TRAP5b and RANKL/OPG Predict Bone Pathology in Patients with Gaucher Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. The Clinical Features of Bone Disease in GD Cohorts
2.3. Measurement of Biomarkers in Plasma Samples
2.4. Statistical Analysis
3. Results
3.1. Bone Pain Is Associated with Osteopenia and Osteoporosis in GD
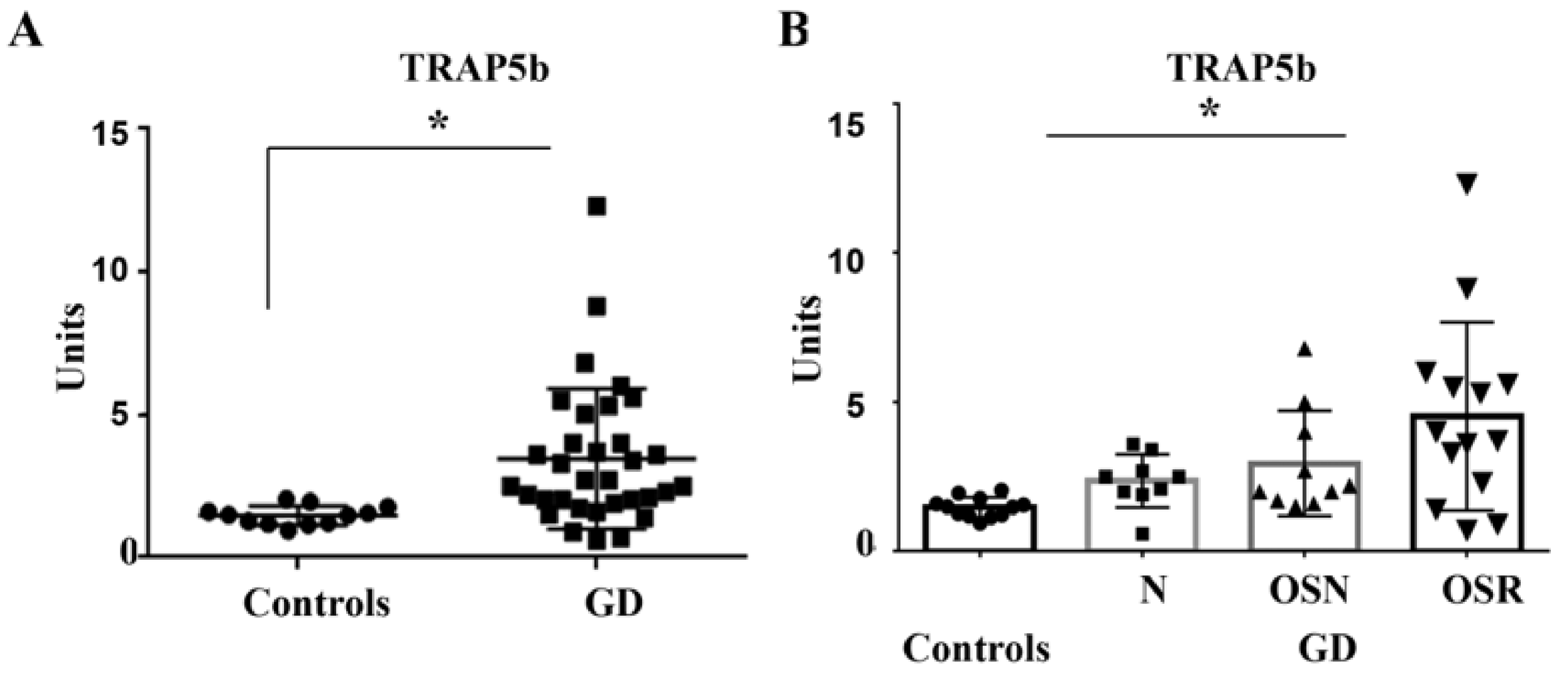
3.2. TRAP5b Is Increased in GD and Correlates with Osteoporosis
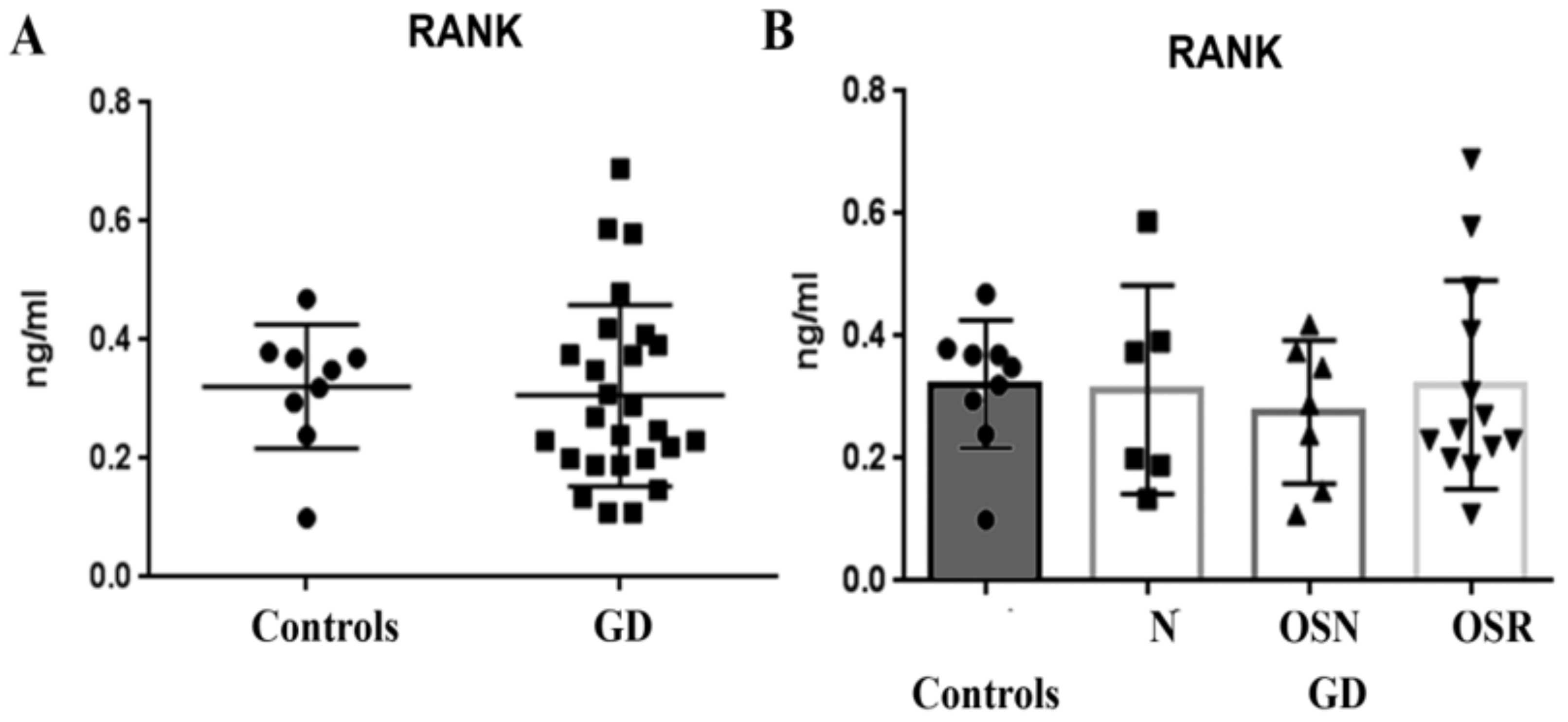
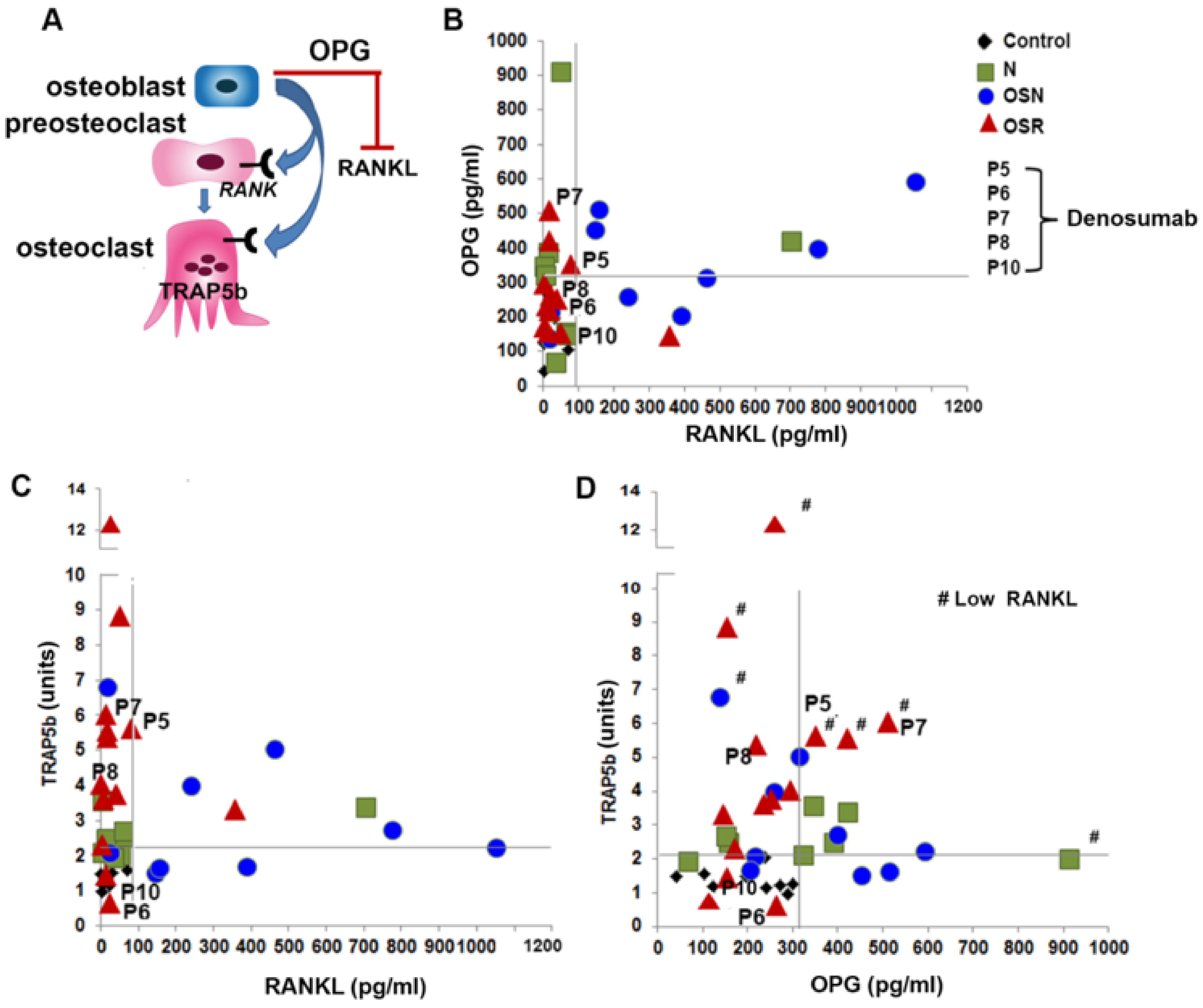
3.3. RANKL, Not RANK Is Elevated in GD and Correlates with Osteopenia
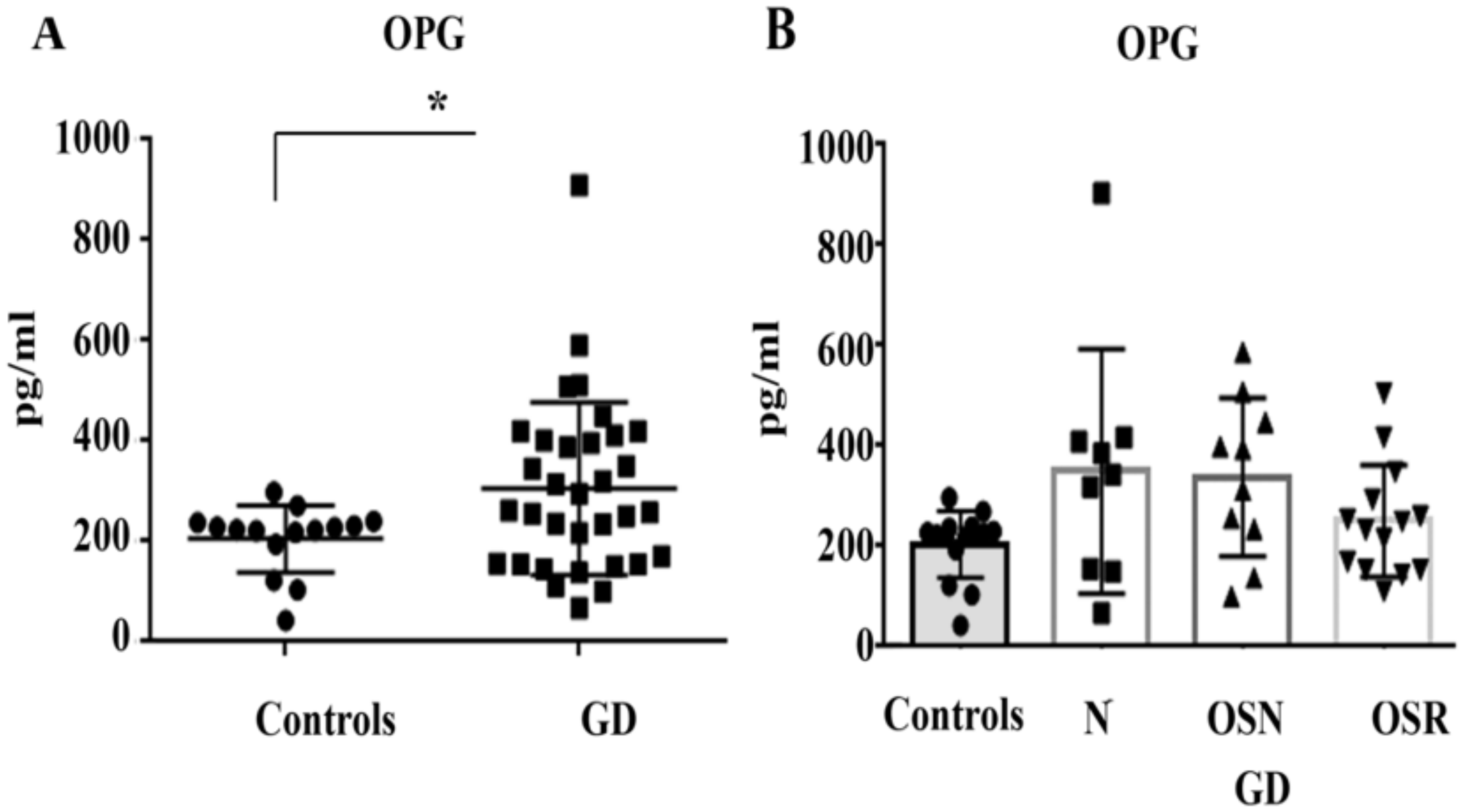
3.4. Elevated OPG Does Not Correlate with OSN or OSR in GD
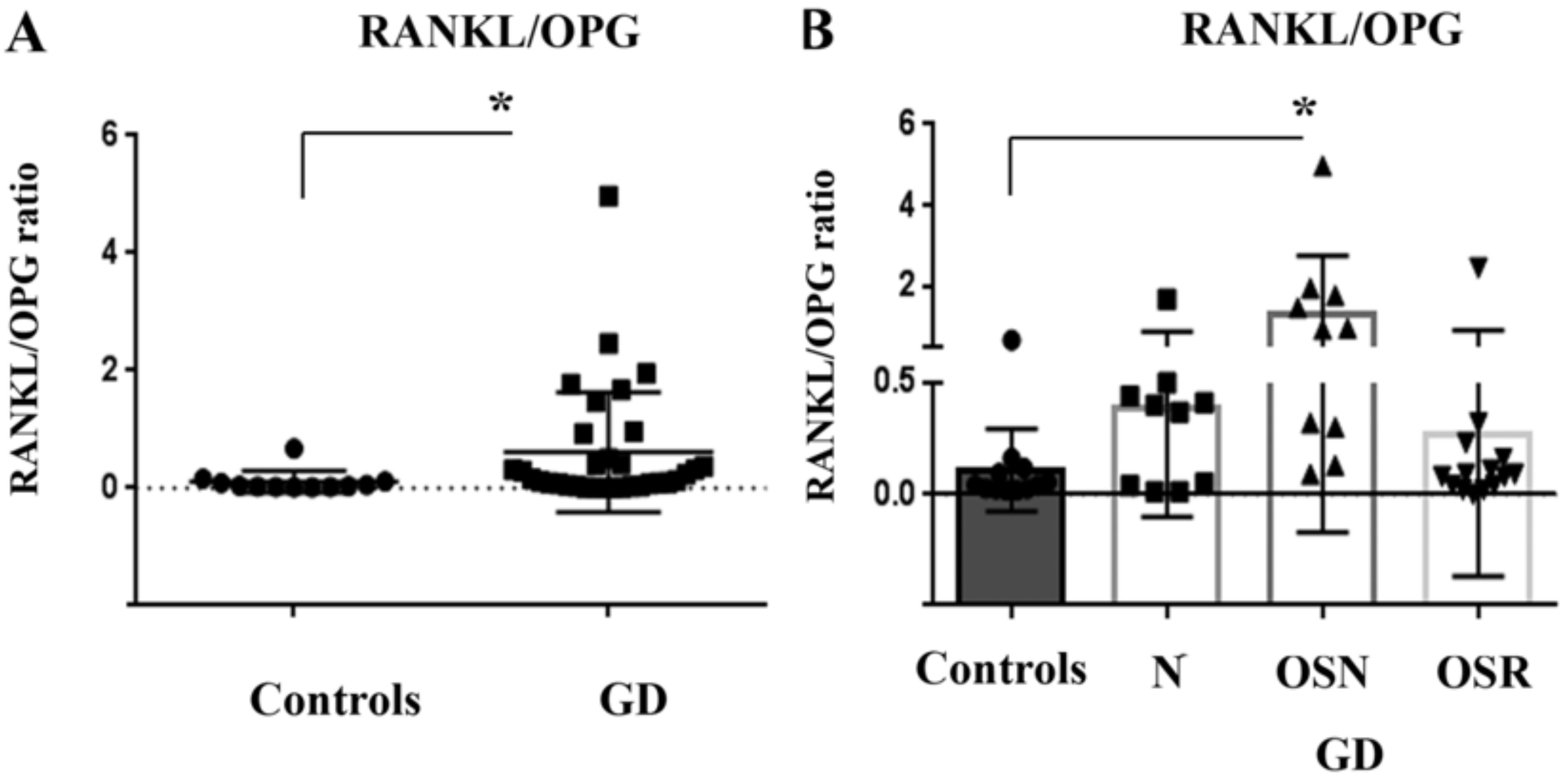
3.5. RANKL/OPG Ratio Is Higher in Patients with GD
3.6. The Relationship between TRAP5b, RANKL, and OPG in GD
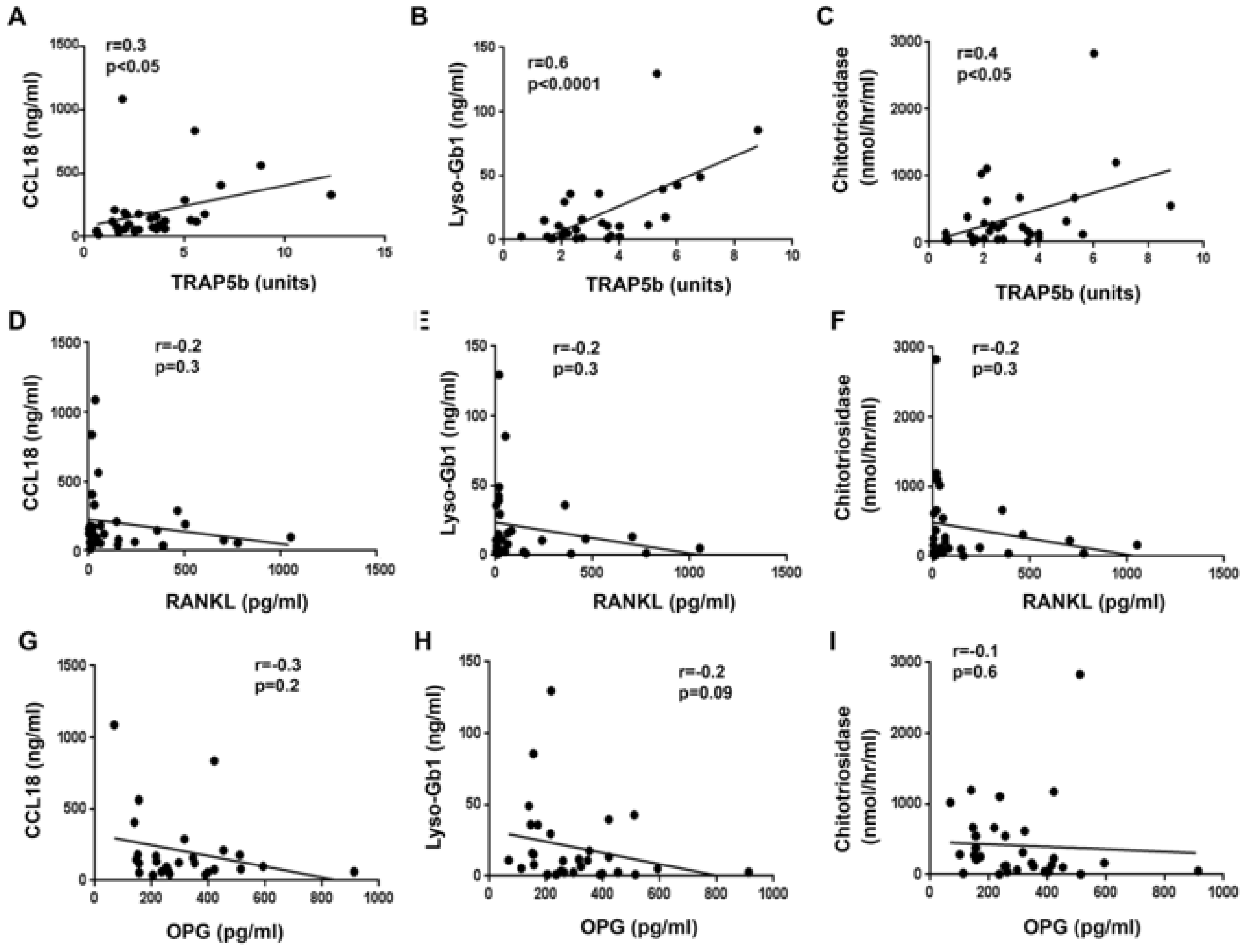
3.7. TRAP5b Positively Correlates with GD Biomarkers: CCL18, Chitotriosidase, and Lyso-Gb1
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pandey, M.K.; Grabowski, G.A. Immunological cells and functions in Gaucher disease. Crit. Rev. Oncog. 2013, 18, 197–220. [Google Scholar] [CrossRef]
- Masi, L.; Brandi, M.L. Gaucher disease: The role of the specialist on metabolic bone diseases. Clin. Cases Min. Bone Metab. 2015, 12, 165–169. [Google Scholar] [CrossRef]
- Mucci, J.M.; Rozenfeld, P. Pathogenesis of Bone Alterations in Gaucher Disease: The Role of Immune System. J. Immunol. Res. 2015, 2015, 192761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ersek, A.; Karadimitris, A.; Horwood, N.J. Effect of glycosphingolipids on osteoclastogenesis and osteolytic bone diseases. Front. Endocrinol. 2012, 3, 106. [Google Scholar] [CrossRef] [Green Version]
