Association between Skin Cancer and Systemic and Ocular Comorbidities in South Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Database Used in the Study
2.2. Selection of Study Samples
2.3. Prevalence and Comorbidities
2.4. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gandini, S.; Palli, D.; Spadola, G.; Bendinelli, B.; Cocorocchio, E.; Stanganelli, I.; Miligi, L.; Masala, G.; Caini, S. Anti-hypertensive drugs and skin cancer risk: A review of the literature and meta-analysis. Crit. Rev. Oncol. Hematol. 2018, 122, 1–9. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin is associated with decreased skin cancer risk in Taiwanese patients with type 2 diabetes. J. Am. Acad. Dermatol. 2018, 78, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Lerman, S.; Amichai, B.; Weinstein, G.; Shalev, V.; Chodick, G. Parkinson’s Disease, Melanoma, and Keratinocyte Carcinoma: A Population-Based Study. Neuroepidemiology 2018, 50, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, I.; Mohr, P.; Zander, N.; Fölster-Holst, R.; Augustin, M. Association of atopy and tentative diagnosis of skin cancer—Results from occupational skin cancer screenings. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 2083–2087. [Google Scholar] [CrossRef]
- Bae, J.M.; Ju, H.J.; Lee, R.W.; Oh, S.H.; Shin, J.H.; Kang, H.Y.; Park, J.H.; Kim, H.J.; Jeong, K.H.; Lee, H.J.; et al. Evaluation for Skin Cancer and Precancer in Patients With Vitiligo Treated With Long-term Narrowband UV-B Phototherapy. JAMA Dermatol. 2020, 156, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Lang, C.; Khadka, J.; Inacio, M.C. Association of Age-Related Cataract With Skin Cancer in an Australian Population. Investig. Ophthalmol. Vis. Sci. 2020, 61, 48. [Google Scholar] [CrossRef]
- Yu, H.C.; Lin, C.L.; Chen, Z.T.; Hu, F.R.; Sung, F.C.; Wang, I.J. Risk of skin cancer in patients with pterygium: A nationwide population-based cohort study in Taiwan. Ocul. Surf. 2014, 12, 69–76. [Google Scholar] [CrossRef]
- Crewe, J.M.; Threlfall, T.; Clark, A.; Sanfilippo, P.G.; Mackey, D.A. Pterygia are indicators of an increased risk of developing cutaneous melanomas. Br. J. Ophthalmol. 2018, 102, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Sng, J.; Koh, D.; Siong, W.C.; Choo, T.B. Skin cancer trends among Asians living in Singapore from 1968 to 2006. J. Am. Acad. Dermatol. 2009, 61, 426–432. [Google Scholar] [CrossRef]
