Do-It-Yourself Preoperative High-Resolution Ultrasound-Guided Flap Design of the Superficial Circumflex Iliac Artery Perforator Flap (SCIP)
Abstract
1. Introduction
2. Methods
2.1. Medical Indication for SCIP Flap
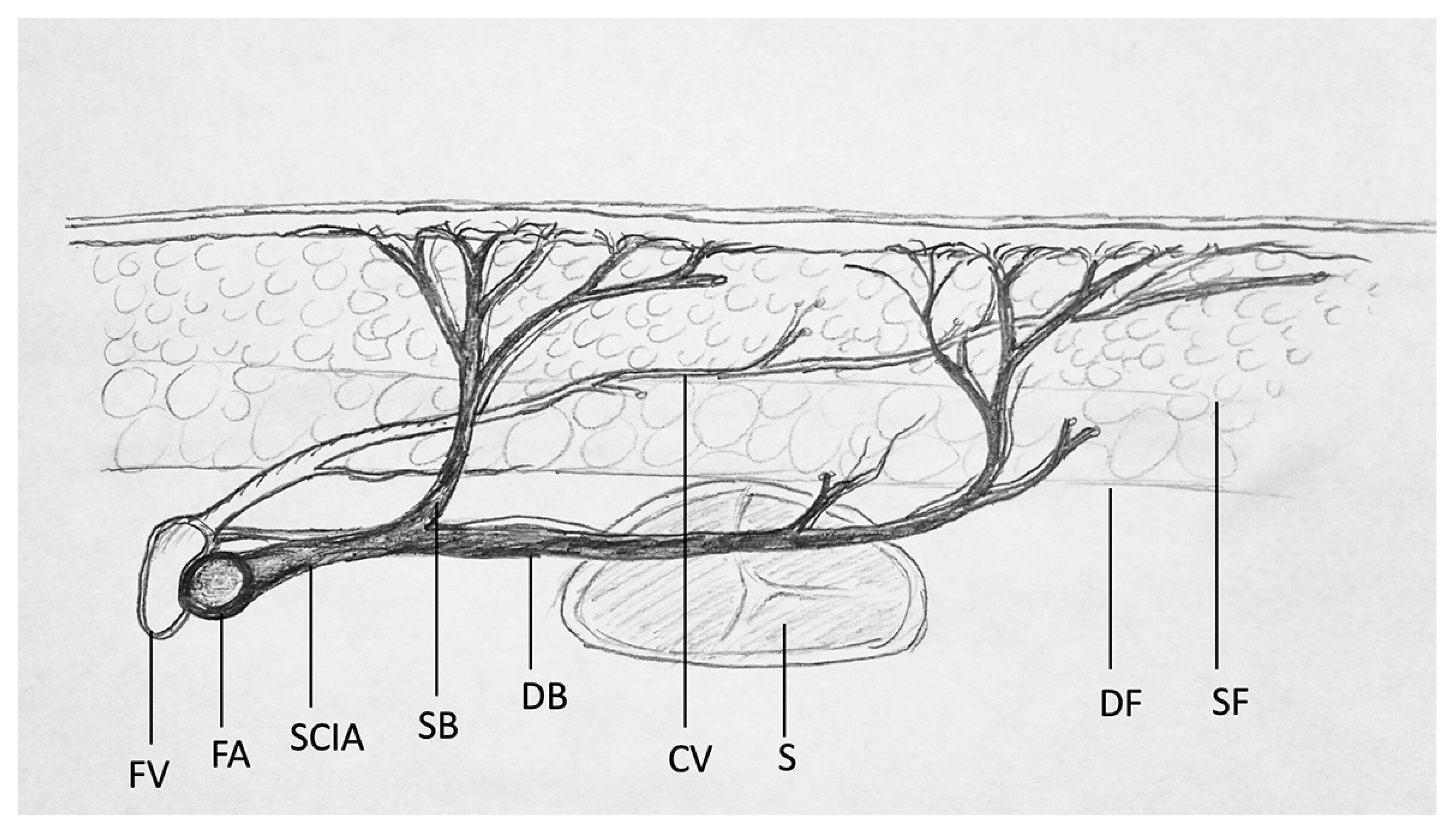
2.2. Ultrasound-Based Flap Design & Perforator Characterization
2.3. Operation Technique
3. Results
- ▪
- Start in B-mode. Choose a linear transducer (9–15 MHz). In this study, the high-resolution transducer ML6-15-D (4–15 MHz) demonstrated the highest level of detail for displaying perforators. US devices offer presets that can be chosen before imaging. The “Thyroid” preset is recommended, as these settings are close to those optimized for the SCIP flap (Table 1). Using transverse cuts, the examiner should visualize the anatomy, assess tissue morphology, and localize the SCIA branching off the femoral artery (FA).
