Ideal Cardiovascular Health in Former Smokers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Smoking Status and Ideal Cardiovascular Health
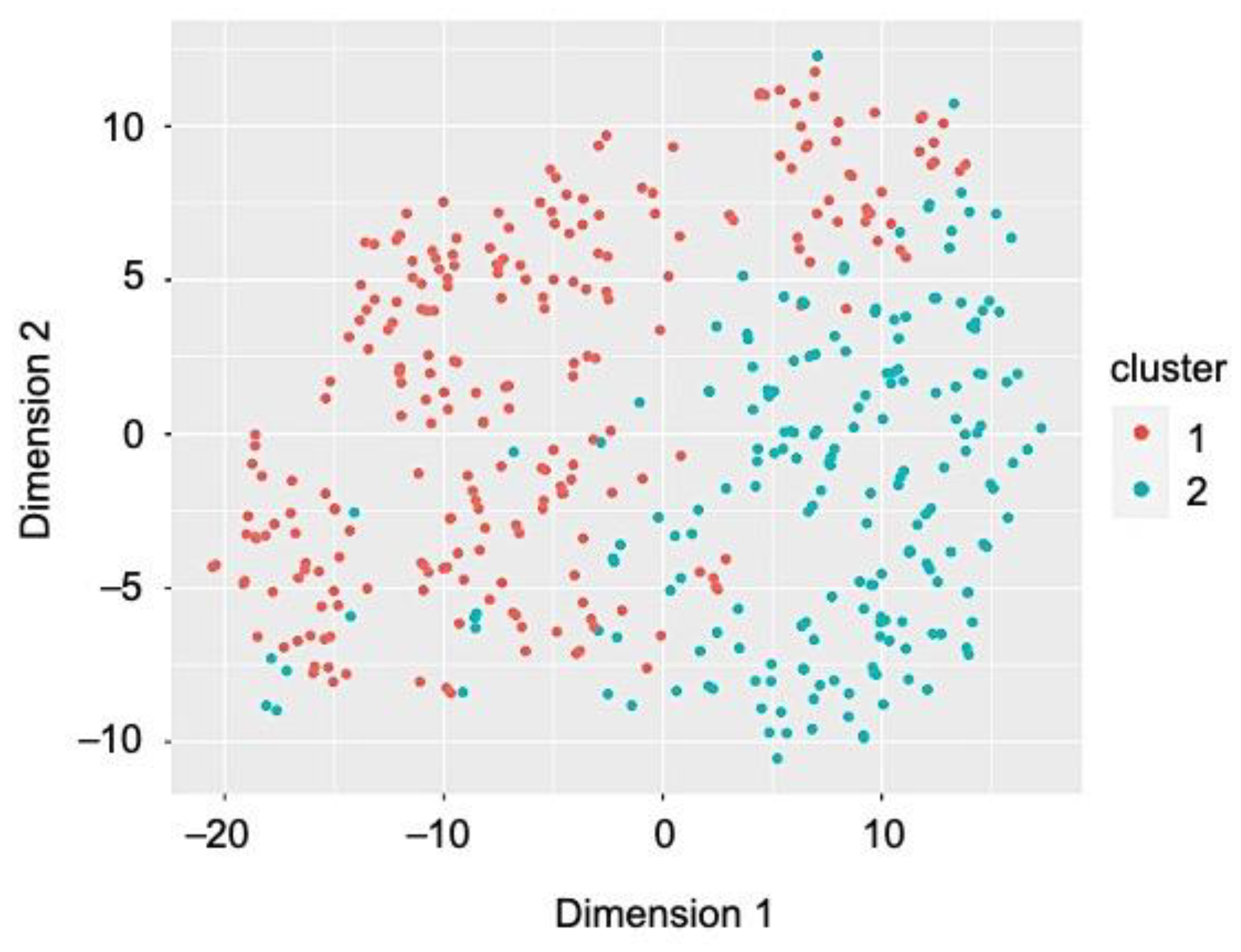
2.3. Cluster Analysis
2.4. Statistical Analysis
3. Results
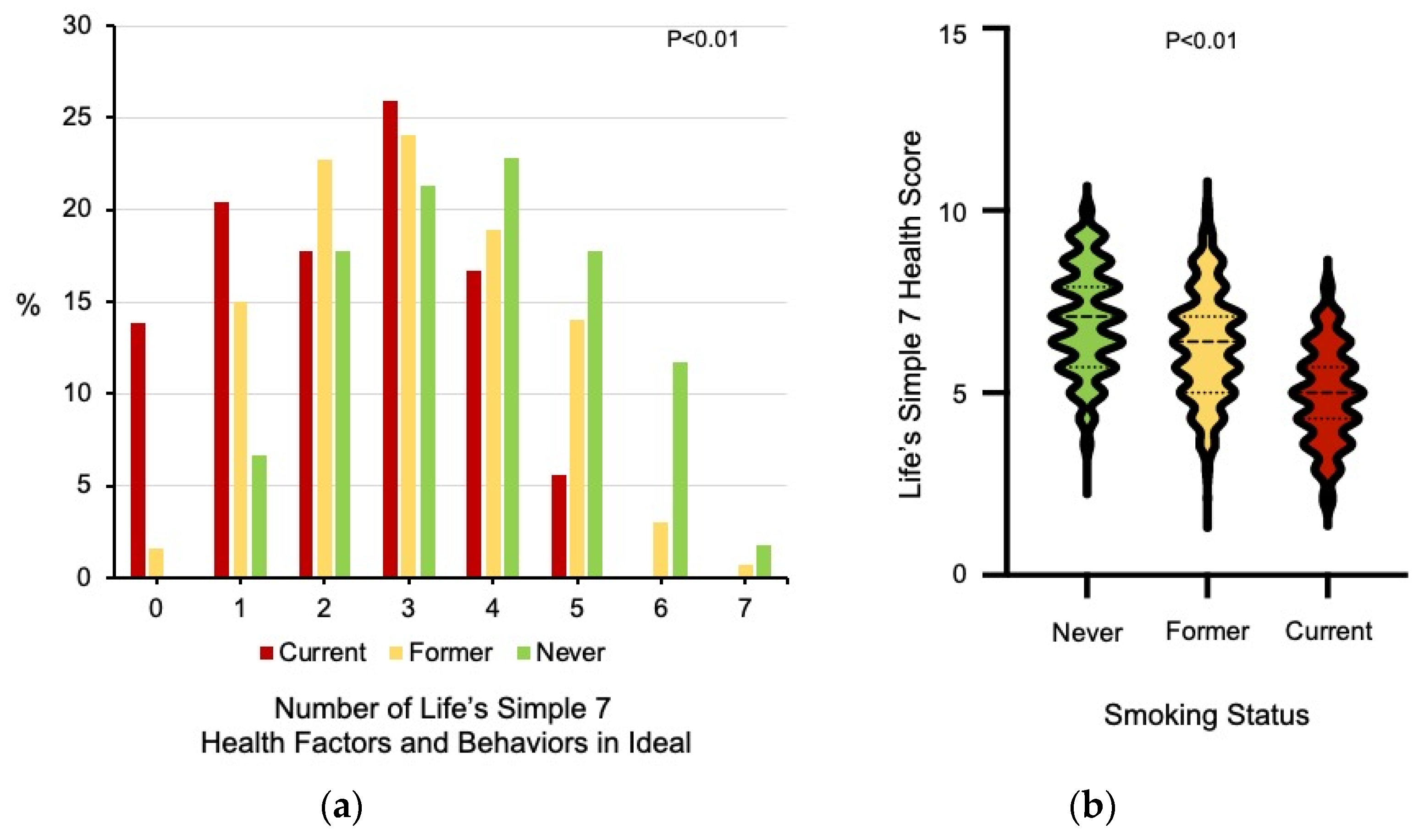
3.1. Differences in Ideal Cardiovascular Health Data among Never, Former, and Current Smokers