- Mistry, P.K.; Liu, J.; Yang, M.; Nottoli, T.; McGrath, J.; Jain, D.; Zhang, K.; Keutzer, J.; Chuang, W.L.; Mehal, W.Z.; et al. Glucocerebrosidase gene-deficient mouse recapitulates Gaucher disease displaying cellular and molecular dysregulation beyond the macrophage. Proc. Natl. Acad. Sci. USA 2010, 107, 19473–19478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, M.C.; Schiffer, C.; Heales, S.; Mehta, A.B.; Hughes, D.A. Impact of sphingolipids on osteoblast and osteoclast activity in Gaucher disease. Mol. Genet. Metab. 2018, 124, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, M.; Limgala, R.P.; Changsila, E.; Kamath, R.; Ioanou, C.; Goker-Alpan, O. Gaucheromas: When macrophages promote tumor formation and dissemination. Blood Cells Mol. Dis. 2016. [Google Scholar] [CrossRef] [PubMed]
- Halleen, J.M.; Alatalo, S.L.; Suominen, H.; Cheng, S.; Janckila, A.J.; Vaananen, H.K. Tartrate-resistant acid phosphatase 5b: A novel serum marker of bone resorption. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2000, 15, 1337–1345. [Google Scholar] [CrossRef]
- Kuo, T.R.; Chen, C.H. Bone biomarker for the clinical assessment of osteoporosis: Recent developments and future perspectives. Biomark. Res. 2017, 5, 18. [Google Scholar] [CrossRef] [Green Version]
- Giuffrida, G.; Cingari, M.R.; Parrinello, N.; Romano, A.; Triolo, A.; Franceschino, M.; Di Raimondo, F. Bone turnover markers in patients with type 1 Gaucher disease. Hematol. Rep. 2012, 4, e21. [Google Scholar] [CrossRef] [Green Version]
- Walsh, M.C.; Choi, Y. Biology of the RANKL-RANK-OPG System in Immunity, Bone, and Beyond. Front. Immunol. 2014, 5, 511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinzone, J.J.; Hall, B.M.; Thudi, N.K.; Vonau, M.; Qiang, Y.W.; Rosol, T.J.; Shaughnessy, J.D., Jr. The role of Dickkopf-1 in bone development, homeostasis, and disease. Blood 2009, 113, 517–525. [Google Scholar] [CrossRef] [Green Version]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, H.; Chen, X.; Gao, S.; Yu, X.; Xiao, J.; Zhang, B.; Liu, X.; Dai, M. Key Triggers of Osteoclast-Related Diseases and Available Strategies for Targeted Therapies: A Review. Front. Med. 2017, 4, 234. [Google Scholar] [CrossRef]
- Gruber, H.E.; Ivey, J.L.; Thompson, E.R.; Chesnut, C.H., 3rd; Baylink, D.J. Osteoblast and osteoclast cell number and cell activity in postmenopausal osteoporosis. Miner. Electrolyte Metab. 1986, 12, 246–254. [Google Scholar] [PubMed]
