OX40L–OX40 Signaling in Atopic Dermatitis
Abstract
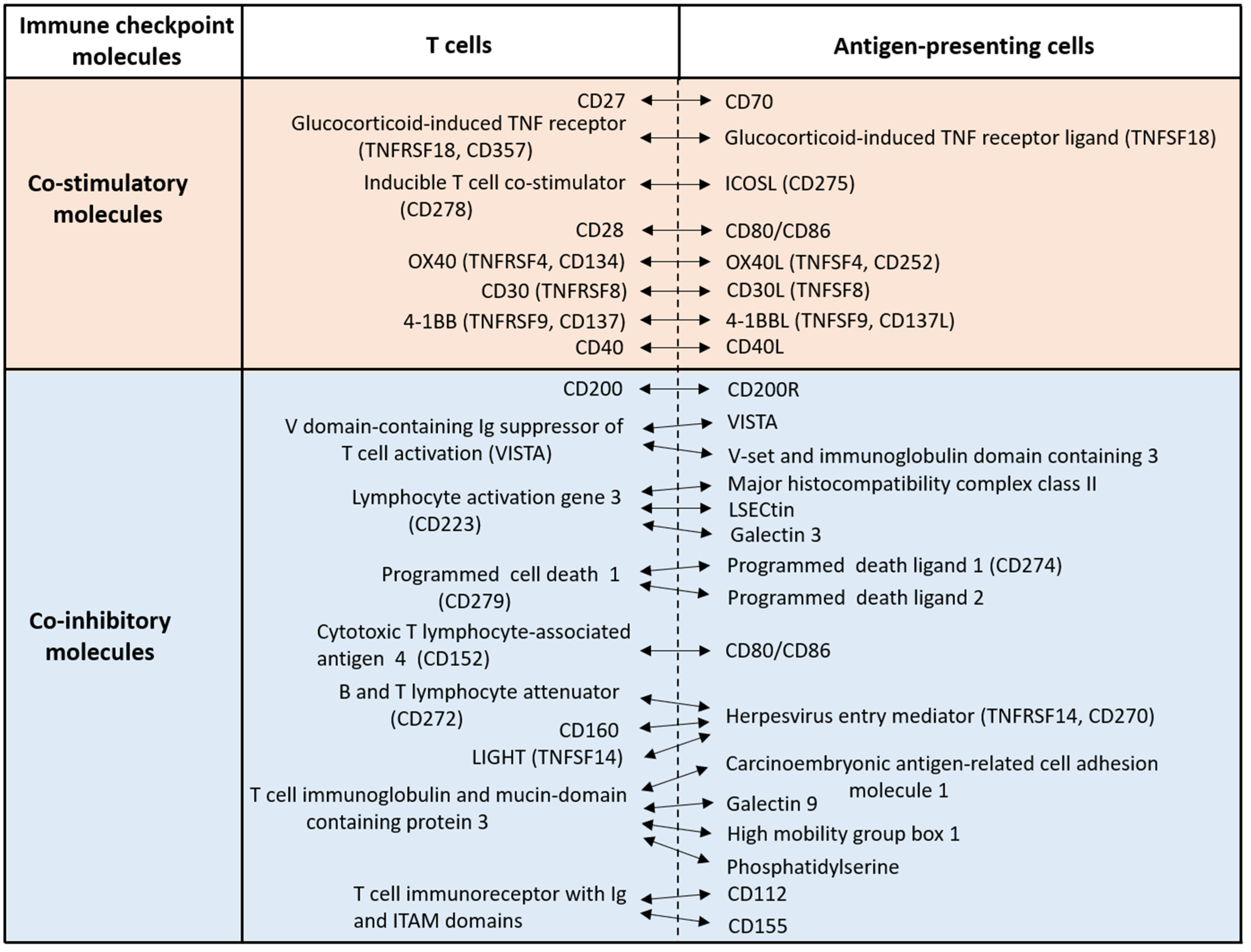
1. Introduction
2. OX40 Expression and Signaling in T Cells
3. OX40–OX40L Axis in Type 1 and Type 2 Immune Responses
4. OX40L–OX40 Axis in Atopic Dermatitis
5. Therapeutic Intervention Targeting the OX40L–OX40 Axis in Atopic Dermatitis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Ethical Approval
Consent to Participate
Consent for Publication
Data Availability Statement
Conflicts of Interest
References
- Smith-Garvin, J.E.; Koretzky, G.A.; Jordan, M.S. T Cell Activation. Annu. Rev. Immunol. 2009, 27, 591–619. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Flies, D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013, 13, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhu, H.X.; Yao, Y.; Bian, Z.H.; Zheng, Y.J.; Li, L.; Moutsopoulos, H.M.; Gershwin, M.E.; Lian, Z.X. Immune checkpoint molecules. Possible future therapeutic implications in autoimmune diseases. J. Autoimmun. 2019, 104, 102333. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Shi, Y.; Haymaker, C.L.; Naing, A.; Ciliberto, G.; Hajjar, J. T-cell agonists in cancer immunotherapy. J. Immunother. Cancer 2020, 8, e000966. [Google Scholar] [CrossRef]
- Nandi, D.; Pathak, S.; Verma, T.; Singh, M.; Chattopadhyay, A.; Thakur, S.; Raghavan, A.; Vijayamahantesh, G.A. T cell costimulation, checkpoint inhibitors and anti-tumor therapy. J. Biosci. 2020, 45, 50. [Google Scholar] [CrossRef]
- Eddy, K.; Chen, S. Overcoming Immune Evasion in Melanoma. Int. J. Mol. Sci. 2020, 21, 8984. [Google Scholar] [CrossRef]