- Oh, C.M.; Cho, H.; Won, Y.J.; Kong, H.J.; Roh, Y.H.; Jeong, K.H.; Jung, K.W. Nationwide Trends in the Incidence of Melanoma and Non-melanoma Skin Cancers from 1999 to 2014 in South Korea. Cancer Res. Treat. 2018, 50, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Sobue, T.; Kitamura, T.; Sawada, N.; Iwasaki, M.; Shimazu, T.; Tsugane, S. Epidemiology of nonmelanoma skin cancer in Japan: Occupational type, lifestyle, and family history of cancer. Cancer Sci. 2020, 111, 4257–4265. [Google Scholar] [CrossRef] [PubMed]
- Guy, G.P., Jr.; Machlin, S.R.; Ekwueme, D.U.; Yabroff, K.R. Prevalence and costs of skin cancer treatment in the U.S., 2002–2006 and 2007–2011. Am. J. Prev. Med. 2015, 48, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; He, P.; Yao, H.; Song, R.; Ma, C.; Cao, M.; Cui, B.; Ning, G. Cancer risk among patients with type 2 diabetes: A real-world study in Shanghai, China. J. Diabetes 2019, 11, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. Rosiglitazone may reduce non-melanoma skin cancer risk in Taiwanese. BMC Cancer 2015, 15, 41. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.A.; Schmidt, M.; Mehnert, F.; Lemeshow, S.; Sørensen, H.T. Use of antihypertensive drugs and risk of skin cancer. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1545–1554. [Google Scholar] [CrossRef]
- Su, K.A.; Habel, L.A.; Achacoso, N.S.; Friedman, G.D.; Asgari, M.M. Photosensitizing antihypertensive drug use and risk of cutaneous squamous cell carcinoma. Br. J. Dermatol. 2018, 179, 1088–1094. [Google Scholar] [CrossRef]
- Frishman, W.H.; Brosnan, B.D.; Grossman, M.; Dasgupta, D.; Sun, A.D. Adverse dermatologic effects of cardiovascular drug therapy: Part I. Cardiol. Rev. 2002, 10, 230–246. [Google Scholar] [CrossRef]
- Park, E.; Lee, Y.; Jue, M.S. Hydrochlorothiazide use and the risk of skin cancer in patients with hypertensive disorder: A nationwide retrospective cohort study from Korea. Korean J. Intern. Med. 2020, 35, 917–928. [Google Scholar] [CrossRef]
- Colt, J.S.; Schwartz, K.; Graubard, B.I.; Davis, F.; Ruterbusch, J.; DiGaetano, R.; Purdue, M.; Rothman, N.; Wacholder, S.; Chow, W.H. Hypertension and risk of renal cell carcinoma among white and black Americans. Epidemiology 2011, 22, 797–804. [Google Scholar] [CrossRef]
- Sanfilippo, K.M.; McTigue, K.M.; Fidler, C.J.; Neaton, J.D.; Chang, Y.; Fried, L.F.; Liu, S.; Kuller, L.H. Hypertension and obesity and the risk of kidney cancer in 2 large cohorts of US men and women. Hypertension 2014, 63, 934–941. [Google Scholar] [CrossRef]
