Timing of Bisphosphonate (Alendronate) Initiation after Surgery for Fragility Fracture: A Population-Based Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Study Samples
2.2. Confounders and Bias
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
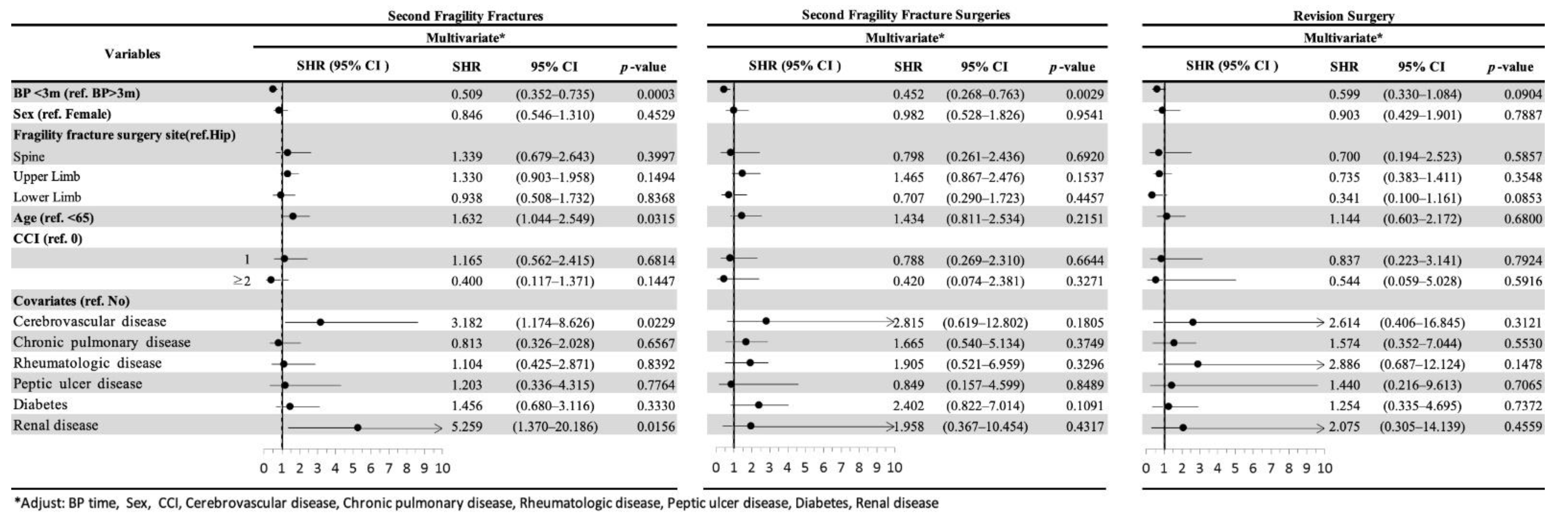
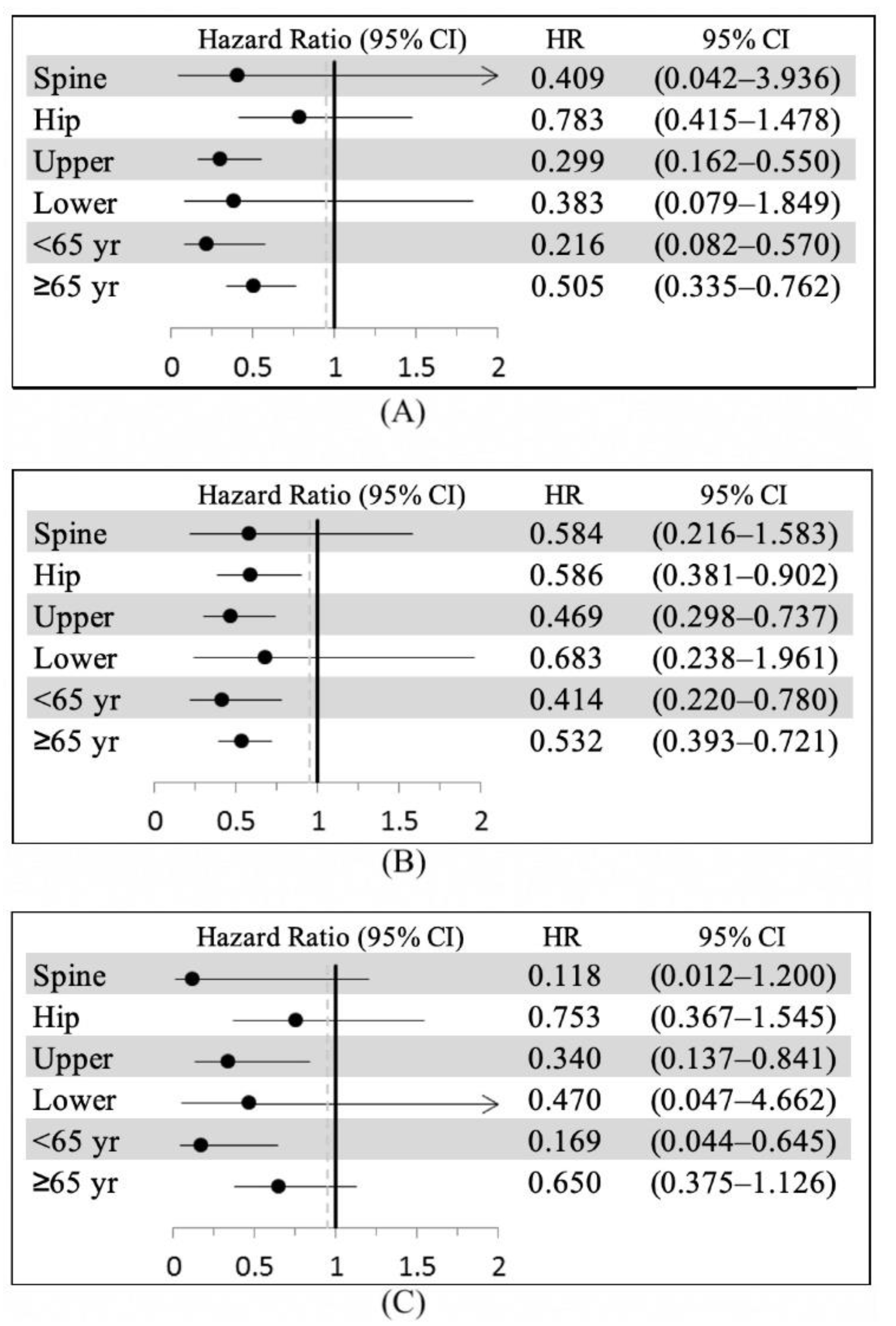
3.2. Second FF and Second FF Surgery
3.3. Adverse Events after FFs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsoumpra, M.K.; Muniz, J.R.; Barnett, B.L.; Kwaasi, A.A.; Pilka, E.S.; Kavanagh, K.L.; Evdokimov, A.; Walter, R.L.; Von Delft, F.; Ebetino, F.H.; et al. The inhibition of human farnesyl pyrophosphate synthase by nitrogen-containing bisphosphonates. Elucidating the role of active site threonine 201 and tyrosine 204 residues using enzyme mutants. Bone 2015, 81, 478–486. [Google Scholar] [CrossRef]
- Cao, Y.; Mori, S.; Mashiba, T.; Westmore, M.S.; Ma, L.; Sato, M.; Akiyama, T.; Shi, L.; Komatsubara, S.; Miyamoto, K.; et al. Raloxifene, estrogen, and alendronate affect the processes of fracture repair differently in ovariectomized rats. J. Bone Miner. Res. 2002, 17, 2237–2246. [Google Scholar] [CrossRef] [PubMed]
- Odvina, C.V.; Zerwekh, J.E.; Rao, D.S.; Maalouf, N.; Gottschalk, F.A.; Pak, C.Y. Severely suppressed bone turnover: A potential complication of alendronate therapy. J. Clin. Endocrinol. Metab. 2005, 90, 1294–1301. [Google Scholar] [CrossRef]
- Bauss, F.; Schenk, R.K.; Hort, S.; Muller-Beckmann, B.; Sponer, G. New model for simulation of fracture repair in full-grown beagle dogs: Model characterization and results from a long-term study with ibandronate. J. Pharmacol. Toxicol. Methods 2004, 50, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Munns, C.F.; Rauch, F.; Zeitlin, L.; Fassier, F.; Glorieux, F.H. Delayed osteotomy but not fracture healing in pediatric osteogenesis imperfecta patients receiving pamidronate. J. Bone Miner. Res. 2004, 19, 1779–1786. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.A.; Tannuri, U.; Guarniero, R. The effect of zoledronate during bone healing. J. Orthop. Traumatol. 2010, 11, 7–12. [Google Scholar] [CrossRef]
- Li, J.; Mori, S.; Kaji, Y.; Mashiba, T.; Kawanishi, J.; Norimatsu, H. Effect of bisphosphonate (incadronate) on fracture healing of long bones in rats. J. Bone Miner. Res. 1999, 14, 969–979. [Google Scholar] [CrossRef]
