mutations in the GGCX and ABCC6 Genes in a Family with Pseudoxanthoma elasticum-like Phenotypes. J. Investig. Dermatol. 2009, 129, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Zarbock, R.; Hendig, D.; Szliska, C.; Kleesiek, K.; Götting, C. Pseudoxanthoma elasticum: Genetic Variations in Antioxidant Genes Are Risk Factors for Early Disease Onset. Clin. Chem. 2007, 53, 1734–1740. [Google Scholar] [CrossRef]
- Bartstra, J.W.; Risseeuw, S.; de Jong, P.A.; van Os, B.; Kalsbeek, L.; Mol, C.; Baas, A.F.; Verschuere, S.; Vanakker, O.; Florijn, R.J.; et al. Genotype-Phenotype Correlation in Pseudoxanthoma elasticum. Atherosclerosis 2021, 324, 18–26. [Google Scholar] [CrossRef]
- Spaide, R.F. Peau d’orange and Angioid Streaks: Manifestations of Bruch Membrane Pathology. Retina 2015, 35, 392–397. [Google Scholar] [CrossRef]
- Murro, V.; Mucciolo, D.P.; Giorgio, D.; Sodi, A.; Boraldi, F.; Quaglino, D.; Virgili, G.; Rizzo, S. Coquille d’oeuf in Young Patients Affected with Pseudoxantoma Elasticum. Ophthalmic Genet. 2019, 40, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Patel, P.; Adkins, T.; Gass, J.D.M. Spectrum of Pattern Dystrophy in Pseudoxanthoma elasticum. Arch. Ophthalmol. 2005, 123, 923–928. [Google Scholar] [CrossRef]
- Finger, R.P.; Charbel Issa, P.; Ladewig, M.S.; Götting, C.; Szliska, C.; Scholl, H.P.N.; Holz, F.G. Pseudoxanthoma elasticum: Genetics, Clinical Manifestations and Therapeutic Approaches. Surv. Ophthalmol. 2009, 54, 272–285. [Google Scholar] [CrossRef]
- Schoenberger, S.D.; Agarwal, A. Geographic Chorioretinal Atrophy in Pseudoxanthoma elasticum. Am. J. Ophthalmol. 2013, 156, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Charbel Issa, P.; Finger, R.P.; Holz, F.G.; Scholl, H.P.N. Multimodal Imaging Including Spectral Domain OCT and Confocal near Infrared Reflectance for Characterization of Outer Retinal Pathology in Pseudoxanthoma elasticum. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5913–5918. [Google Scholar] [CrossRef]
- Gliem, M.; Müller, P.L.; Birtel, J.; Hendig, D.; Holz, F.G.; Charbel Issa, P. Frequency, Phenotypic Characteristics and Progression of Atrophy Associated with a Diseased Bruch’s Membrane in Pseudoxanthoma elasticum. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3323–3330. [Google Scholar] [CrossRef]
- Marchese, A.; Rabiolo, A.; Corbelli, E.; Carnevali, A.; Cicinelli, M.V.; Giuffrè, C.; Querques, G.; Bandello, F. Ultra-Widefield Imaging in Patients with Angioid Streaks Secondary to Pseudoxanthoma elasticum. Ophthalmol. Retin. 2017, 1, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Charbel Issa, P.; Finger, R.P.; Götting, C.; Hendig, D.; Holz, F.G.; Scholl, H.P.N. Centrifugal Fundus Abnormalities in Pseudoxanthoma elasticum. Ophthalmology 2010, 117, 1406–1414. [Google Scholar] [CrossRef]
- Gliem, M.; Fimmers, R.; Müller, P.L.; Brinkmann, C.K.; Finger, R.P.; Hendig, D.; Holz, F.G.; Charbel Issa, P. Choroidal Changes Associated with Bruch Membrane Pathology in Pseudoxanthoma elasticum. Am. J. Ophthalmol. 2014, 158, 198–207. [Google Scholar] [CrossRef]