- Schraufstatter, I.U.; Zhao, M.; Khaldoyanidi, S.K.; Discipio, R.G. The chemokine CCL18 causes maturation of cultured monocytes to macrophages in the M2 spectrum. Immunology 2012, 135, 287–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollak, C.E.; van Weely, S.; van Oers, M.H.; Aerts, J.M. Marked elevation of plasma chitotriosidase activity. A novel hallmark of Gaucher disease. J. Clin. Investig. 1994, 93, 1288–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Revel-Vilk, S.; Fuller, M.; Zimran, A. Value of Glucosylsphingosine (Lyso-Gb1) as a Biomarker in Gaucher Disease: A Systematic Literature Review. Int. J. Mol. Sci. 2020, 21, 7159. [Google Scholar] [CrossRef] [PubMed]
- Wenstrup, R.J.; Roca-Espiau, M.; Weinreb, N.J.; Bembi, B. Skeletal aspects of Gaucher disease: A review. Br. J. Radiol. 2002, 75 (Suppl. 1), A2–A12. [Google Scholar] [CrossRef]
- Hughes, D.; Mikosch, P.; Belmatoug, N.; Carubbi, F.; Cox, T.; Goker-Alpan, O.; Kindmark, A.; Mistry, P.; Poll, L.; Weinreb, N.; et al. Gaucher Disease in Bone: From Pathophysiology to Practice. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2019, 34, 996–1013. [Google Scholar] [CrossRef]
- Iseme, R.A.; McEvoy, M.; Kelly, B.; Agnew, L.; Walker, F.R.; Attia, J. Is osteoporosis an autoimmune mediated disorder? Bone Rep. 2017, 7, 121–131. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, G.; Xu, W.; Tao, P.; Lv, X.; Wang, Y. Tartrate-resistant acid phosphatase 5b is a marker of osteoclast number and volume in RAW 264.7 cells treated with receptor-activated nuclear kappaB ligand. Exp. Ther. Med. 2015, 9, 143–146. [Google Scholar] [CrossRef] [Green Version]
- Janckila, A.J.; Slone, S.P.; Lear, S.C.; Martin, A.; Yam, L.T. Tartrate-resistant acid phosphatase as an immunohistochemical marker for inflammatory macrophages. Am. J. Clin. Pathol. 2007, 127, 556–566. [Google Scholar] [CrossRef]