- ▪
- Color Flow (CF)-mode should then be initiated. This mode allows for color-coded imaging of the intravascular blood flow. The “PRF/Scale” should be moved between 0.7 and 1.3 kHz/5 and 9 cm/s to be able to visualize smaller vessels with low flow (perforators, superficial arteries and veins). By micro-rotation and careful sliding of the transducer, the examiner should verify the anatomy, asses the vascular axis of the superficial branch of SCIA, and confirm the positions of one or several perforators branching off the superficial branch. The emergence points of the perforator penetrating the deep as well as the superficial fascia should be marked with a permanent marker to allow for a precise flap design and to simplify microsurgical dissection. An additional cutaneous vein may be marked and traced deep to the femoral vein at the level of the saphenous hiatus.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parrett, B.M.; Talbot, S.G.; Pribaz, J.J.; Lee, B.T. A Review of Local and Regional Flaps for Distal Leg Reconstruction. J. Reconstr. Microsurg. 2009, 25, 445–455. [Google Scholar] [CrossRef]
- Suh, H.S.; Oh, T.S.; Lee, H.S.; Lee, S.H.; Cho, Y.P.; Park, J.R.; Hong, J.P. A New Approach for Reconstruction of Diabetic Foot Wounds Using the Angiosome and Supermicrosurgery Concept. Plast. Reconstr. Surg. 2016, 138, 702e–709e. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, Y.J.; Oh, D.Y.; Jun, Y.J.; Rhie, J.W.; Moon, S.H. Reconstruction of wide soft tissue defects with extended an-terolateral thigh perforator flap turbocharged technique with anteromedial thigh perforator. Microsurgery 2019, 1–7. [Google Scholar] [CrossRef]
- Suh, H.S.P.; Jeong, H.H.; Choi, D.H.; Hong, J.P.J.P. Study of the Medial Superficial Perforator of the Superficial Circumflex Iliac Artery Perforator Flap Using Computed Tomographic Angiography and Surgical Anatomy in 142 Patients. Plast. Reconstr. Surg. 2017, 139, 738–748. [Google Scholar] [CrossRef]
- Suh, Y.C.; Hong, J.P.; Suh, H.P. Elevation Technique for Medial Branch based Superficial Circumflex Iliac Artery Perforator flap. Handchir. Mikrochir. Plast. Chir. 2018, 50, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Fuse, Y.; Yoshimatsu, H.; Yamamoto, T. Lateral approach to the deep branch of the superficial circumflex iliac artery for harvesting a SCIP flap. Microsurgery 2018, 38, 589–590. [Google Scholar] [CrossRef] [PubMed]
- Berner, J.E.; Nikkhah, D.; Zhao, J.; Prousskaia, E.; Teo, T.C. The Versatility of the Superficial Circumflex Iliac Artery Perforator Flap: A Single Surgeon’s 16-Year Experience for Limb Reconstruction and a Systematic Review. J. Reconstr. Microsurg. 2020, 36, 093–103. [Google Scholar] [CrossRef] [PubMed]
- Iida, T. Superficial Circumflex Iliac Perforator (SCIP) Flap: Variations of the SCIP Flap and Their Clinical Applications. J. Reconstr. Microsurg. 2014, 30, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Pereira, N.; Cámbara, Á.; Kufeke, M.; Roa, R. Post-traumatic lymphedema treatment with superficial circumflex iliac artery perforator lymphatic free flap: A case report. Microsurgery 2019, 39, 354–359. [Google Scholar] [CrossRef]