- Li, C.; Mori, S.; Li, J.; Kaji, Y.; Akiyama, T.; Kawanishi, J.; Norimatsu, H. Long-term effect of incadronate disodium (YM-175) on fracture healing of femoral shaft in growing rats. J. Bone Miner. Res. 2001, 16, 429–436. [Google Scholar] [CrossRef]
- Greiner, S.H.; Wildemann, B.; Back, D.A.; Alidoust, M.; Schwabe, P.; Haas, N.P.; Schmidmaier, G. Local application of zoledronic acid incorporated in a poly(D,L-lactide)-coated implant accelerates fracture healing in rats. Acta Orthop. 2008, 79, 717–725. [Google Scholar] [CrossRef]
- Rozental, T.D.; Vazquez, M.A.; Chacko, A.T.; Ayogu, N.; Bouxsein, M.L. Comparison of radiographic fracture healing in the distal radius for patients on and off bisphosphonate therapy. J. Hand Surg. Am. 2009, 34, 595–602. [Google Scholar] [CrossRef]
- Amanat, N.; Brown, R.; Bilston, L.E.; Little, D.G. A single systemic dose of pamidronate improves bone mineral content and accelerates restoration of strength in a rat model of fracture repair. J. Orthop. Res. 2005, 23, 1029–1034. [Google Scholar] [CrossRef]
- Little, D.G.; McDonald, M.; Bransford, R.; Godfrey, C.B.; Amanat, N. Manipulation of the anabolic and catabolic responses with OP-1 and zoledronic acid in a rat critical defect model. J. Bone Miner. Res. 2005, 20, 2044–2052. [Google Scholar] [CrossRef]
- Bransford, R.; Goergens, E.; Briody, J.; Amanat, N.; Cree, A.; Little, D. Effect of zoledronic acid in an L6-L7 rabbit spine fusion model. Eur. Spine J. 2007, 16, 557–562. [Google Scholar] [CrossRef]
- Li, C.; Wang, H.R.; Li, X.L.; Zhou, X.G.; Dong, J. The relation between zoledronic acid infusion and interbody fusion in patients undergoing transforaminal lumbar interbody fusion surgery. Acta Neurochir. 2012, 154, 731–738. [Google Scholar] [CrossRef]
- Kim, T.Y.; Ha, Y.C.; Kang, B.J.; Lee, Y.K.; Koo, K.H. Does early administration of bisphosphonate affect fracture healing in patients with intertrochanteric fractures? J. Bone Jt. Surg. Br. 2012, 94, 956–960. [Google Scholar] [CrossRef]
- Gong, H.S.; Song, C.H.; Lee, Y.H.; Rhee, S.H.; Lee, H.J.; Baek, G.H. Early initiation of bisphosphonate does not affect healing and outcomes of volar plate fixation of osteoporotic distal radial fractures. J. Bone Jt. Surg. Am. 2012, 94, 1729–1736. [Google Scholar] [CrossRef]
- Gray, R. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann. Stat. 1998, 1998, 1141–1154. [Google Scholar] [CrossRef]