- Pradella, S.D.; Slone, S.P.; Wu, Y.Y.; Chao, T.Y.; Parthasarathy, R.N.; Yam, L.T.; Janckila, A.J. Applications and performance of monoclonal antibodies to human tartrate resistant acid phosphatase. J. Immunol. Methods 2011, 372, 162–170. [Google Scholar] [CrossRef]
- Troy, K.; Cuttner, J.; Reilly, M.; Grabowski, G.; Desnick, R. Tartrate-resistant acid phosphatase staining of monocytes in Gaucher disease. Am. J. Hematol. 1985, 19, 237–244. [Google Scholar] [CrossRef]
- Stirnemann, J.; Belmatoug, N.; Vincent, C.; Fain, O.; Fantin, B.; Mentre, F. Bone events and evolution of biologic markers in Gaucher disease before and during treatment. Arthritis Res. Ther. 2010, 12, R156. [Google Scholar] [CrossRef] [Green Version]
- Dai, R.; Wu, Z.; Chu, H.Y.; Lu, J.; Lyu, A.; Liu, J.; Zhang, G. Cathepsin K: The Action in and Beyond Bone. Front. Cell Dev. Biol. 2020, 8, 433. [Google Scholar] [CrossRef]
- Goto, T.; Yamaza, T.; Tanaka, T. Cathepsins in the osteoclast. J. Electron. Microsc. 2003, 52, 551–558. [Google Scholar] [CrossRef]
- Moran, M.T.; Schofield, J.P.; Hayman, A.R.; Shi, G.P.; Young, E.; Cox, T.M. Pathologic gene expression in Gaucher disease: Up-regulation of cysteine proteinases including osteoclastic cathepsin K. Blood 2000, 96, 1969–1978. [Google Scholar] [CrossRef]
- Lobatoa, J.B.; Parejo, P.D.; Vázquez, R.J.; Jiménez, L.M. Cathepsin K as a biomarker of bone involvement in type 1 Gaucher disease. Med. Clín. 2015, 145, 6. [Google Scholar] [CrossRef]
- Redlich, K.; Smolen, J.S. Inflammatory bone loss: Pathogenesis and therapeutic intervention. Nat. Rev. Drug Discov. 2012, 11, 234–250. [Google Scholar] [CrossRef]
- Nair, S.; Boddupalli, C.S.; Verma, R.; Liu, J.; Yang, R.; Pastores, G.M.; Mistry, P.K.; Dhodapkar, M.V. Type II NKT-TFH cells against Gaucher lipids regulate B-cell immunity and inflammation. Blood 2015, 125, 1256–1271. [Google Scholar] [CrossRef] [Green Version]
- Lukas, J.; Cozma, C.; Yang, F.; Kramp, G.; Meyer, A.; Nesslauer, A.M.; Eichler, S.; Bottcher, T.; Witt, M.; Brauer, A.U.; et al. Glucosylsphingosine Causes Hematological and Visceral Changes in Mice-Evidence for a Pathophysiological Role in Gaucher Disease. Int. J. Mol. Sci. 2017, 18, 2192. [Google Scholar] [CrossRef] [Green Version]