- Goh, T.L.H.; Park, S.W.; Cho, J.Y.; Choi, J.W.; Hong, J.P. The Search for the Ideal Thin Skin Flap. Plast. Reconstr. Surg. 2015, 135, 592–601. [Google Scholar] [CrossRef]
- Karakawa, R.; Iida, T.; Yoshimatsu, H.; Kanayama, K.; Yamasoba, T. Functional and Aesthetic Reconstruction for Microtia Using the Combination of Superficial Circumflex Iliac Artery Perforator Superthin Flap Transfer and Skin Grafting. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2312. [Google Scholar] [CrossRef] [PubMed]
- Myung, Y.; Yim, S.; Kim, B.-K. A comparison of axial circumference between superficial circumflex iliac artery perforator flap and other workhorse flaps in dorsal foot reconstruction. J. Plast. Surg. Hand Surg. 2017, 51, 381–386. [Google Scholar] [CrossRef]
- Zubler, C.; Haberthür, D.; Hlushchuk, R.; Djonov, V.; Constantinescu, M.A.; Olariu, R. The anatomical reliability of the superficial circumflex iliac artery perforator (SCIP) flap. Ann. Anat. Anat. Anz. 2021, 234, 151624. [Google Scholar] [CrossRef]
- Gandolfi, S.; Postel, F.; Auquit-Auckbur, I.; Boissière, F.; Pelissier, P.; Casoli, V.; Duparc, F. Vascularization of the superficial circumflex iliac perforator flap (SCIP flap): An anatomical study. Surg. Radiol. Anat. 2020, 42, 473–481. [Google Scholar] [CrossRef]
- Yoshimatsu, H.; Yamamoto, T.; Hayashi, A.; Fuse, Y.; Karakawa, R.; Iida, T.; Narushima, M.; Tanakura, K.; Weninger, W.J.; Tzou, C.H.J. Use of the transverse branch of the superficial circumflex iliac artery as a landmark facilitating identification and dissection of the deep branch of the superficial circumflex iliac artery for free flap pedicle: Anatomical study and clinical applications. Microsurgery 2019, 39, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Gentileschi, S.; Servillo, M.; De Bonis, F.; Albanese, R.; Pino, V.; Mangialardi, M.L.; Valente, I.; Garganese, G.; Scambia, G.; Salgarello, M.; et al. Radioanatomical Study of the Pedicle of the Superficial Circumflex Iliac Perforator Flap. J. Reconstr. Microsurg. 2019, 35, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Pereira, N.; Parada, L.; Kufeke, M.; Troncoso, E.; Roa, R. A New Planning Method to Easily Harvest the Superficial Circumflex Iliac Artery Perforator Flap. J. Reconstr. Microsurg. 2020, 36, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, K.; Harima, M.; Kato, M.; Yamamoto, T.; Yamashita, S.; Narushima, M.; Iida, T.; Koshima, I. Preoperative color Doppler ultrasound assessment in planning of SCIP flaps. J. Plast. Reconstr. Aesthet. Surg. 2015, 68, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; He, Y.; Tian, Z.; Feng, S.; Zhang, Y. Superficial circumflex iliac artery perforator flap aided by color Doppler sonography mapping for like-with-like buccal reconstruction. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 119, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y. Doppler ultrasonography of the lower extremity arteries: Anatomy and scanning guidelines. Ultrasonography 2017, 36, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Saba, L.; Atzeni, M.; Rozen, W.M.; Alonso-Burgos, A.; Bura, R.; Piga, M.; Ribuffo, D. Non-invasive vascular imaging in perforator flap surgery. Acta Radiol. 2013, 54, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Kehrer, A.; Heidekrueger, P.I.; Lonic, D.; Taeger, C.D.; Klein, S.; Lamby, P.; Sachanadani, N.S.; Jung, E.M.; Prantl, L.; Da Silva, N.P.B. High-Resolution Ultrasound-Guided Perforator Mapping and Characterization by the Microsurgeon in Lower Limb Reconstruction. J. Reconstr. Microsurg. 2021, 37, 75–82. [Google Scholar] [CrossRef]