- Fine, J.P.; Gray, R.J. A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- Lewiecki, E.M. Bisphosphonates for the treatment of osteoporosis: Insights for clinicians. Ther. Adv. Chronic. Dis. 2010, 1, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Anastasilakis, A.D.; Polyzos, S.A.; Makras, P.; Aubry-Rozier, B.; Kaouri, S.; Lamy, O. Clinical Features of 24 Patients With Rebound-Associated Vertebral Fractures After Denosumab Discontinuation: Systematic Review and Additional Cases. J. Bone Miner. Res. 2017, 32, 1291–1296. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, E.F.; Lyles, K.W.; Colón-Emeric, C.S.; Pieper, C.F.; Magaziner, J.S.; Adachi, J.D.; Hyldstrup, L.; Recknor, C.; Nordsletten, L.; Lavecchia, C.; et al. Antifracture efficacy and reduction of mortality in relation to timing of the first dose of zoledronic acid after hip fracture. J. Bone Miner. Res. 2009, 24, 1308–1313. [Google Scholar] [CrossRef]
- Lyles, K.W.; Colón-Emeric, C.S.; Magaziner, J.S.; Adachi, J.D.; Pieper, C.F.; Mautalen, C.; Hyldstrup, L.; Recknor, C.; Nordsletten, L.; Moore, K.A.; et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N. Engl. J. Med. 2007, 357, 1799–1809. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, S.; Itsubo, T.; Nakamura, K.; Fujinaga, Y.; Sato, N.; Imaeda, T.; Kadoya, M.; Kato, H. Effect of early administration of alendronate after surgery for distal radial fragility fracture on radiological fracture healing time. Bone Jt. J. 2013, 95, 1544–1550. [Google Scholar] [CrossRef]
- Sing, C.-W.; Wong, A.Y.; Kiel, D.P.; Cheung, E.Y.; Lam, J.K.; Cheung, T.T.; Chan, E.W.; Kung, A.W.; Wong, I.C.; Cheung, C.-L. Association of Alendronate and Risk of Cardiovascular Events in Patients with Hip Fracture. J. Bone Miner. Res. 2018, 33, 1422–1434. [Google Scholar] [CrossRef]
- Guney, E.; Kisakol, G.; Ozgen, A.G.; Yilmaz, C.; Kabalak, T. Effects of bisphosphonates on lipid metabolism. Neuroendocrinol. Lett. 2008, 29, 252–255. [Google Scholar]
- Alishiri, G.; Heshmat-Ghahdarijani, K.; Hashemi, M.; Zavar, R.; Farahani, M.M. Alendronate slows down aortic stenosis progression in osteoporotic patients: An observational prospective study. J. Res. Med. Sci. 2020, 25, 65. [Google Scholar] [CrossRef]
- Takeuchi, K.; Kato, S.; Amagase, K. Gastric ulcerogenic and healing impairment actions of alendronate, a nitrogen-containing bisphosphonate—Prophylactic effects of rebamipide. Curr. Pharm. Des. 2011, 17, 1602–1611. [Google Scholar] [CrossRef]