- Gliem, M.; Zaeytijd, J.D.; Finger, R.P.; Holz, F.G.; Leroy, B.P.; Charbel Issa, P. An Update on the Ocular Phenotype in Patients with Pseudoxanthoma elasticum. Front. Genet. 2013, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Auw-Haedrich, C.; Staubach, F.; Witschel, H. Optic Disk Drusen. Surv. Ophthalmol. 2002, 47, 515–532. [Google Scholar] [CrossRef]
- Meislik, J.; Neldner, K.; Reeve, E.B.; Ellis, P.P. Atypical Drusen in Pseudoxanthoma elasticum. Ann. Ophthalmol. 1979, 11, 653–656. [Google Scholar]
- Yan, Y.; Zhou, X.; Chu, Z.; Stell, L.; Shariati, M.A.; Wang, R.K.; Liao, Y.J. Vision Loss in Optic Disc Drusen Correlates with Increased Macular Vessel Diameter and Flux and Reduced Peripapillary Vascular Density. Am. J. Ophthalmol. 2020, 218, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Pipelart, V.; Leroux, B.; Leruez, S.; Henni, S.; Navasiolava, N.; Martin, L.; Ebran, J.-M. A Study of Optic Nerve Head Drusen in 38 Pseudoxanthoma elasticum (PXE) Patients (64 Eyes). Location of Optic Nerve Head Drusen in PXE. J. Fr. Ophtalmol. 2019, 42, 262–268. [Google Scholar] [CrossRef]
- Gass, J.D.M. “Comet” Lesion: An Ocular Sign of Pseudoxanthoma elasticum. Retina 2003, 23, 729–730. [Google Scholar] [CrossRef] [PubMed]



| Intron/ Exon | Nucleotide Variation | Amino Acid Variation | Ref. | Intron/ Exon | Nucleotide Variation | Amino Acid Variation | Ref. |
|---|---|---|---|---|---|---|---|
| IVS1 | c.36+1dupG | Loss of splice donor site | N | 21 | c.2678C>A | p.Ser893Ter | [19] |
| IVS1 | c.36+1G>C | Loss of splice donor site | [27] | 21 | c.2728_2746dupTGGATGACCCTGACAGGGC | p.Trp918Ter | [28] |
| 2 | c.113G>C | p.Trp38Ser | [29] | IVS21 | c.2787+1G>T | Loss of splice donor site | [30] |
| 2 | c.117_118insC | p.Met42HisfsTer59 | [27] | 22 | c.2836_2860delinsTCTGCCTCT | p.Leu946SerfsTer18 | N |
| 2 | c.196dupT | p.Ser66PhefsTer35 | [31] | 22 | c.2848G>A | p.Ala950Thr | [20] |
| 5 | c.557delT | p.Leu186ArgfsTer46 | [28] | 22 | c.2900G>A | p.Trp967Ter | N |
| 6 | c.613G>T | p.Glu205Ter | N | 23 | c.3037G>A | p.Gly1013Arg | N |
| IVS7 | c.794+1G>A | Loss of splice donor site | N | 23 | c.3088C>T | p.Arg1030Ter | [17] |
| 8 | c.913C>T | p.Gln305Ter | [28] | 23 | c.3109G>A | p.Glu1037Lys | [27] |
| 8 | c.940G>A | p.Gly314Arg | [28] | 23 | c.3142_3144delTTC | p.Phe1048del | [27] |
| 8 | c.951C>A | p.Ser317Arg | [32] | 24 | c.3340C>T | p.Arg1114Cys | [14] |
| 8 | c.956T>A | p.Ile319Asn | [33] | 24 | c.3341G>A | p.Arg1114His | [13] |
| 8 | c.960delC | p.Ser321ValfsTer35 | [34] | 24 | c.3380T>C | p.Met1127Thr | [14] |
| 8 | c.989delA | p.Lys330SerfsTer26 | [28] | 24 | c.3389C>T | p.Thr1130Met | [35] |
| 9 | c.1091C>G | p.Thr364Arg | [31] | 24 | c.3398G>A | p.Gly1133Asp | [20] |
| 9 | c.1132C>T | p.Gln378Ter | [31] | 24 | c.3412C>T | p.Arg1138Trp | [36] |
| 9 | c.1145G>A | p.Arg382Gln | [37] | 24 | c.3413G>A | p.Arg1138Gln | [36] |
| 9 | c.1160G>T | p.Gly387Val | N | 24 | c.3421C>T | p.Arg1141Ter | [12] |
| 9 | c.1171A>G | p.Arg391Gly | [38] | 24 | c.3490C>T | p.Arg1164Ter | [39] |
| 9 | c.1174A>G | p.Lys392Glu | N | 24 | c.3491G>A | p.Arg1164Gln | [40] |
| 9 | c.1175A>G | p.Lys392Arg | N | 24 | c.3307-940_3506+660del | p.? | [18] |
| 10 | c.1220G>A | p.Gly407Asp | N | 25 | c.3542G>A | p.Gly1181Asp | [41] |
| 10 | c.1220G>T | p.Gly407Val | [28] | 25 | c.3544dupC | p.Leu1182ProfsTer96 | N |