- Nair, S.; Branagan, A.R.; Liu, J.; Boddupalli, C.S.; Mistry, P.K.; Dhodapkar, M.V. Clonal Immunoglobulin against Lysolipids in the Origin of Myeloma. N. Engl. J. Med. 2016, 374, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Cawley, K.M.; Bustamante-Gomez, N.C.; Guha, A.G.; MacLeod, R.S.; Xiong, J.; Gubrij, I.; Liu, Y.; Mulkey, R.; Palmieri, M.; Thostenson, J.D.; et al. Local Production of Osteoprotegerin by Osteoblasts Suppresses Bone Resorption. Cell Rep. 2020, 32, 108052. [Google Scholar] [CrossRef]
- O’Brien, C.A. Control of RANKL gene expression. Bone 2010, 46, 911–919. [Google Scholar] [CrossRef] [Green Version]
- Sinder, B.P.; Pettit, A.R.; McCauley, L.K. Macrophages: Their Emerging Roles in Bone. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2015, 30, 2140–2149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirraco, R.P.; Reis, R.L.; Marques, A.P. Effect of monocytes/macrophages on the early osteogenic differentiation of hBMSCs. J. Tissue Eng. Regen. Med. 2013, 7, 392–400. [Google Scholar] [CrossRef] [Green Version]
- Pereira, M.; Petretto, E.; Gordon, S.; Bassett, J.H.D.; Williams, G.R.; Behmoaras, J. Common signalling pathways in macrophage and osteoclast multinucleation. J. Cell Sci. 2018, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mucci, J.M.; Cuello, M.F.; Kisinovsky, I.; Larroude, M.; Delpino, M.V.; Rozenfeld, P.A. Proinflammatory and proosteoclastogenic potential of peripheral blood mononuclear cells from Gaucher patients: Implication for bone pathology. Blood Cells Mol. Dis. 2015, 55, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Reed, M.; Baker, R.J.; Mehta, A.B.; Hughes, D.A. Enhanced differentiation of osteoclasts from mononuclear precursors in patients with Gaucher disease. Blood Cells Mol. Dis. 2013, 51, 185–194. [Google Scholar] [CrossRef]