- Han, H.; Guo, W.; Shi, W.; Yu, Y.; Zhang, Y.; Ye, X.; He, J. Hypertension and breast cancer risk: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 44877. [Google Scholar] [CrossRef] [PubMed]
- Masoudkabir, F.; Sarrafzadegan, N.; Gotay, C.; Ignaszewski, A.; Krahn, A.D.; Davis, M.K.; Franco, C.; Mani, A. Cardiovascular disease and cancer: Evidence for shared disease pathways and pharmacologic prevention. Atherosclerosis 2017, 263, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Cavo, A.; Rubagotti, A.; Zanardi, E.; Fabbroni, C.; Zinoli, L.; Di Meglio, A.; Arboscello, E.; Bellodi, A.; Spallarossa, P.; Cattrini, C.; et al. Abiraterone acetate and prednisone in the pre- and post-docetaxel setting for metastatic castration-resistant prostate cancer: A mono-institutional experience focused on cardiovascular events and their impact on clinical outcomes. Ther. Adv. Med. Oncol. 2018, 10, 1758834017745819. [Google Scholar] [CrossRef] [PubMed]
- Tini, G.; Sarocchi, M.; Tocci, G.; Arboscello, E.; Ghigliotti, G.; Novo, G.; Brunelli, C.; Lenihan, D.; Volpe, M.; Spallarossa, P. Arterial hypertension in cancer: The elephant in the room. Int. J. Cardiol. 2019, 281, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Modenese, A.; Korpinen, L.; Gobba, F. Solar Radiation Exposure and Outdoor Work: An Underestimated Occupational Risk. Int. J. Environ. Res. Public Health 2018, 15, 2063. [Google Scholar] [CrossRef] [PubMed]
- Rezvan, F.; Khabazkhoob, M.; Hooshmand, E.; Yekta, A.; Saatchi, M.; Hashemi, H. Prevalence and risk factors of pterygium: A systematic review and meta-analysis. Surv. Ophthalmol. 2018, 63, 719–735. [Google Scholar] [CrossRef]
- Asbell, P.A.; Dualan, I.; Mindel, J.; Brocks, D.; Ahmad, M.; Epstein, S. Age-related cataract. Lancet 2005, 365, 599–609. [Google Scholar] [CrossRef]
- Rim, T.H.; Kim, M.H.; Kim, W.C.; Kim, T.I.; Kim, E.K. Cataract subtype risk factors identified from the Korea National Health and Nutrition Examination survey 2008–2010. BMC Ophthalmol. 2014, 14, 4. [Google Scholar] [CrossRef]
- Taylor, H.R.; Keeffe, J.E. World blindness: A 21st century perspective. Br. J. Ophthalmol. 2001, 85, 261–266. [Google Scholar] [CrossRef]
- Tomany, S.C.; Cruickshanks, K.J.; Klein, R.; Klein, B.E.; Knudtson, M.D. Sunlight and the 10-year incidence of age-related maculopathy: The Beaver Dam Eye Study. Arch. Ophthalmol. 2004, 122, 750–757. [Google Scholar] [CrossRef]