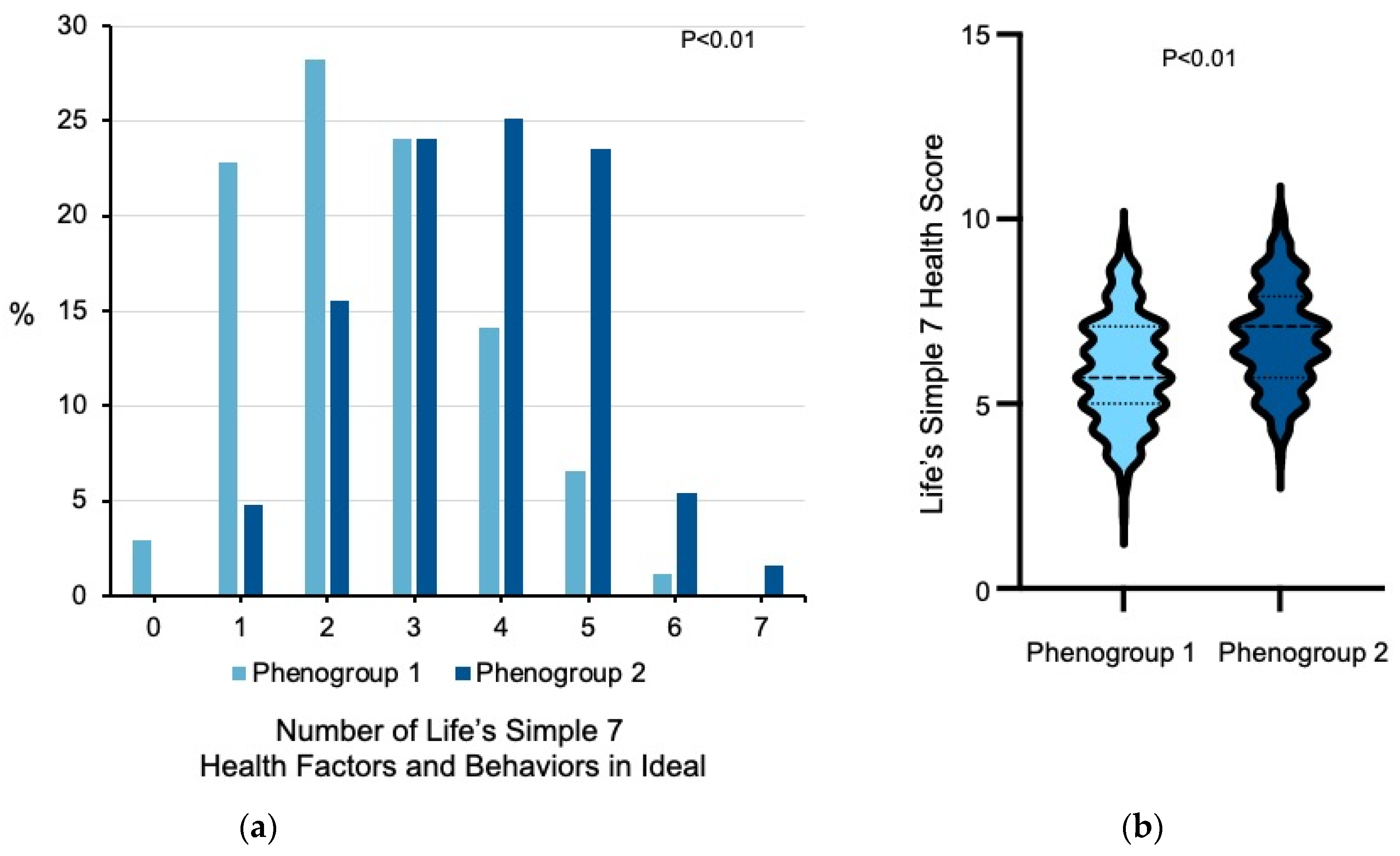
3.2. Heterogeneity among Former Smokers
3.3. Digital Health Data and Life’s Simple 7 Health Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thun, M.J.; Carter, B.D.; Feskanich, D.; Freedman, N.D.; Prentice, R.; Lopez, A.D.; Hartge, P.; Gapstur, S.M. 50-year trends in smoking-related mortality in the United States. N. Engl. J. Med. 2013, 368, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Thun, M.J.; Lopez, A.D.; Hartge, P. Smoking-related mortality in the United States. N. Engl. J. Med. 2013, 368, 1753. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Tobacco Fact Sheets. 2020. Available online: www.who.int (accessed on 1 March 2021).