- Willsmore, Z.N.; Coumbe, B.G.T.; Crescioli, S.; Reci, S.; Gupta, A.; Harris, R.J.; Chenoweth, A.; Chauhan, J.; Bax, H.J.; McCraw, A.; et al. Combined anti-PD-1 and anti-CTLA-4 checkpoint blockade: Treatment of melanoma and immune mechanisms of action. Eur. J. Immunol. 2021, 51, 544–556. [Google Scholar] [CrossRef]
- Hallqvist, A.; Rohlin, A.; Raghavan, S. Immune checkpoint blockade and biomarkers of clinical response in non–small cell lung cancer. Scand. J. Immunol. 2020, 92, e12980. [Google Scholar] [CrossRef]
- Alves Costa Silva, C.; Facchinetti, F.; Routy, B.; Derosa, L. New pathways in immune stimulation: Targeting OX40. ESMO Open 2020, 5, e000573. [Google Scholar] [CrossRef]
- Croft, M.; So, T.; Duan, W.; Soroosh, P. The significance of OX40 and OX40L to T-cell biology and immune disease. Immunol. Rev. 2009, 229, 173–191. [Google Scholar] [CrossRef]
- Croft, M. Control of Immunity by the TNFR-Related Molecule OX40 (CD134). Annu. Rev. Immunol. 2010, 28, 57–78. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Pavel, A.B.; Zhou, L.; Estrada, Y.D.; Zhang, N.; Xu, H.; Peng, X.; Wen, H.-C.; Govas, P.; Gudi, G.; et al. GBR 830, an anti-OX40, improves skin gene signatures and clinical scores in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019, 144, 482–493.e7. [Google Scholar] [CrossRef] [PubMed]
- Available online:. Available online: https://www.kyowakirin.com/media_center/news_releases/2021/pdf/e20210218_01.pdf (accessed on 16 March 2021).