- Awad, F.; Assrawi, E.; Louvrier, C.; Jumeau, C.; Giurgea, I.; Amselem, S.; Karabina, S.A. Photoaging and skin cancer: Is the inflammasome the missing link? Mech. Ageing Dev. 2018, 172, 131–137. [Google Scholar] [CrossRef]
- Godic, A.; Poljšak, B.; Adamic, M.; Dahmane, R. The role of antioxidants in skin cancer prevention and treatment. Oxid. Med. Cell Longev. 2014, 2014, 860479. [Google Scholar] [CrossRef]
- Bose, A.; Petsko, G.A.; Eliezer, D. Parkinson’s Disease and Melanoma: Co-Occurrence and Mechanisms. J. Parkinsons Dis. 2018, 8, 385–398. [Google Scholar] [CrossRef]
- Hagen, J.W.; Pugliano-Mauro, M.A. Nonmelanoma Skin Cancer Risk in Patients With Inflammatory Bowel Disease Undergoing Thiopurine Therapy: A Systematic Review of the Literature. Dermatol. Surg. 2018, 44, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Z.; Liu, Z.C.; Liao, W.X.; Wei, J.X.; Huang, X.W.; Yang, C.; Xia, Y.H.; Li, L.; Ye, C.; Dai, S.X. Risk of skin cancers in thiopurines-treated and thiopurines-untreated patients with inflammatory bowel disease: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2019, 34, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Goydos, J.S.; Shoen, S.L. Acral Lentiginous Melanoma. Cancer Treat. Res. 2016, 167, 321–329. [Google Scholar] [PubMed]
- Ito, T.; Wada, M.; Nagae, K.; Nakano-Nakamura, M.; Nakahara, T.; Hagihara, A.; Furue, M.; Uchi, H. Acral lentiginous melanoma: Who benefits from sentinel lymph node biopsy? J. Am. Acad. Dermatol. 2015, 72, 71–77. [Google Scholar] [CrossRef] [PubMed]
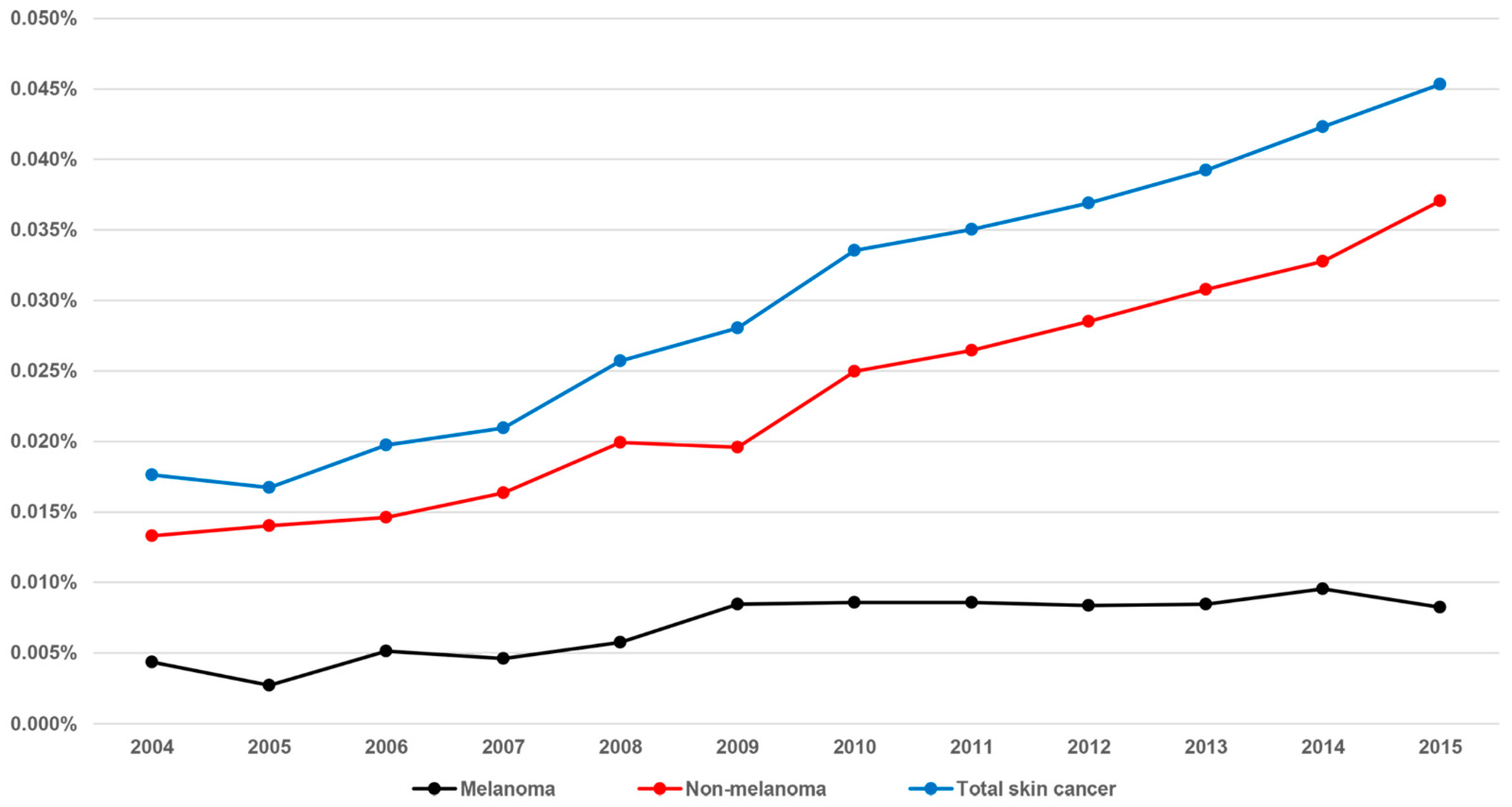
| Year | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Population | 804,790 | 848,240 | 876,546 | 892,730 | 902,580 | 923,373 | 828,801 | 933,289 | 943,205 | 945,707 | 954,779 | 957,535 |
| Total skin cancer | ||||||||||||
| Prevalence | 142 | 142 | 173 | 187 | 232 | 259 | 278 | 327 | 348 | 371 | 404 | 434 |
| Per 10,000 persons | 1.8 | 1.7 | 2.0 | 2.1 | 2.6 | 2.8 | 3.4 | 3.5 | 3.7 | 3.9 | 4.2 | 4.5 |
| NMSC | ||||||||||||
| Prevalence | 107 | 119 | 128 | 146 | 180 | 181 | 207 | 247 | 269 | 291 | 313 | 355 |
| Per 10,000 persons | 1.3 | 1.4 | 1.5 | 1.6 | 2.0 | 2.0 | 2.5 | 2.6 | 2.9 | 3.1 | 3.3 | 3.7 |
| Melanoma | ||||||||||||
| Prevalence | 35 | 23 | 45 | 41 | 52 | 78 | 71 | 80 | 79 | 80 | 91 | 79 |
| Per 10,000 persons | 0.4 | 0.3 | 0.5 | 0.5 | 0.6 | 0.8 | 0.9 | 0.9 | 0.8 | 0.8 | 1.0 | 0.8 |
| Total n (%) | Cancer n (%) | No Cancer n (%) | p-Value | |