| 10 | c.1255C>T | p.Arg419Trp | N | 25 | c.3563C>G | p.Thr1188Arg | N |
| 10 | c.1256G>A | p.Arg419Gln | [42] | 26 | c.3661C>T | p.Arg1221Cys | [43] |
| 10 | c.1284C>G | p.Asn428Lys | [19] | 26 | c.3662G>A | p.Arg1221His | [20] |
| 10 | c.1308G>A | p.Trp436Ter | [28] | 26 | c.3677T>C | p.Leu1226Pro | [33] |
| 10 | c.1318T>G | p.Cys440Gly | [14] | 26 | c.3700G>A | p.Glu1234Lys | [44] |
| 12 | c.1484T>A | p.Leu495His | [32] | 26 | c.3712G>T | p.Asp1238Tyr | [38] |
| 12 | c.1526C>G | p.Ala509Gly | [19] | 26 | c.3735G>A | p.Glu1245= | N |
| 12 | c.1552C>T | p.Arg518Ter | [34] | IVS26 | c.3736-1G>A | Loss of splice acceptor site | [36] |
| 12 | c.1553G>A | p.Arg518Gln | [45] | 27 | c.3774_3775insC | p.Trp1259LeufsTer19 | [20] |
| IVS13 | c.1779+1G>C | Loss of splice donor site | [28] | 27 | c.3823C>T | p.Arg1275Ter | [14] |
| 14 | c.1798C>T | p.Arg600Cys | [14] | 27 | c.3871delG | p.Ala1291GlnfsTer68 | [28] |
| 14 | c.1799G>A | p.Arg600His | [41] | 27 | c.3880_3882delAAG | p.Lys1294del | [20] |
| 14 | c.1857dupC | p.Ser620LeufsTer121 | [20] | 28 | c.3892G>A | p.Val1298Ile | N |
| 16 | c.1961C>T | p.Pro654Leu | [37] | 28 | c.3902C>T | p.Thr1301Ile | [17] |
| 16 | c.1987G>A | p.Gly663Ser | [46] | 28 | c.3904G>A | p.Gly1302Arg | [17] |
| 16 | c.1987G>T | p.Gly663Cys | [20] | 28 | c.3940C>T | p.Arg1314Trp | [12] |
| 16 | c.1999delG | p.Ala667GlnfsTer21 | [20] | 28 | c.3989T>C | p.Ile1330Thr | [37] |
| 17 | c.2018T>C | p.Leu673Pro | [17] | 29 | c.4015C>T | p.Arg1339Cys | [39] |
| 17 | c.2093A>C | p.Gln698Pro | [20] | 29 | c.4036C>T | p.Pro1346Ser | [14] |
| 17 | c.2095G>A | p.Glu699Lys | [37] | 29 | c.4041G>A | p.Gln1347= | [28] |
| 17 | c.2153C>A | p.Asp718Gly | [47] | 29 | c.4055T>C | p.Phe1352Ser | [48] |
| IVS17 | c.2248-2_2248-1delAG | Loss of splice acceptor site | [49] | 29 | c.4070G>C | p.Arg1357Pro | [28] |
| IVS17 | c.2247+1G>A | Loss of splice donor site | [27] | 29 | c.4159_4171dupCTGCCCGGCCAGC | p.Leu1391ProfsTer10 | N |
| 18 | c.2263G>A | p.Gly755Arg | [20] | 29 | c.4182delG | p.Lys1394AsnfsTer9 | [13] |
| 18 | c.2264G>A | p.Gly755Glu | [27] | 29 | c.4198G>A | p.Glu1400Lys | [38] |
| 18 | c.2266G>A | p.Gly756Ser | [28] | IVS29 | c.4208+1G>A | Loss of splice donor site | [28] |
| 18 | c.2278C>T | p.Arg760Trp | [50] | 30 | c.4318delA | p.Met1440CysfsTer24 | [14] |
| 18 | c.2294G>A | p.Arg765Gln | [17] | 30 | c.4361T>C | p.Leu1454Pro | N |
| 18 | c.2307_2308insA | p.Ala771GlyfsTer8 | N | 30 | c.4403G>A | p.Arg1468Gln | N |
| 18 | c.2329G>A | p.Asp777Asn | [20] | 30 | c.del30 | p.? | [51] |
| 18 | c.2383G>T | p.Val795Phe | [27] | 1_31 | c.1_4511del | p.? | [52] |
| 19 | c.2419C>T | p.Arg807Trp | [32] | 11_18 | c.dup11-18 | p.? | N |
| 19 | c.2428G>A | p.Val810Met | [14] | 23_29 | c.2996_4208del | p.? | [17] |
| 19 | c.2458G>C | p.Ala820Pro | [33] | 24_27 | c.3307-1006_3735+1582del | p.? | [18] |
| 19 | c.2477T>C | p.Leu826Pro | [53] | 25_27 | c.3507_3882del | p.? | [49] |
| 19 | c.2504G>A | p.Gly835Asp | N |
| Age | N. of Pt | Pt with PO | %/ Decade | Pt with CO | %/ Decade | Pt with AS | %/ Decade | Pt with CL | %/ Decade | Pt with CNV | %/ Decade | Pt with PD | %/ Decade | Pt with PPA | %/ Decade | Pt with ODD | %/ Decade |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <10 | 2 | 2 | 100 | 2 | 100 | 0 | 0 | 2 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 20–29 | 7 | 7 | 100 | 7 | 100 | 6 | 85.7 | 5 | 71.4 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 14.3 |