- Kehrer, A.; Hsu, M.-Y.; Chen, Y.-T.; Sachanandani, N.; Tsao, C.-K. Simplified profunda artery perforator (PAP) flap design using power Doppler ultrasonography (PDU): A prospective study. Microsurgery 2018, 38, 512–523. [Google Scholar] [CrossRef]
- Cheng, H.-T.; Lin, F.-Y.; Chang, S.C.-N. Diagnostic Efficacy of Color Doppler Ultrasonography in Preoperative Assessment of Anterolateral Thigh Flap Cutaneous Perforators. Plast. Reconstr. Surg. 2013, 131, 471e–473e. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Lu, L.; Lazzeri, D.; Zhang, Y.X.; Wang, D.; Innocenti, M.; Qian, Y.; Agostini, T.; Levin, L.S.; Messmer, C. Contrast-Enhanced Ultrasound Combined with Three-Dimensional Reconstruction in Preoperative Perforator Flap Planning. Plast. Reconstr. Surg. 2013, 131, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Kehrer, A.; Sachanadani, N.S.; da Silva, N.P.B.; Lonic, D.; Heidekrueger, P.; Taeger, C.D.; Klein, S.; Jung, E.M.; Prantl, L.; Hong, J.P. Step-by-step guide to ultrasound-based design of alt flaps by the microsurgeon—Basic and advanced applica-tions and device settings. J. Plast. Reconstr. Aesthet. Surg. 2019, 73, 1081–1090. [Google Scholar] [CrossRef]
- Hong, J.P.; Sun, S.H.; Ben-Nakhi, M. Modified Superficial Circumflex Iliac Artery Perforator Flap and Supermicrosurgery Technique for Lower Extremity Reconstruction. Ann. Plast. Surg. 2013, 71, 380–383. [Google Scholar] [CrossRef]
- Koshima, I.; Nanba, Y.; Tsutsui, T.; Takahashi, Y.; Urushibara, K.; Inagawa, K.; Hamasaki, T.; Moriguchi, T. Superficial Circumflex Iliac Artery Perforator Flap for Reconstruction of Limb Defects. Plast. Reconstr. Surg. 2004, 113, 233–240. [Google Scholar] [CrossRef]
- Cheng, H.-T.; Lin, F.-Y.; Chang, S.C.-N. Diagnostic Efficacy of Preoperative 64-Section Multidetector Computed Tomographic Angiography in Identifying the Cutaneous Perforators in the Anterolateral Thigh Flap. Plast. Reconstr. Surg. 2012, 130, 771e–772e. [Google Scholar] [CrossRef] [PubMed]
- Mathes, D.W.; Neligan, P.C. Preoperative Imaging Techniques for Perforator Selection in Abdomen-Based Microsurgical Breast Reconstruction. Clin. Plast. Surg. 2010, 37, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Yoshimatsu, H.; Karakawa, R.; Fuse, Y.; Okada, A.; Hayashi, A.; Yano, T. Use of Preoperative High-Resolution Ultrasound System to Facilitate Elevation of the Superficial Circumflex Iliac Artery Perforator Flap. J. Reconstr. Microsurg. 2021. [Google Scholar] [CrossRef]