- Magal, I.; Lebel, E.; Altarescu, G.; Itzchaki, M.; Rudensky, B.; Foldes, A.J.; Zimran, A.; Elstein, D. Serum levels of osteoprotegerin and osteoprotegerin polymorphisms in Gaucher disease. Br. J. Haematol. 2006, 133, 93–97. [Google Scholar] [CrossRef]
- Zimmermann, A.; Popp, R.A.; Rossmann, H.; Bucerzan, S.; Nascu, I.; Leucuta, D.; Weber, M.M.; Grigorescu-Sido, P. Gene variants of osteoprotegerin, estrogen-, calcitonin- and vitamin D-receptor genes and serum markers of bone metabolism in patients with Gaucher disease type 1. Ther. Clin. Risk Manag. 2018, 14, 2069–2080. [Google Scholar] [CrossRef] [Green Version]
- Azizieh, F.Y.; Shehab, D.; Jarallah, K.A.; Gupta, R.; Raghupathy, R. Circulatory Levels of RANKL, OPG, and Oxidative Stress Markers in Postmenopausal Women With Normal or Low Bone Mineral Density. Biomark. Insights 2019, 14. [Google Scholar] [CrossRef]
- Coury, F.; Peyruchaud, O.; Machuca-Gayet, I. Osteoimmunology of Bone Loss in Inflammatory Rheumatic Diseases. Front. Immunol. 2019, 10, 679. [Google Scholar] [CrossRef] [Green Version]
- Weitzmann, M.N. The Role of Inflammatory Cytokines, the RANKL/OPG Axis, and the Immunoskeletal Interface in Physiological Bone Turnover and Osteoporosis. Science 2013, 2013, 125705. [Google Scholar] [CrossRef]
- Li, J.; Hsu, H.C.; Mountz, J.D. Managing macrophages in rheumatoid arthritis by reform or removal. Curr. Rheumatol. Rep. 2012, 14, 445–454. [Google Scholar] [CrossRef] [Green Version]
- Takayanagi, H.; Oda, H.; Yamamoto, S.; Kawaguchi, H.; Tanaka, S.; Nishikawa, T.; Koshihara, Y. A new mechanism of bone destruction in rheumatoid arthritis: Synovial fibroblasts induce osteoclastogenesis. Biochem. Biophys. Res. Commun. 1997, 240, 279–286. [Google Scholar] [CrossRef]
- Devigili, G.; De Filippo, M.; Ciana, G.; Dardis, A.; Lettieri, C.; Rinaldo, S.; Macor, D.; Moro, A.; Eleopra, R.; Bembi, B. Chronic pain in Gaucher disease: Skeletal or neuropathic origin? Orphanet J. Rare Dis. 2017, 12, 148. [Google Scholar] [CrossRef] [Green Version]