|---|---|---|---|---|
| Age | 1.000 | |||
| <50 | 1032 | 172 (16.7) | 860 (83.3) | |
| 50–59 | 1014 | 169 (16.7) | 845 (83.3) | |
| 60–69 | 1350 | 225 (16.7) | 1125 (83.3) | |
| 70–79 | 1872 | 312 (16.7) | 1560 (83.3) | |
| >80 | 1944 | 324 (16.7) | 1620 (83.3) | |
| Sex | 1.000 | |||
| Male | 3252 | 542 (16.7) | 2710 (83.3) | |
| Female | 3960 | 660 (16.7) | 3300 (83.3) | |
| Income | 1.000 | |||
| 70–100 percentile (low) | 3804 | 634 (16.7) | 3170 (83.3) | |
| 40–70 percentile | 1740 | 290 (16.7) | 1450 (83.3) | |
| >40 percentile (high) | 1668 | 278 (16.7) | 1390 (83.3) | |
| Residential area | 1.000 | |||
| City resident | 2994 | 499 (16.7) | 2495 (83.3) | |
| Rural resident | 4218 | 703 (16.7) | 3515 (83.3) | |
| CCI | 1.000 | |||
| <3 | 4044 | 674 (16.7) | 3370 (83.3) | |
| ≥3 | 3168 | 528 (16.7) | 2640 (83.3) | |
| Diabetes mellitus | 0.965 | |||
| Yes | 2824 | 470 (16.6) | 2354 (83.4) | |
| No | 4388 | 732 (16.7) | 3656 (83.3) | |
| Hypertension | 0.001 * | |||
| Yes | 4288 | 765 (17.8) | 3523 (82.2) | |
| No | 2924 | 437 (14.9) | 2487 (85.1) | |
| Hyperlipidemia | 0.049 * | |||
| Yes | 3402 | 598 (17.6) | 2804 (82.4) | |
| No | 3810 | 604 (15.9) | 3206 (84.1) | |
| Parkinson’s disease | 0.686 | |||
| Yes | 151 | 27 (17.9) | 124 (82.1) | |
| No | 7061 | 1175 (16.6) | 5886 (83.4) | |
| Coronary artery disease | 0.019 * | |||
| Yes | 259 | 57 (22.0) | 202 (78.0) | |
| No | 6953 | 1145 (16.5) | 5808 (83.5) | |
| Myocardial infarction | 0.828 | |||
| Yes | 156 | 27 (17.3) | 129 (82.7) | |
| No | 7056 | 1175 (16.7) | 5881 (83.3) | |
| Inflammatory bowel disease | 0.788 | |||
| Yes | 2843 | 478 (16.8) | 2365 (83.2) | |
| No | 4369 | 724 (16.6) | 3645 (83.4) | |
| Stroke | 0.408 | |||
| Yes | 107 | 21 (19.6) | 86 (80.4) | |
| No | 7105 | 1181 (16.6) | 5924 (83.4) | |
| COPD | 0.284 | |||
| Yes | 506 | 93 (18.4) | 413 (81.6) | |
| No | 6706 | 1109 (16.5) | 5597 (83.5) | |
| Cataract | 0.153 | |||
| Yes | 2927 | 510 (17.4) | 2417 (82.6) | |
| No | 4285 | 692 (16.1) | 3593 (83.9) | |
| Macular degeneration | 0.019 * | |||
| Yes | 233 | 52 (22.3) | 181 (77.7) | |
| No | 6979 | 1150 (16.5) | 5829 (83.5) | |
| Pterygium | 0.131 | |||
| Yes | 343 | 47 (13.7) | 296 (86.3) | |
| No | 6869 | 1155 (16.8) | 5714 (83.2) |
| OR (95% CI) | p-Value | |
|---|---|---|
| Age | ||
| <50 | Ref. | |
| 50–59 | 0.896 (0.703–1.140) | 0.302 |
| 60–69 | 0.842 (0.662–1.072) | 0.772 |