- Wang, T.W.; Asman, K.; Gentzke, A.S.; Cullen, K.A.; Holder-Hayes, E.; Reyes-Guzman, C.; Jamal, A.; Neff, L.; King, B.A. Tobacco Product Use among Adults—United States, 2017. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 1225–1232. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef]
- Chang, C.M.; Corey, C.G.; Rostron, B.L.; Apelberg, B.J. Systematic review of cigar smoking and all cause and smoking related mortality. BMC Public Health 2015, 15, 390. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Hong, Y.; Labarthe, D.; Mozaffarian, D.; Appel, L.J.; Van Horn, L.; Greenlund, K.; Daniels, S.; Nichol, G.; Tomaselli, G.F.; et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 2010, 121, 586–613. [Google Scholar] [CrossRef]
- Enserro, D.M.; Vasan, R.S.; Xanthakis, V. Twenty-Year Trends in the American Heart Association Cardiovascular Health Score and Impact on Subclinical and Clinical Cardiovascular Disease: The Framingham Offspring Study. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef]
- Corlin, L.; Short, M.I.; Vasan, R.S.; Xanthakis, V. Association of the Duration of Ideal Cardiovascular Health Through Adulthood With Cardiometabolic Outcomes and Mortality in the Framingham Offspring Study. JAMA Cardiol. 2020, 5, 549–556. [Google Scholar] [CrossRef]
- Nguyen, A.T.H.; Saeed, A.; Bambs, C.E.; Swanson, J.; Emechebe, N.; Mansuri, F.; Talreja, K.; Reis, S.E.; Kip, K.E. Usefulness of the American Heart Association’s Ideal Cardiovascular Health Measure to Predict Long-term Major Adverse Cardiovascular Events (From the Heart SCORE Study). Am. J. Cardiol. 2021, 138, 20–25. [Google Scholar] [CrossRef]
- Ramirez-Velez, R.; Saavedra, J.M.; Lobelo, F.; Celis-Morales, C.A.; Pozo-Cruz, B.D.; Garcia-Hermoso, A. Ideal Cardiovascular Health and Incident Cardiovascular Disease Among Adults: A Systematic Review and Meta-analysis. Mayo Clin. Proc. 2018, 93, 1589–1599. [Google Scholar] [CrossRef]
- Ommerborn, M.J.; Blackshear, C.T.; Hickson, D.A.; Griswold, M.E.; Kwatra, J.; Djousse, L.; Clark, C.R. Ideal Cardiovascular Health and Incident Cardiovascular Events: The Jackson Heart Study. Am. J. Prev. Med. 2016, 51, 502–506. [Google Scholar] [CrossRef]
- Mons, U.; Muezzinler, A.; Gellert, C.; Schottker, B.; Abnet, C.C.; Bobak, M.; de Groot, L.; Freedman, N.D.; Jansen, E.; Kee, F.; et al. Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: Meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ 2015, 350, h1551. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Sang, Y.; Chen, J.; Ballew, S.H.; Kalbaugh, C.A.; Salameh, M.J.; Blaha, M.J.; Allison, M.; Heiss, G.; Selvin, E.; et al. Cigarette Smoking, Smoking Cessation, and Long-Term Risk of 3 Major Atherosclerotic Diseases. J. Am. Coll. Cardiol. 2019, 74, 498–507. [Google Scholar] [CrossRef]