- Kehrer, A.; Mandlik, V.; Taeger, C.; Geis, S.; Prantl, L.; Jung, E.-M. Postoperative control of functional muscle flaps for facial palsy reconstruction: Ultrasound guided tissue monitoring using contrast enhanced ultrasound (CEUS) and ultrasound elastography. Clin. Hemorheol. Microcirc. 2017, 67, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Kehrer, A.; Lonic, D.; Heidekrueger, P.; Bosselmann, T.; Taeger, C.D.; Lamby, P.; Kehrer, M.; Jung, E.M.; Prantl, L.; Da Silva, N.P.B. Feasibility study of preoperative microvessel evaluation and characterization in perforator flaps using various modes of color-coded duplex sonography (CCDS). Microsurgery 2020, 40, 750–759. [Google Scholar] [CrossRef]
- Dusseldorp, M.B.J.R.; Pennington, F.F.M.D.G. Quantifying Blood Flow in the DIEP Flap. Plast. Reconstr. Surg. Glob. Open 2014, 2, e228. [Google Scholar] [CrossRef]
- Debelmas, A.; Camuzard, O.; Aguilar, P.; Qassemyar, Q. Reliability of Color Doppler Ultrasound Imaging for the Assessment of Anterolateral Thigh Flap Perforators. Plast. Reconstr. Surg. 2018, 141, 762–766. [Google Scholar] [CrossRef]
- Alzaraa, A.; Gravante, G.; Chung, W.Y.; Al-Leswas, D.; Morgan, B.; Dennison, A.; Lloyd, D. Contrast-enhanced ultrasound in the preoperative, intraoperative and postoperative assessment of liver lesions. Hepatol. Res. 2013, 43, 809–819. [Google Scholar] [CrossRef]
- Hallock, G.G. Doppler sonography and color duplex imaging for planning a perforator flap. Clin. Plast. Surg. 2003, 30, 347–357. [Google Scholar] [CrossRef]
- Lin, C.-T.; Huang, J.-S.; Hsu, K.-C.; Yang, K.-C.; Chen, J.-S.; Chen, L.-W. Different Types of Suprafascial Courses in Thoracodorsal Artery Skin Perforators. Plast. Reconstr. Surg. 2008, 121, 840–848. [Google Scholar] [CrossRef]
- Miller, J.R.; Potpari, Z.; Colen, L.B.; Sorrell, K.; Carraway, J.H. The Accuracy of Duplex Ultrasonography in the Planning of Skin Flaps in the Lower Extremity. Plast. Reconstr. Surg. 1995, 95, 1221–1227. [Google Scholar] [CrossRef]
- Haddock, N.; Greaney, P.; Otterburn, D.; Levine, S.; Allen, R.J. Predicting perforator location on preoperative imaging for the profunda artery perforator flap. Microsurgery 2012, 32, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Cina, A.; Salgarello, M.; Barone-Adesi, L.; Rinaldi, P.; Bonomo, L. Planning Breast Reconstruction with Deep Inferior Epigastric Artery Perforating Vessels: Multidetector CT Angiography versus Color Doppler US 1. Radiology 2010, 255, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Schiltz, D.; Geis, S.; Kehrer, A.; Dolderer, J.; Prantl, L.; Taeger, C.D. Video Tutorial for Clinical Flap-Monitoring in Plastic Surgery. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1478. [Google Scholar] [CrossRef] [PubMed]




| Qualitative Characterization | |
|---|---|
| B-mode (B) | |
| Probe selection | linear (optimal 15 MHz) |
| Frequency (Frq) | 15 MHz |
| Gain (Gn) | 25–35 dB |
| Depth (D) | 2–3 cm |
| Focus | 1–2 cm |
| Color flow (CF) | |
| Wall Filter (WF) | minimum |
| Gain (Gn) | 10–20 dB |
| Frequency (Frq) | 5–7.5 MHz |
| PRS/Scale | 5–9 cm/s |
| Quantitative Characterization | |
| Pulse Wave (PW) | |
| Frequency (Frq) | 6.3 MHz |
| Gain (Gn) | 30–35 dB |
| Wall Filter (WF) | 100–200 Hz |
| PRS/Scale | 6–12 cm/s |
| Patient No. | Sex | Age (years) | BMI (kg/m2) | Tissue Defect Area |
|---|---|---|---|---|
| 1 | F | 47 | 29.64 | ankle |
| 2 | M | 58 | 40.91 | forefoot |
| 3 | M | 68 | 31.74 | forefoot |