| 70–79 | 0.754 (0.586–0.971) | 0.171 |
| >80 | 0.676 (0.519–0.881) | 0.006 * |
| Sex | ||
| Male | 1.025 (0.899–1.169) | 0.713 |
| Female | Ref. | |
| Income | ||
| 70–100 percentile (low) | Ref. | |
| 40–70 percentile | 1.009 (0.862–1.180) | 0.942 |
| >40 percentile (high) | 1.006 (0.857–1.181) | 0.983 |
| City | ||
| City resident | 1.020 (0.896–1.161) | 0.761 |
| Rural resident | Ref. | |
| CCI | ||
| <3 | Ref. | |
| ≥3 | 0.968 (0.724–1.293) | 0.824 |
| Diabetes mellitus | ||
| Yes | 0.923 (0.686–1.242) | 0.597 |
| No | Ref. | |
| Hypertension | ||
| Yes | 1.380 (1.111–1.539) | 0.001 * |
| No | Ref. | |
| Hyperlipidemia | ||
| Yes | 1.060 (0.920–1.221) | 0.422 |
| No | Ref. | |
| Parkinson’s disease | ||
| Yes | 1.026 (0.660–1.593) | 0.910 |
| No | Ref. | |
| Coronary artery disease | ||
| Yes | 1.320 (0.956–1.821) | 0.091 |
| No | Ref. | |
| Myocardial infarction | ||
| Yes | 0.926 (0.594–1.442) | 0.733 |
| No | Ref. | |
| Irritable bowel disease | ||
| Yes | 0.967 (0.848–1.102) | 0.614 |
| No | Ref. | |
| Stroke | ||
| Yes | 1.158 (0.689–1.946) | 0.580 |
| No | Ref. | |
| COPD | ||
| Yes | 1.101 (0.858–1.412) | 0.449 |
| No | Ref. | |
| Cataract | ||
| Yes | 1.055 (0.899–1.238) | 0.515 |
| No | Ref. | |
| Macular degeneration | ||
| Yes | 1.500 (1.080–2.082) | 0.016 * |
| No | Ref. | |
| Pterygium | ||
| Yes | 0.789 (0.571–1.091) | 0.152 |
| No | Ref. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.H.; Ro, J.-S.; Chung, K.Y.; Lee, S.H.; Park, Y.L.; Kim, J.E.; Lee, S.H. Association between Skin Cancer and Systemic and Ocular Comorbidities in South Korea. J. Clin. Med. 2021, 10, 2451. https://doi.org/10.3390/jcm10112451
Lee SH, Ro J-S, Chung KY, Lee SH, Park YL, Kim JE, Lee SH. Association between Skin Cancer and Systemic and Ocular Comorbidities in South Korea. Journal of Clinical Medicine. 2021; 10(11):2451. https://doi.org/10.3390/jcm10112451
Chicago/Turabian StyleLee, Sul Hee, Jun-Soo Ro, Kee Yang Chung, Sang Hoon Lee, Young Lip Park, Jung Eun Kim, and Si Hyung Lee. 2021. "Association between Skin Cancer and Systemic and Ocular Comorbidities in South Korea" Journal of Clinical Medicine 10, no. 11: 2451. https://doi.org/10.3390/jcm10112451
APA StyleLee, S. H., Ro, J.-S., Chung, K. Y., Lee, S. H., Park, Y. L., Kim, J. E., & Lee, S. H. (2021). Association between Skin Cancer and Systemic and Ocular Comorbidities in South Korea. Journal of Clinical Medicine, 10(11), 2451. https://doi.org/10.3390/jcm10112451