- Duncan, M.S.; Freiberg, M.S.; Greevy, R.A., Jr.; Kundu, S.; Vasan, R.S.; Tindle, H.A. Association of Smoking Cessation With Subsequent Risk of Cardiovascular Disease. JAMA 2019, 322, 642–650. [Google Scholar] [CrossRef]
- Leopold, J.A.; Davis, R.B.; Antman, E.M. Data from digital health devices informs ideal cardiovascular health. J. Pers. Med. 2021, 11, 189. [Google Scholar] [CrossRef]
- Sanchez, E. Life’s Simple 7: Vital But Not Easy. J. Am. Heart Assoc. 2018, 7, e009324. [Google Scholar] [CrossRef]
- Angell, S.Y.; McConnell, M.V.; Anderson, C.A.M.; Bibbins-Domingo, K.; Boyle, D.S.; Capewell, S.; Ezzati, M.; de Ferranti, S.; Gaskin, D.J.; Goetzel, R.Z.; et al. The American Heart Association 2030 Impact Goal: A Presidential Advisory From the American Heart Association. Circulation 2020, 141, e120–e138. [Google Scholar] [CrossRef] [PubMed]
- Escofier, B.; Pages, J. Multiple factor analysis (AFMULT package). Comput. Stat. Data Anal. 1994, 18, 121–140. [Google Scholar] [CrossRef]
- Gower, J.C. A general coefficient of similarity and some of its properties. Biometrics 1971, 27, 857–871. [Google Scholar] [CrossRef]
- Kaufman, L.; Rousseeuw, P. Finding Groups in Data: An Introduction to Cluster Analysis, 2nd ed.; Wiley: New York, NY, USA, 1990. [Google Scholar]
- Chow, C.K.; Jolly, S.; Rao-Melacini, P.; Fox, K.A.; Anand, S.S.; Yusuf, S. Association of diet, exercise, and smoking modification with risk of early cardiovascular events after acute coronary syndromes. Circulation 2010, 121, 750–758. [Google Scholar] [CrossRef]
- Kimokoti, R.W.; Newby, P.K.; Gona, P.; Zhu, L.; Jasuja, G.K.; Pencina, M.J.; McKeon-O’Malley, C.; Fox, C.S.; D’Agostino, R.B.; Millen, B.E. Diet quality, physical activity, smoking status, and weight fluctuation are associated with weight change in women and men. J. Nutr. 2010, 140, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Troiano, R.P.; Pamuk, E.R.; Kuczmarski, R.J.; Campbell, S.M. The influence of smoking cessation on the prevalence of overweight in the United States. N. Engl. J. Med. 1995, 333, 1165–1170. [Google Scholar] [CrossRef]
- Williamson, D.F.; Madans, J.; Anda, R.F.; Kleinman, J.C.; Giovino, G.A.; Byers, T. Smoking cessation and severity of weight gain in a national cohort. N. Engl. J. Med. 1991, 324, 739–745. [Google Scholar] [CrossRef]
- Hu, E.A.; Toledo, E.; Diez-Espino, J.; Estruch, R.; Corella, D.; Salas-Salvado, J.; Vinyoles, E.; Gomez-Gracia, E.; Aros, F.; Fiol, M.; et al. Lifestyles and risk factors associated with adherence to the Mediterranean diet: A baseline assessment of the PREDIMED trial. PLoS ONE 2013, 8, e60166. [Google Scholar] [CrossRef] [PubMed]