- Furue, M. Regulation of skin barrier function via competition between AHR axis versus IL-13/IL-4‒JAK‒STAT6/STAT3 axis: Pathogenic and therapeutic implications in atopic dermatitis. J. Clin. Med. 2020, 9, 3741. [Google Scholar] [CrossRef]
- Nakahara, T.; Kido-Nakahara, M.; Tsuji, G.; Furue, M. Basics and recent advances in the pathophysiology of atopic dermatitis. J. Dermatol. 2021, 48, 130–139. [Google Scholar] [CrossRef]
- Gramaglia, I.; Jember, A.; Pippig, S.D.; Weinberg, A.D.; Killeen, N.; Croft, M. The OX40 Costimulatory Receptor Determines the Development of CD4 Memory by Regulating Primary Clonal Expansion. J. Immunol. 2000, 165, 3043–3050. [Google Scholar] [CrossRef]
- Bansal-Pakala, P.; Halteman, B.S.; Cheng, M.H.; Croft, M. Costimulation of CD8 T Cell Responses by OX40. J. Immunol. 2004, 172, 4821–4825. [Google Scholar] [CrossRef] [PubMed]
- Webb, G.J.; Hirschfield, G.M.; Lane, P.J. OX40, OX40L and Autoimmunity: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 50, 312–332. [Google Scholar] [CrossRef] [PubMed]
- Vu, M.D.; Xiao, X.; Gao, W.; Degauque, N.; Chen, M.; Kroemer, A.; Killeen, N.; Ishii, N.; Li, X.C. OX40 costimulation turns off Foxp3+ Tregs. Blood 2007, 110, 2501–2510. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, X.; Lan, P.; Li, J.; Dou, Y.; Chen, W.; Ishii, N.; Chen, S.; Xia, B.; Chen, K.; et al. OX40 Costimulation Inhibits Foxp3 Expression and Treg Induction via BATF3-Dependent and Independent Mechanisms. Cell Rep. 2018, 24, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Jacquemin, C.; Augusto, J.F.; Scherlinger, M.; Gensous, N.; Forcade, E.; Douchet, I.; Levionnois, E.; Richez, C.; Lazaro, E.; Duffau, P.; et al. OX40L/OX40 axis impairs follicular and natural Treg function in human SLE. JCI Insight 2018, 3, e122167. [Google Scholar] [CrossRef]
- Kopf, M.; Ruedl, C.; Schmitz, N.; Gallimore, A.; Lefrang, K.; Ecabert, B.; Odermatt, B.; Bachmann, M.F. OX40-Deficient Mice Are Defective in Th Cell Proliferation but Are Competent in Generating B Cell and CTL Responses after Virus Infection. Immunity 1999, 11, 699–708. [Google Scholar] [CrossRef]
- Gajdasik, D.W.; Gaspal, F.; Halford, E.E.; Fiancette, R.; Dutton, E.E.; Willis, C.; Rückert, T.; Romagnani, C.; Gerard, A.; Bevington, S.L.; et al. Th1 responses in vivo require cell-specific provision of OX40L dictated by environmental cues. Nat. Commun. 2020, 11, 3421. [Google Scholar] [CrossRef]
- Furue, M.; Ulzii, D.; Vu, Y.H.; Tsuji, G.; Kido-Nakahara, M.; Nakahara, T. Pathogenesis of atopic dermatitis: Current paradigm. Iran. J. Immunol. 2019, 16, 97–107. [Google Scholar] [PubMed]
- Boguniewicz, M.; Beck, L.A.; Sher, L.; Guttman-Yassky, E.; Thaçi, D.; Blauvelt, A.; Worm, M.; Corren, J.; Soong, W.; Lio, P.; et al. Dupilumab Improves Asthma and Sinonasal Outcomes in Adults with Moderate to Severe Atopic Dermatitis. J. Allergy Clin. Immunol. Pract. 2021, 9, 1212–1223.e6. [Google Scholar] [CrossRef]
- Jember, A.G.; Zuberi, R.; Liu, F.T.; Croft, M. Development of Allergic Inflammation in a Murine Model of Asthma Is Dependent on the Costimulatory Receptor Ox40. J. Exp. Med. 2001, 193, 387–392. [Google Scholar] [CrossRef]
- Park, S.C.; Shim, D.; Kim, H.; Bak, Y.; Choi, D.Y.; Yoon, J.H.; Kim, C.H.; Shin, S.J. Fms-like tyrosine kinase 3-independent dendritic cells are major mediators of Th2 immune responses in allergen-induced asthmatic mice. Int. J. Mol. Sci. 2020, 21, 9508. [Google Scholar] [CrossRef]