- Peng, Y.L.; Hu, H.Y.; Luo, J.C.; Hou, M.C.; Lin, H.C.; Lee, F.Y. Alendronate, a bisphosphonate, increased upper and lower gastrointestinal bleeding: Risk factor analysis from a nationwide population-based study. Osteoporos. Int. 2014, 25, 1617–1623. [Google Scholar] [CrossRef]
- Miller, P.D.; Jamal, S.A.; Evenepoel, P.; Eastell, R.; Boonen, S. Renal safety in patients treated with bisphosphonates for osteoporosis: A review. J. Bone Miner. Res. 2013, 28, 2049–2059. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.-Y.; Tsai, K.-S.; Peng, J.-K.; Chen, C.-H.; Lin, G.-T.; Lin, C.-H.; Tu, S.-T.; Mao, I.-C.; Gau, Y.-L.; Liu, H.-C.; et al. The development of Taiwan Fracture Liaison Service network. Osteoporos. Sarcopenia 2018, 4, 47–52. [Google Scholar] [CrossRef]
- Wang, C.Y.; Fu, S.H.; Yang, R.S.; Chen, L.K.; Shen, L.J.; Hsiao, F.Y. Timing of anti-osteoporosis medications initiation after a hip fracture affects the risk of subsequent fracture: A nationwide cohort study. Bone 2020, 138, 115452. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.-H.; Lee, M.-C.; Chou, M.-C. Accuracy of cause-of-death coding in Taiwan: Types of miscoding and effects on mortality statistics. Int. J. Epidemiol. 2000, 29, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Saita, Y.; Ishijima, M.; Mogami, A.; Kubota, M.; Baba, T.; Kaketa, T.; Nagao, M.; Sakamoto, Y.; Sakai, K.; Homma, Y.; et al. The incidence of and risk factors for developing atypical femoral fractures in Japan. J. Bone Miner. Metab. 2015, 33, 311–318. [Google Scholar] [CrossRef]
- Lee, Y.K.; Ahn, S.; Kim, K.M.; Suh, C.S.; Koo, K.H. Incidence Rate of Atypical Femoral Fracture after Bisphosphonates Treatment in Korea. J. Korean Med. Sci. 2018, 33, e38. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, F.-Y.; Huang, W.-F.; Chen, Y.-M.; Wen, Y.-W.; Kao, Y.-H.; Chen, L.-K.; Tsai, Y.-W. Hip and subtrochanteric or diaphyseal femoral fractures in alendronate users: A 10-year, nationwide retrospective cohort study in Taiwanese women. Clin. Ther. 2011, 33, 1659–1667. [Google Scholar] [CrossRef]


| Variables | Total | EIBP | LIBP | p Value | ||||
|---|---|---|---|---|---|---|---|---|
| (n = 524) | (n = 262) | (n = 262) | ||||||
| Count | % | Count | % | Count | % | |||
| Sex | ||||||||
| Male | 115 | 21.946 | 56 | 21.374 | 59 | 22.519 | 0.752 | |
| Female | 409 | 78.053 | 206 | 78.626 | 203 | 77.481 | ||
| Age (years) | ||||||||
| <65 | 129 | 24.618 | 65 | 24.809 | 64 | 24.427 | 0.919 | |
| ≥65 | 395 | 75.382 | 197 | 75.191 | 198 | 75.573 | ||