- Berg, C.J.; Thomas, J.L.; An, L.C.; Guo, H.; Collins, T.; Okuyemi, K.S.; Ahluwalia, J.S. Change in smoking, diet, and walking for exercise in Blacks. Health Educ. Behav. 2012, 39, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Nduaguba, S.O.; Ford, K.H.; Rascati, K. The Role of Physical Activity in the Association Between Smoking Status and Quality of Life. Nicotine Tob. Res. 2019, 21, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
| Never Smoker (n = 1025) | Former Smoker (n = 428) | Current Smoker (n = 108) | p-Value | ||
|---|---|---|---|---|---|
| Age (yrs) | 43.4 ± 13.7 | 46.9 ± 11.5 | 41.0 ± 10.1 | <0.01 | |
| Gender (% female) | 79.8 | 79.7 | 85.2 | 0.40 | |
| Race and Ethnicity (no.) | <0.02 | ||||
| Asian | 37 | 4 | 1 | ||
| Black | 46 | 11 | 3 | ||
| Hispanic | 46 | 20 | 2 | ||
| White | 868 | 372 | 97 | ||
| Other | 28 | 21 | 5 | ||
| Region (no.) | 0.71 | ||||
| Northeast | 145 | 65 | 17 | ||
| South | 400 | 182 | 40 | ||
| Midwest | 258 | 96 | 24 | ||
| West | 222 | 85 | 27 | ||
| Diagnosed with Cardiovascular Disease (%) | 29.6 | 50.0 | 45.4 | <0.01 | |
| Diabetes mellitus (%) | 8.9 | 13.3 | 12.0 | <0.04 | |
| Hypertension (%) | 46.1 | 59.1 | 49.1 | <0.01 | |
| Hypercholesterolemia (%) | 49.5 | 63.6 | 50.9 | <0.01 | |
| Medications (%) | |||||
| Diabetes mellitus | 7.8 | 11.2 | 8.3 | 0.11 | |
| Hypertension | 27.3 | 45.3 | 30.6 | <0.01 | |
| Hypercholesterolemia | 16.9 | 30.1 | 17.6 | <0.01 | |
| CLINICAL DATA | |||||
| Weight (kg) | 81.7 ± 23.1 | 89.7 ± 26.1 | 86.5 ± 25.6 | <0.01 | |
| BMI (kg/m2) | 29.1 ± 7.9 | 31.7 ± 8.7 | 31.3 ± 8.0 | <0.01 | |
| Systolic blood pressure (mmHg) * | 117.1 ± 12.0 | 120.1 ± 14.1 | 119.3 ± 12.9 | <0.01 | |
| Diastolic blood pressure (mmHg) * | 73.2 ± 8.5 | 74.0 ± 9.1 | 72.6 ± 8.0 | 0.13 | |
| Total cholesterol (mmol/L) * | 187 ± 29 | 192 ± 29 | 193 ± 27 | <0.01 | |
| Blood glucose (mmol/L) * | 98.0 ± 17.0 | 102.0 ± 22.0 | 99.0 ± 16.0 | <0.01 | |
| DIET | |||||
| Vegetables/day (cups) | 1.9 ± 1.3 | 1.9 ± 1.3 | 1.4 ± 1.3 | <0.01 | |
| Fruit/day (cups) | 1.4 ± 1.1 | 1.2 ± 1.0 | 1.0 ± 1.1 | <0.01 | |
| Fish (servings/week) | 1.0 ± 1.0 | 0.9 ± 1.0 | 0.7 ± 0.9 | <0.02 | |
| Whole grains (servings/day) | 1.7 ± 1.2 | 1.5 ± 1.2 | 1.4 ± 1.1 | <0.01 | |
| Sugar-sweetened beverages (servings/week) | 2.1 ± 3.1 | 2.3 ± 3.2 | 5.4 ± 4.8 | <0.01 | |
| Avoid prepackaged foods (%) | 52.7 | 54.0 | 40.7 | <0.05 | |
| Avoid eating out (%) | 37.0 | 41.4 | 28.7 | <0.05 | |
| Avoid salt at home (%) | 55.9 | 59.1 | 53.7 | 0.43 | |
| EXERCISE | |||||
| Moderate exercise (min/week) | 195.5 ± 200.5 | 217.9 ± 235.8 | 229.9 ± 255.7 | 0.08 | |
| Vigorous exercise (min/week) | 77.2 ± 121.5 | 52.3 ± 107.2 | 33.2 ± 94.5 | <0.01 | |
| LIFE’S SIMPLE 7 | |||||
| Smoking score (%) | <0.01 | ||||
| Poor | 0 | 0 | 100 | ||
| Intermediate | 0 | 13.8 | 0 | ||
| Ideal | 100 | 86.2 | 0 | ||
| Activity score (%) | <0.02 | ||||
| Poor | 1.5 | 2.8 | 2.8 | ||
| Intermediate | 35.8 | 39.3 | 49.1 | ||