- Wu, J.; Cui, Y.; Zhu, W.; Bai, S.; Zhao, N.; Liu, B. Critical role of OX40/OX40L in ILC2-mediated activation of CD4+T cells during respiratory syncytial virus infection in mice. Int. Immunopharmacol. 2019, 76, 105784. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.P.; Xu, W.; Zhao, F.P.; Pan, H.Z.; Feng, H.J.; Xu, S.E.; Zhao, C.; Bao, Y.L.; Jiang, L.; Huang, Y.; et al. Effect of Blocking the OX40/OX40L Signaling Pathway by siRNA Interference on Animal Experimental Study of Allergic Rhinitis. Arch. Med. Res. 2019, 50, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Kumamoto, Y.; Linehan, M.; Weinstein, J.S.; Laidlaw, B.J.; Craft, J.E.; Iwasaki, A. CD301b⁺ dermal dendritic cells drive T helper 2 cell-mediated immunity. Immunity 2013, 39, 733–743. [Google Scholar] [CrossRef]
- Wu, J.; Hayes, B.W.; Phoenix, C.; Macias, G.S.; Miao, Y.; Choi, H.W.; Hughes, F.M., Jr.; Todd Purves, J.; Lee Reinhardt, R.; Abraham, S.N. A highly polarized TH2 bladder response to infection promotes epithelial repair at the expense of preventing new infections. Nat. Immunol. 2020, 21, 671–683. [Google Scholar] [CrossRef]
- Xiao, X.; Shi, X.; Fan, Y.; Wu, C.; Zhang, X.; Minze, L.; Liu, W.; Ghobrial, R.M.; Lan, P.; Li, X.C. The costimulatory receptor OX40 inhibits interleukin-17 expression through activation of repressive chromatin remodeling pathways. Immunity 2016, 44, 1271–1283. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Shang, X.; Benson, J.; Merle Elloso, M.; Schantz, A.; Bracht, M.; Orlovsky, Y.; Sweet, R. Negative regulation of IL-17 production by OX40/OX40L interaction. Cell. Immunol. 2008, 253, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhong, W.; Hinrichs, D.; Wu, X.; Weinberg, A.; Hall, M.; Spencer, D.; Wegmann, K.; Rosenbaum, J.T. Activation of OX40 Augments Th17 Cytokine Expression and Antigen-Specific Uveitis. Am. J. Pathol. 2010, 177, 2912–2920. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Jiang, T.T.; Chaturvedi, V.; Kinder, J.M.; Ertelt, J.M.; Rowe, J.H.; Steinbrecher, K.A.; Way, S.S. Commensal microbes drive intestinal inflammation by IL-17-producing CD4+ T cells through ICOSL and OX40L costimulation in the absence of B7-1 and B7-2. Proc. Natl. Acad. Sci. USA 2014, 111, 10672–10677. [Google Scholar] [CrossRef] [PubMed]
- Watts, T.H. TNF/TNFR family members in costimulation of T cell responses. Annu. Rev. Immunol. 2005, 23, 23–68. [Google Scholar] [CrossRef]
- Gaspal, F.; Bekiaris, V.; Kim, M.Y.; Withers, D.R.; Bobat, S.; MacLennan, I.C.; Anderson, G.; Lane, P.J.; Cunningham, A.F. Critical synergy of CD30 and OX40 signals in CD4 T cell homeostasis and Th1 immunity to Salmonella. J. Immunol. 2008, 180, 2824–2829. [Google Scholar] [CrossRef]
- Gaspal, F.; Withers, D.; Saini, M.; Bekiaris, V.; McConnell, F.M.; White, A.; Khan, M.; Yagita, H.; Walker, L.S.; Anderson, G.; et al. Abrogation of CD30 and OX40 signals prevents autoimmune disease in FoxP3-deficient mice. J. Exp. Med. 2011, 208, 1579–1584. [Google Scholar] [CrossRef]
- Gracias, D.T.; Sethi, G.S.; Mehta, A.K.; Miki, H.; Gupta, R.K.; Yagita, H.; Croft, M. Combination blockade of OX40L and CD30L inhibits allergen-driven memory TH2 cell reactivity and lung inflammation. J. Allergy Clin. Immunol. 2020, 147, 2316–2329. [Google Scholar] [CrossRef]