| CCI | ||||||||
| 0 | 327 | 62.405 | 163 | 62.214 | 164 | 62.595 | 0.968 | |
| 1 | 123 | 23.473 | 61 | 23.282 | 62 | 23.664 | ||
| ≥2 | 74 | 14.122 | 38 | 14.504 | 36 | 13.740 | ||
| Covariates | ||||||||
| Cerebrovascular disease | 14 | 2.672 | 11 | 4.198 | 3 | 1.145 | 0.030 | |
| Chronic pulmonary disease | 43 | 8.206 | 21 | 8.015 | 22 | 8.397 | 0.874 | |
| Rheumatologic disease | 21 | 4.008 | 8 | 3.053 | 13 | 4.962 | 0.265 | |
| Peptic ulcer disease | 20 | 3.817 | 10 | 3.817 | 10 | 3.817 | 1.000 | |
| Diabetes | 104 | 19.847 | 51 | 19.466 | 53 | 20.229 | 0.827 | |
| Diabetes with chronic complications | 26 | 4.962 | 13 | 4.962 | 13 | 4.962 | 1.000 | |
| Renal disease | 13 | 2.481 | 6 | 2.290 | 7 | 2.672 | 0.779 | |
| Obesity | 5 | 0.954 | 3 | 1.145 | 2 | 0.763 | 0.744 | |
| History of fragility fracture | 42 | 8.015 | 22 | 8.396 | 20 | 7.633 | 0.615 | |
| Fragility fracture surgery site | ||||||||
| Spine | 38 | 7.252 | 19 | 7.252 | 19 | 7.252 | 1.000 | |
| Hip | 224 | 42.748 | 112 | 42.748 | 112 | 42.748 | ||
| Upper limb | 188 | 35.878 | 94 | 35.878 | 94 | 35.878 | ||
| Lower limb | 74 | 14.122 | 37 | 14.122 | 37 | 14.122 | ||
| Concomitant drug | ||||||||
| Proton pump inhibitor | 4 | 0.331 | 3 | 0.320 | 1 | 0.368 | 0.905 | |
| Steroid | 30 | 2.481 | 19 | 2.028 | 11 | 4.044 | 0.059 | |
| Antiepileptic | 4 | 0.331 | 3 | 0.320 | 1 | 0.368 | 0.905 | |
| Follow-up time (mean years) | 4.314 | 4.2456 | 4.3824 | |||||
| Variables | EIBP | LIBP | Log-Rank p Value | Chi-Square p Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| (n = 262) | (n = 262) | ||||||||
| Count | % | Incidence * | Count | % | Incidence * | ||||
| Site of second fragility fracture | 50 | 19.084 | 5.173 | 85 | 32.443 | 9.693 | 0.0005 | 0.0005 | |
| Trunk | <5 | <5.000 | <5 | <5.000 | |||||
| Spine | 8 | 16.000 | 22 | 25.882 | |||||
| Hip | 22 | 44.000 | 31 | 36.471 | |||||
| Upper Limb | 16 | 32.000 | 23 | 27.059 | |||||
| Lower Limb | <5 | <5.000 | <5 | <5.000 | |||||
| Site of second fracture surgery | 24 | 9.160 | 2.323 | 49 | 18.702 | 4.943 | 0.0021 | 0.0016 | |
| Spine | <5 | <5 | 5 | 10.204 | |||||
| Hip | 19 | 79.167 | 24 | 48.980 | |||||
| Upper Limb | 4 | 16.667 | 14 | 28.571 | |||||
| Lower Limb | <5 | <5 | 5 | 10.204 | |||||
| Site of revision surgery | 18 | 6.870 | 1.705 | 30 | 11.450 | 2.858 | 0.0789 | 0.0692 | |
| Spine | <5 | <5.000 | <5 | <10.000 | |||||
| Hip | 15 | 83.333 | 11 | 36.667 | |||||