| Ideal | 62.7 | 57.9 | 48.1 | ||
| Diet score (%) | <0.01 | ||||
| Poor | 41.5 | 45.3 | 64.8 | ||
| Intermediate | 49.4 | 45.1 | 33.3 | ||
| Ideal | 9.1 | 9.6 | 1.9 | ||
| Weight score (%) | <0.01 | ||||
| Poor | 37.7 | 50.2 | 54.6 | ||
| Intermediate | 25.0 | 23.8 | 18.5 | ||
| Ideal | 37.3 | 26.0 | 26.9 | ||
| Blood glucose score (%) | <0.01 | ||||
| Poor | 2.9 | 5.8 | 4.6 | ||
| Intermediate | 33.8 | 40.4 | 36.1 | ||
| Ideal | 63.3 | 53.7 | 59.3 | ||
| Cholesterol score (%) | <0.01 | ||||
| Poor | 2.1 | 3.7 | 1.9 | ||
| Intermediate | 46.0 | 59.6 | 48.1 | ||
| Ideal | 51.9 | 36.7 | 50.0 | ||
| Blood pressure score (%) | <0.01 | ||||
| Poor | 4.8 | 9.4 | 6.5 | ||
| Intermediate | 49.8 | 59.3 | 51.9 | ||
| Ideal | 45.4 | 31.3 | 41.6 | ||
| LS7 Health Score | 7.0 ± 1.4 | 6.3 ± 1.5 | 4.9 ± 1.3 | <0.01 | |
| Phenogroup 1 (n = 241) | Phenogroup 2 (n = 187) | p-Value | |
|---|---|---|---|
| Age (yrs) | 49.7 ± 11.1 | 43.4 ± 11.2 | <0.01 |
| Gender (% female) | 77.6 | 82.4 | 0.23 |
| Race and Ethnicity (no.) | 0.49 | ||
| Asian | 3 | 1 | |
| Black | 7 | 4 | |
| Hispanic | 9 | 11 | |
| White | 213 | 159 | |
| Other | 9 | 12 | |
| Region (no.) | 0.59 | ||
| Northeast | 38 | 27 | |
| South | 102 | 80 | |
| Midwest | 58 | 38 | |
| West | 43 | 42 | |
| Diagnosed with Cardiovascular Disease (%) | 72.2 | 21.4 | <0.01 |
| Diabetes mellitus (%) | 19.2 | 4.81 | <0.01 |
| Hypertension (%) | 82.2 | 29.4 | <0.01 |
| Hypercholesterolemia (%) | 80.9 | 41.2 | <0.01 |
| Medications (%) | |||
| Diabetes mellitus | 17.0 | 3.7 | <0.01 |
| Hypertension | 74.2 | 8.0 | <0.01 |
| Hypercholesterolemia | 46.5 | 9.1 | <0.01 |
| CLINICAL DATA | |||
| Weight (kg) | 92.3 ± 26.8 | 86.2 ± 24.8 | <0.02 |
| BMI (kg/m2) | 32.6 ± 9.0 | 30.5 ± 8.2 | <0.02 |
| Systolic blood pressure (mmHg) * | 122.2 ± 14.9 | 117.4 ± 12.5 | <0.01 |
| Diastolic blood pressure (mmHg) * | 74.9 ± 10.0 | 72.9 ± 7.8 | <0.03 |
| Total cholesterol (mmol/L) * | 190.6 ± 31.6 | 192.8 ± 26.6 | 0.45 |
| Blood glucose (mmol/L) * | 104.9 ± 22.7 | 99.2 ± 19.8 | <0.01 |
| DIET | |||
| Vegetables/day (cups) | 1.9 ± 1.3 | 1.9 ± 1.3 | 0.80 |
| Fruit/day (cups) | 1.4 ± 1.1 | 1.1 ± 0.8 | <0.02 |
| Fish (servings/week) | 1.0 ± 1.0 | 0.8 ± 0.9 | <0.03 |
| Whole grains (servings/day) | 1.5 ± 1.2 | 1.5 ± 1.2 | 0.92 |
| Sugar-sweetened beverages (servings/week) | 1.9 ± 2.9 | 2.8 ± 3.6 | <0.01 |
| Avoid prepackaged foods (%) | 65.6 | 39.0 | <0.01 |
| Avoid eating out (%) | 56.0 | 22.5 | <0.01 |
| Avoid salt at home (%) | 78.0 | 34.8 | <0.01 |
| EXERCISE | |||
| Moderate exercise (min/week) | 200.4 ± 217.1 | 240.5 ± 256.7 | 0.08 |
| Vigorous exercise (min/week) | 43.0 ± 92.7 | 64.2 ± 122.6 | <0.05 |
| LIFE’S SIMPLE 7 | |||
| Smoking score (%) | <0.06 | ||
| Poor | 0 | 0 | |
| Intermediate | 16.6 | 10.2 | |