- Goker-Alpan, O. Therapeutic approaches to bone pathology in Gaucher disease: Past, present and future. Mol. Genet. Metab. 2011, 104, 438–447. [Google Scholar] [CrossRef]
- Limgala, R.P.; Goker-Alpan, O. Effect of Substrate Reduction Therapy in Comparison to Enzyme Replacement Therapy on Immune Aspects and Bone Involvement in Gaucher Disease. Biomolecules 2020, 10, 526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimmel, D.B. Mechanism of action, pharmacokinetic and pharmacodynamic profile, and clinical applications of nitrogen-containing bisphosphonates. J. Dent. Res. 2007, 86, 1022–1033. [Google Scholar] [CrossRef]
- Cox, T.M.; Aerts, J.M.; Belmatoug, N.; Cappellini, M.D.; vom Dahl, S.; Goldblatt, J.; Grabowski, G.A.; Hollak, C.E.; Hwu, P.; Maas, M.; et al. Management of non-neuronopathic Gaucher disease with special reference to pregnancy, splenectomy, bisphosphonate therapy, use of biomarkers and bone disease monitoring. J. Inherit. Metab. Dis. 2008, 31, 319–336. [Google Scholar] [CrossRef]
- Giuffrida, G.; Cappellini, M.D.; Carubbi, F.; Di Rocco, M.; Iolascon, G. Management of bone disease in Gaucher disease type 1: Clinical practice. Adv. Ther. 2014, 31, 1197–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.; Hanley, D.A.; McNeil, C.; Boyd, S. Improvement in Bone Mineral Density and Architecture in a Patient with Gaucher Disease Using Teriparatide. JIMD Rep. 2015, 22, 23–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dekker, N.; van Dussen, L.; Hollak, C.E.; Overkleeft, H.; Scheij, S.; Ghauharali, K.; van Breemen, M.J.; Ferraz, M.J.; Groener, J.E.; Maas, M.; et al. Elevated plasma glucosylsphingosine in Gaucher disease: Relation to phenotype, storage cell markers, and therapeutic response. Blood 2011, 118, e118–e127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, K.N.; Lie, J.D.; Wan, C.K.V.; Cameron, M.; Austel, A.G.; Nguyen, J.K.; Van, K.; Hyun, D. Osteoporosis: A Review of Treatment Options. Pharm. Ther. 2018, 43, 92–104. [Google Scholar]

| N | OSN | OSR | |
|---|---|---|---|
| T-score (average ± STDEV) | 0.03 ± 0.2 | −1.07 ± 0.2 | −2.96 ± 0.8 |