| 4 | F | 17 | 23.34 | ankle |
| 5 | M | 43 | 28.29 | ankle |
| 6 | M | 56 | 26.34 | forefoot |
| 7 | F | 22 | 20.76 | head |
| 8 | M | 49 | 24.84 | hand |
| 9 | M | 9 | 24.97 | forefoot |
| 10 | F | 25 | 18.65 | forefoot |
| 11 | M | 58 | 26.43 | ankle |
| 12 | M | 55 | 25.36 | forefoot |
| CCDS Characteristics | Intraoperative Characteristics | |||||||
|---|---|---|---|---|---|---|---|---|
| Qualitative Characteristics | Quantitative Characteristics | |||||||
| Patient No. | Mapped Perforator | Perforator Diameter CCDS (mm) | PS cm/s | ED cm/s | RI | Intraoperatively Confirmed Anatomy | Flap Size (cm) | Recipient Vessel |
| 1 | medial | 1.3 | 14.7 | 3.1 | 0.79 | yes | 15 ×6 | anterior tibial artery |
| 2 | medial | 1.7 | 24.5 | 6.8 | 0.72 | yes | 22 × 8 | anterior tibial artery |
| 3 | medial | 1.2 | 17.9 | 5.7 | 0.68 | yes | 16 × 7 | anterior tibial artery |
| 4 | medial | 2.3 | 16.4 | 2.1 | 0.87 | yes | 8 × 10 | dorsalis pedis artery |
| 5 | medial | 1.6 | 9.9 | 4.5 | 0.55 | yes | 20 × 7 | posterior tibial artery |
| 6 | medial | 1.2 | 17.9 | 5.7 | 0.68 | yes | / | dorsalis pedis artery |
| 7 | medial | 1.9 | 17.7 | 4.9 | 0.61 | yes | 4 × 5 | superficial temporal artery |
| 8 | medial | 1.2 | 20.6 | 5.2 | 0.58 | yes | 13 × 6 | perforator of radial artery |
| 9 | medial | 1.4 | 11 | 4.6 | 0.64 | yes | 15 × 4.5 | dorsalis pedis artery |
| 10 | medial | 1.3 | 16.3 | 5.3 | 0.68 | yes | / | anterior tibial artery |
| 11 | medial | 2.2 | 12.6 | 2.6 | 0.79 | yes | 11 × 18 | posterior tibial artery |
| 12 | medial | 1.2 | 22.3 | 4.9 | 0.78 | yes | 15 × 6 | anterior tibial artery |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiltz, D.; Lenhard, J.; Klein, S.; Anker, A.; Lonic, D.; Heidekrueger, P.I.; Prantl, L.; Jung, E.-M.; Platz Batista Da Silva, N.; Kehrer, A. Do-It-Yourself Preoperative High-Resolution Ultrasound-Guided Flap Design of the Superficial Circumflex Iliac Artery Perforator Flap (SCIP). J. Clin. Med. 2021, 10, 2427. https://doi.org/10.3390/jcm10112427
Schiltz D, Lenhard J, Klein S, Anker A, Lonic D, Heidekrueger PI, Prantl L, Jung E-M, Platz Batista Da Silva N, Kehrer A. Do-It-Yourself Preoperative High-Resolution Ultrasound-Guided Flap Design of the Superficial Circumflex Iliac Artery Perforator Flap (SCIP). Journal of Clinical Medicine. 2021; 10(11):2427. https://doi.org/10.3390/jcm10112427
Chicago/Turabian StyleSchiltz, Daniel, Jasmin Lenhard, Silvan Klein, Alexandra Anker, Daniel Lonic, Paul I. Heidekrueger, Lukas Prantl, Ernst-Michael Jung, Natascha Platz Batista Da Silva, and Andreas Kehrer. 2021. "Do-It-Yourself Preoperative High-Resolution Ultrasound-Guided Flap Design of the Superficial Circumflex Iliac Artery Perforator Flap (SCIP)" Journal of Clinical Medicine 10, no. 11: 2427. https://doi.org/10.3390/jcm10112427
APA StyleSchiltz, D., Lenhard, J., Klein, S., Anker, A., Lonic, D., Heidekrueger, P. I., Prantl, L., Jung, E.-M., Platz Batista Da Silva, N., & Kehrer, A. (2021). Do-It-Yourself Preoperative High-Resolution Ultrasound-Guided Flap Design of the Superficial Circumflex Iliac Artery Perforator Flap (SCIP). Journal of Clinical Medicine, 10(11), 2427. https://doi.org/10.3390/jcm10112427