- Esaki, H.; Brunner, P.M.; Renert-Yuval, Y.; Czarnowicki, T.; Huynh, T.; Tran, G.; Lyon, S.; Rodriguez, G.; Immaneni, S.; Johnson, D.B.; et al. Early-onset pediatric atopic dermatitis is T(H)2 but also T(H)17 polarized in skin. J. Allergy Clin. Immunol. 2016, 138, 1639–1651. [Google Scholar] [CrossRef] [PubMed]
- Hammad, H.; Lambrecht, B.N. Barrier Epithelial Cells and the Control of Type 2 Immunity. Immunity 2015, 43, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Aktar, M.K.; Kido-Nakahara, M.; Furue, M.; Nakahara, T. Mutual upregulation of endothelin-1 and IL-25 in atopic dermatitis. Allergy 2015, 70, 846–854. [Google Scholar] [CrossRef]
- Soumelis, V.; Reche, P.A.; Kanzler, H.; Yuan, W.; Edward, G.; Homey, B.; Gilliet, M.; Ho, S.; Antonenko, S.; Lauerma, A.; et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002, 3, 673–680. [Google Scholar] [CrossRef]
- Oyoshi, M.K.; Larson, R.P.; Ziegler, S.F.; Geha, R.S. Mechanical injury polarizes skin dendritic cells to elicit a T(H)2 response by inducing cutaneous thymic stromal lymphopoietin expression. J. Allergy Clin. Immunol. 2010, 126, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Gilliet, M.; Soumelis, V.; Watanabe, N.; Hanabuchi, S.; Antonenko, S.; De Waal-Malefyt, R.; Liu, Y.J. Human Dendritic Cells Activated by TSLP and CD40L Induce Proallergic Cytotoxic T Cells. J. Exp. Med. 2003, 197, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Wang, Y.H.; Duramad, O.; Hori, T.; Delespesse, G.J.; Watanabe, N.; Qin, F.X.; Yao, Z.; Cao, W.; Liu, Y.J. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J. Exp. Med. 2005, 202, 1213–1223. [Google Scholar] [CrossRef]
- Liu, Y.J.; Soumelis, V.; Watanabe, N.; Ito, T.; Wang, Y.-H.; de Waal Malefyt, R.; Omori, M.; Zhou, B.; Ziegler, S.F. TSLP: An Epithelial Cell Cytokine that Regulates T Cell Differentiation by Conditioning Dendritic Cell Maturation. Annu. Rev. Immunol. 2007, 25, 193–219. [Google Scholar] [CrossRef]
- Seshasayee, D.; Lee, W.P.; Zhou, M.; Shu, J.; Suto, E.; Zhang, J.; Diehl, L.; Austin, C.D.; Meng, Y.G.; Tan, M.; et al. In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J. Clin. Invest. 2007, 117, 3868–3878. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; French, D.; Mao, W.; Maruoka, M.; Risser, P.; Lee, J.; Foster, J.; Aggarwal, S.; Nicholes, K.; Guillet, S.; et al. Forced expression of murine IL-17E induces growth retardation, jaundice, a Th2-biased response, and multiorgan inflammation in mice. J. Immunol. 2001, 167, 6559–6567. [Google Scholar] [CrossRef] [PubMed]
- Hurst, S.D.; Muchamuel, T.; Gorman, D.M.; Gilbert, J.M.; Clifford, T.; Kwan, S.; Menon, S.; Seymour, B.; Jackson, C.; Kung, T.T.; et al. New IL-17 family members promote Th1 or Th2 responses in the lung: In vivo function of the novel cytokine IL-25. J. Immunol. 2002, 169, 443–453. [Google Scholar] [CrossRef]
- Zheng, R.; Chen, F.H.; Gao, W.X.; Wang, D.; Yang, Q.T.; Wang, K.; Lai, Y.Y.; Deng, J.; Jiang, L.J.; Sun, Y.Q.; et al. The T(H)2-polarizing function of atopic interleukin 17 receptor B-positive dendritic cells up-regulated by lipopolysaccharide. Ann. Allergy Asthma Immunol. 2017, 118, 474–482. [Google Scholar] [CrossRef]
- Cayrol, C.; Duval, A.; Schmitt, P.; Roga, S.; Camus, M.; Stella, A.; Burlet-Schiltz, O.; Gonzalez-de-Peredo, A.; Girard, J.P. Environmental allergens induce allergic inflammation through proteolytic maturation of IL-33. Nat. Immunol. 2018, 19, 375–385. [Google Scholar] [CrossRef]