| Upper Limb | <5 | <15.000 | 14 | 46.667 | |||||
| Lower Limb | <5 | <5.000 | <5 | <5.000 | |||||
| Death | 35 | 13.359 | 3.146 | 51 | 19.466 | 4.442 | 0.1351 | 0.0591 | |
| Complication | |||||||||
| Respiratory failure | 14 | 5.344 | 1.271 | 36 | 13.740 | 3.208 | 0.0025 | 0.0011 | |
| Pneumonia | 81 | 30.916 | 8.577 | 93 | 35.496 | 9.974 | 0.3032 | 0.2657 | |
| Acute cardiac event | 55 | 20.992 | 5.554 | 65 | 24.809 | 6.631 | 0.3111 | 0.2985 | |
| CVA and SCI, nerve injury | 69 | 26.336 | 7.211 | 67 | 25.573 | 6.948 | 0.8965 | 0.842 | |
| Renal failure | 6 | 2.290 | 0.543 | 18 | 6.870 | 1.597 | 0.0174 | 0.0122 | |
| UGI bleeding | 170 | 64.885 | 11.738 | 199 | 75.954 | 35.809 | 0.0361 | 0.0055 | |
| Acute postoperative hemorrhage | 11 | 4.198 | 0.989 | 12 | 4.580 | 1.045 | 0.8927 | 0.8311 | |
| Urinary tract infection | 97 | 37.023 | 11.210 | 115 | 43.893 | 13.123 | 0.2457 | 0.1091 | |
| Sepsis | 32 | 12.214 | 2.989 | 38 | 14.504 | 3.445 | 0.6182 | 0.441 | |
| Osteomyelitis | 5 | 1.908 | 0.454 | 13 | 4.962 | 1.170 | 0.0604 | 0.055 | |
| Postoperative Infection | 5 | 1.908 | 0.457 | 13 | 4.962 | 1.182 | 0.059 | 0.055 | |
| Nonunion/malunion | 6 | 2.290 | 0.549 | 12 | 4.580 | 1.094 | 0.1581 | 0.1501 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.-H.; Lin, Y.-S.; Wu, C.; Lee, C.-Y.; Chen, Y.-C.; Huang, T.-J.; Cheng, J.-S. Timing of Bisphosphonate (Alendronate) Initiation after Surgery for Fragility Fracture: A Population-Based Cohort Study. J. Clin. Med. 2021, 10, 2541. https://doi.org/10.3390/jcm10122541
Wu M-H, Lin Y-S, Wu C, Lee C-Y, Chen Y-C, Huang T-J, Cheng J-S. Timing of Bisphosphonate (Alendronate) Initiation after Surgery for Fragility Fracture: A Population-Based Cohort Study. Journal of Clinical Medicine. 2021; 10(12):2541. https://doi.org/10.3390/jcm10122541
Chicago/Turabian StyleWu, Meng-Huang, Yu-Sheng Lin, Christopher Wu, Ching-Yu Lee, Yi-Chia Chen, Tsung-Jen Huang, and Jur-Shan Cheng. 2021. "Timing of Bisphosphonate (Alendronate) Initiation after Surgery for Fragility Fracture: A Population-Based Cohort Study" Journal of Clinical Medicine 10, no. 12: 2541. https://doi.org/10.3390/jcm10122541
APA StyleWu, M.-H., Lin, Y.-S., Wu, C., Lee, C.-Y., Chen, Y.-C., Huang, T.-J., & Cheng, J.-S. (2021). Timing of Bisphosphonate (Alendronate) Initiation after Surgery for Fragility Fracture: A Population-Based Cohort Study. Journal of Clinical Medicine, 10(12), 2541. https://doi.org/10.3390/jcm10122541