| 30–39 | 11 | 11 | 100 | 11 | 100 | 10 | 90.9 | 9 | 81.8 | 2 | 18.2 | 3 | 27.3 | 1 | 9.1 | 0 | 0 |
| 40–49 | 12 | 11 | 91.7 | 11 | 91.7 | 12 | 100 | 10 | 83.3 | 5 | 41.7 | 3 | 25.0 | 1 | 8.3 | 1 | 8.3 |
| 50–59 | 11 | 10 | 90.9 | 10 | 90.9 | 11 | 100 | 7 | 63.6 | 10 | 90.9 | 8 | 72.7 | 5 | 45.5 | 1 | 9.1 |
| 60–69 | 10 | 4 | 40 | 4 | 40 | 10 | 100 | 7 | 70 | 10 | 100 | 8 | 80 | 7 | 70 | 0 | 0 |
| Sum | 53 | 45 | 45 | 49 | 40 | 27 | 22 | 14 | 3 | ||||||||
| Mean | 84.9 | 84.9 | 92.5 | 75.5 | 50.9 | 41.5 | 26.4 | 5.7 |
| Organ System Findings | |
|---|---|
| Skin | |
| S0 | No sign |
| S1 | Papules/bumps |
| S2 | Plaques of coalesced papules |
| S3 | Lax and redundant skin |
| Eyes (score of RE + score of LE) | |
| E0 | No sign |
| E1 | Peau d’orange and/or Coquille d’oeuf |
| E2 | Angioid streaks and/or comet lesion with or without comet tail |
| E3 | Bleeding and/or scarring and/or pattern dystrophy-like changes |
| E4 | Extensive atrophic area involving the entire posterior pole |
| Gastrointestinal | |
| G0 | No sign |
| G1 | Gastrointestinal bleeding as related to PXE |
| Vascular | |
| V0 | No sign |
| V1 | Weak or absent pulse or peripheral artery disease revealed by vascular imaging |
| V2 | Intermittent claudication |
| V3 | Vascular surgery or stroke/transient ischaemic attack (TIA) |
| Cardiac | |
| C0 | No sign |
| C1 | Chest pain/angina/abnormal EKG or abnormal stress test with no symptom or mitral insufficiency |
| C2 | Heart attack |
| Renal | |
| R0 | No sign |
| R1 | Nephrolithiasis |
| In bold, the features are added. RE= right eye; LE= left eye; EKG= electrocardiogram. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boraldi, F.; Murro, V.; Lofaro, F.D.; Mucciolo, D.P.; Costa, S.; Pavese, L.; Quaglino, D. Phenotypic Features and Genetic Findings in a Cohort of Italian Pseudoxanthoma Elasticum Patients and Update of the Ophthalmologic Evaluation Score. J. Clin. Med. 2021, 10, 2710. https://doi.org/10.3390/jcm10122710
Boraldi F, Murro V, Lofaro FD, Mucciolo DP, Costa S, Pavese L, Quaglino D. Phenotypic Features and Genetic Findings in a Cohort of Italian Pseudoxanthoma Elasticum Patients and Update of the Ophthalmologic Evaluation Score. Journal of Clinical Medicine. 2021; 10(12):2710. https://doi.org/10.3390/jcm10122710
Chicago/Turabian StyleBoraldi, Federica, Vittoria Murro, Francesco Demetrio Lofaro, Dario Pasquale Mucciolo, Sonia Costa, Laura Pavese, and Daniela Quaglino. 2021. "Phenotypic Features and Genetic Findings in a Cohort of Italian Pseudoxanthoma Elasticum Patients and Update of the Ophthalmologic Evaluation Score" Journal of Clinical Medicine 10, no. 12: 2710. https://doi.org/10.3390/jcm10122710
APA StyleBoraldi, F., Murro, V., Lofaro, F. D., Mucciolo, D. P., Costa, S., Pavese, L., & Quaglino, D. (2021). Phenotypic Features and Genetic Findings in a Cohort of Italian Pseudoxanthoma Elasticum Patients and Update of the Ophthalmologic Evaluation Score. Journal of Clinical Medicine, 10(12), 2710. https://doi.org/10.3390/jcm10122710