| Z-score (average ± STDEV) | −0.2 | −1.6 | −2.73 ± 0.4 |
| Bone pain | 4/9 (44%) | 4/10 (40%) | 10/14 (71%) |
| Bone surgery | 0/9 (0%) | 1/10 (10%) | 6/14 (42%) |
| Pathologic fractures | 0/9 (0%) | 2/10 (20%) | 3/14 (21%) |
| Bone marrow infiltration | 7/9 (77%) | 7/10 (67%) | 8/14 (57%) |
| EM-flask deformity | 5/9 (55%) | 3/10 (30%) | 8/14 (57%) |
| Cystic changes | 0/9 (0%) | 0/10 (0%) | 1/14 (7%) |
| Osteonecrosis | 3/9 (33%) | 1/10 (10%) | 4/14 (28%) |
| TRAP5b | OPG | RANKL | ||
|---|---|---|---|---|
| CCL18 | N | N | N | N |
| OSN | positive correlation (R = 0.7; p = 0.02) | N | N | |
| OSR | positive correlation (R = 0.5, p = 0.03) | N | N | |
| CHITO | N | N | N | N |
| OSN | positive correlation (R = 0.8, p = 0.006) | negative correlation (R = −0.6, p = 0.03) | N | |
| OSR | positive correlation (R = 0.2, p = 0.05) | OSN + OSR (R = −0.4, p = 0.02 *) | N | |
| Lyso-Gb1 | N | N | N | N |
| OSN | positive correlation (R = 0.7, p = 0.02) | negative correlation (R = −0.6, p = 0.03) | N | |
| OSR | positive correlation (R = 0.6, p = 0.02) | N | N |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, M.; Dao, J.; Noll, L.; Fikry, J.; Goker-Alpan, O. TRAP5b and RANKL/OPG Predict Bone Pathology in Patients with Gaucher Disease. J. Clin. Med. 2021, 10, 2217. https://doi.org/10.3390/jcm10102217
Ivanova M, Dao J, Noll L, Fikry J, Goker-Alpan O. TRAP5b and RANKL/OPG Predict Bone Pathology in Patients with Gaucher Disease. Journal of Clinical Medicine. 2021; 10(10):2217. https://doi.org/10.3390/jcm10102217
Chicago/Turabian StyleIvanova, Margarita, Julia Dao, Lauren Noll, Jacqueline Fikry, and Ozlem Goker-Alpan. 2021. "TRAP5b and RANKL/OPG Predict Bone Pathology in Patients with Gaucher Disease" Journal of Clinical Medicine 10, no. 10: 2217. https://doi.org/10.3390/jcm10102217
APA StyleIvanova, M., Dao, J., Noll, L., Fikry, J., & Goker-Alpan, O. (2021). TRAP5b and RANKL/OPG Predict Bone Pathology in Patients with Gaucher Disease. Journal of Clinical Medicine, 10(10), 2217. https://doi.org/10.3390/jcm10102217