- Dickel, H.; Gambichler, T.; Kamphowe, J.; Altmeyer, P.; Skrygan, M. Standardized tape stripping prior to patch testing induces upregulation of Hsp90, Hsp70, IL-33, TNF-α and IL-8/CXCL8 mRNA: New insights into the involvement of ‘alarmins’. Contact Dermat. 2010, 63, 215–222. [Google Scholar] [CrossRef]
- Halim, T.Y.F.; Rana, B.M.J.; Walker, J.A.; Kerscher, B.; Knolle, M.D.; Jolin, H.E.; Serrao, E.M.; Haim-Vilmovsky, L.; Teichmann, S.A.; Rodewald, H.R.; et al. Tissue-Restricted Adaptive Type 2 Immunity Is Orchestrated by Expression of the Costimulatory Molecule OX40L on Group 2 Innate Lymphoid Cells. Immunity 2018, 48, 1195–1207.e6. [Google Scholar] [CrossRef]
- Nechama, M.; Kwon, J.; Wei, S.; Kyi, A.T.; Welner, R.S.; Ben-Dov, I.Z.; Arredouani, M.S.; Asara, J.M.; Chen, C.H.; Tsai, C.Y.; et al. The IL-33-PIN1-IRAK-M axis is critical for type 2 immunity in IL-33-induced allergic airway inflammation. Nat. Commun. 2018, 9, 1603. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Fariñas, M.; Dhingra, N.; Gittler, J.; Shemer, A.; Cardinale, I.; de Guzman Strong, C.; Krueger, J.G.; Guttman-Yassky, E. Intrinsic atopic dermatitis shows similar TH2 and higher TH17 immune activation compared with extrinsic atopic dermatitis. J. Allergy Clin. Immunol. 2013, 132, 361–370. [Google Scholar] [CrossRef]
- Noda, S.; Suárez-Fariñas, M.; Ungar, B.; Kim, S.J.; de Guzman Strong, C.; Xu, H.; Peng, X.; Estrada, Y.D.; Nakajima, S.; Honda, T.; et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J. Allergy Clin. Immunol. 2015, 136, 1254–1264. [Google Scholar] [CrossRef]
- Ilves, T.; Harvima, I.T. OX40 ligand and OX40 are increased in atopic dermatitis lesions but do not correlate with clinical severity. J. Eur. Acad. Dermatol. Venereol. 2013, 27, e197–e205. [Google Scholar] [CrossRef]
- Fujita, H.; Shemer, A.; Suárez-Fariñas, M.; Johnson-Huang, L.M.; Tintle, S.; Cardinale, I.; Fuentes-Duculan, J.; Novitskaya, I.; Carucci, J.A.; Krueger, J.G.; et al. Lesional dendritic cells in patients with chronic atopic dermatitis and psoriasis exhibit parallel ability to activate T-cell subsets. J. Allergy Clin. Immunol. 2011, 128, 574–582.e12. [Google Scholar] [CrossRef]
- Elsner, J.S.; Carlsson, M.; Stougaard, J.K.; Nygaard, U.; Buchner, M.; Fölster-Holst, R.; Hvid, M.; Vestergaard, C.; Deleuran, M.; Deleuran, B. The OX40 Axis is Associated with Both Systemic and Local Involvement in Atopic Dermatitis. Acta Derm. Venereol. 2020, 100, adv00099–5. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Iizuka, H.; Nemoto, O.; Shimabe, M.; Furukawa, Y.; Kikuta, N.; Ootaki, K. Safety, tolerability and efficacy of repeated intravenous infusions of KHK4083, a fully human anti-OX40 monoclonal antibody, in Japanese patients with moderate to severe atopic dermatitis. J. Dermatol. Sci. 2020, 99, 82–89. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furue, M.; Furue, M. OX40L–OX40 Signaling in Atopic Dermatitis. J. Clin. Med. 2021, 10, 2578. https://doi.org/10.3390/jcm10122578
Furue M, Furue M. OX40L–OX40 Signaling in Atopic Dermatitis. Journal of Clinical Medicine. 2021; 10(12):2578. https://doi.org/10.3390/jcm10122578
Chicago/Turabian StyleFurue, Masutaka, and Mihoko Furue. 2021. "OX40L–OX40 Signaling in Atopic Dermatitis" Journal of Clinical Medicine 10, no. 12: 2578. https://doi.org/10.3390/jcm10122578
APA StyleFurue, M., & Furue, M. (2021). OX40L–OX40 Signaling in Atopic Dermatitis. Journal of Clinical Medicine, 10(12), 2578. https://doi.org/10.3390/jcm10122578