| Ideal | 83.4 | 89.9 | |
| Activity score (%) | 0.24 | ||
| Poor | 3.3 | 2.1 | |
| Intermediate | 41.1 | 36.9 | |
| Ideal | 55.6 | 61.0 | |
| Diet score (%) | <0.01 | ||
| Poor | 34.9 | 58.8 | |
| Intermediate | 51.9 | 36.4 | |
| Ideal | 13.3 | 4.8 | |
| Weight score (%) | <0.01 | ||
| Poor | 55.6 | 43.3 | |
| Intermediate | 23.7 | 24.1 | |
| Ideal | 20.7 | 32.6 | |
| Blood glucose score (%) | <0.01 | ||
| Poor | 8.7 | 2.1 | |
| Intermediate | 49.0 | 29.4 | |
| Ideal | 42.3 | 68.5 | |
| Cholesterol score (%) | <0.01 | ||
| Poor | 3.7 | 3.7 | |
| Intermediate | 77.2 | 36.9 | |
| Ideal | 19.1 | 59.4 | |
| Blood pressure score (%) | <0.01 | ||
| Poor | 12.9 | 4.8 | |
| Intermediate | 73.0 | 41.7 | |
| Ideal | 14.1 | 53.5 | |
| LS7 Health Score | 5.9 ± 1.4 | 6.8 ± 1.3 | <0.01 |
| Never Smokers (n = 269) | Former Smokers (n = 101) | p-Value | |
| Steps per day | 7896.2 ± 4776.3 | 7723.1 ± 4465.8 | 0.75 |
| Never Smokers (n = 204) | Former Smokers (n = 85) | p-Value | |
| Weight (kg) | 78.9 ± 19.4 | 85.7 ± 22.9 | <0.01 |
| Absolute change in weight (kg) Reported vs. Measured | −0.8 ± 3.9 | 0.01 ± 5.2 | 0.25 |
| BMI (kg/m2) | 27.8 ± 6.3 | 30.4 ± 8.1 | <0.01 |
| Absolute change in BMI (kg/m2) Reported vs. Measured | −0.3 ± 1.4 | 0.1 ± 2.0 | 0.27 |
| Healthy Weight Score (%) | <0.04 | ||
| Poor | 30.9 | 44.7 | |
| Intermediate | 31.4 | 27.1 | |
| Ideal | 37.7 | 28.2 | |
| Never Smokers (n = 259) | Former Smokers (n = 93) | p-Value | |
| Moderate exercise (min/week) | 149.9 ± 190.8 | 98.3 ± 117.4 | <0.02 |
| Absolute change in moderate exercise (min/week) Reported vs. Measured | 54.5 ± 250.6 | 146.5 ± 244.6 | <0.01 |
| Vigorous exercise (min/week) | 165.8 ± 230.9 | 161.4 ± 227.9 | 0.87 |
| Absolute change in vigorous exercise (min/week) Reported vs. Measured | −70.4 ± 233.6 | −92.7 ± 201.8 | 0.41 |
| Physical Activity Score (%) | 0.06 | ||
| Poor | 0.0 | 0.0 | |
| Intermediate | 36.3 | 47.3 | |
| Ideal | 63.7 | 52.7 | |
| Never Smokers (n = 193) | Former Smokers (n = 77) | p-Value | |
| Recalculated | |||
| LS7 Health Score | 7.2 ± 1.3 | 6.5 ± 1.3 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leopold, J.A.; Antman, E.M. Ideal Cardiovascular Health in Former Smokers. J. Clin. Med. 2021, 10, 2450. https://doi.org/10.3390/jcm10112450
Leopold JA, Antman EM. Ideal Cardiovascular Health in Former Smokers. Journal of Clinical Medicine. 2021; 10(11):2450. https://doi.org/10.3390/jcm10112450
Chicago/Turabian StyleLeopold, Jane A., and Elliott M. Antman. 2021. "Ideal Cardiovascular Health in Former Smokers" Journal of Clinical Medicine 10, no. 11: 2450. https://doi.org/10.3390/jcm10112450
APA StyleLeopold, J. A., & Antman, E. M. (2021). Ideal Cardiovascular Health in Former Smokers. Journal of Clinical Medicine, 10(11), 2450. https://doi.org/10.3390/jcm10112450







