Improvement of Superficial and Deep Cutaneous Microcirculation Due to Axillary Plexus Anesthesia Impaired by Smoking
Abstract
:1. Introduction
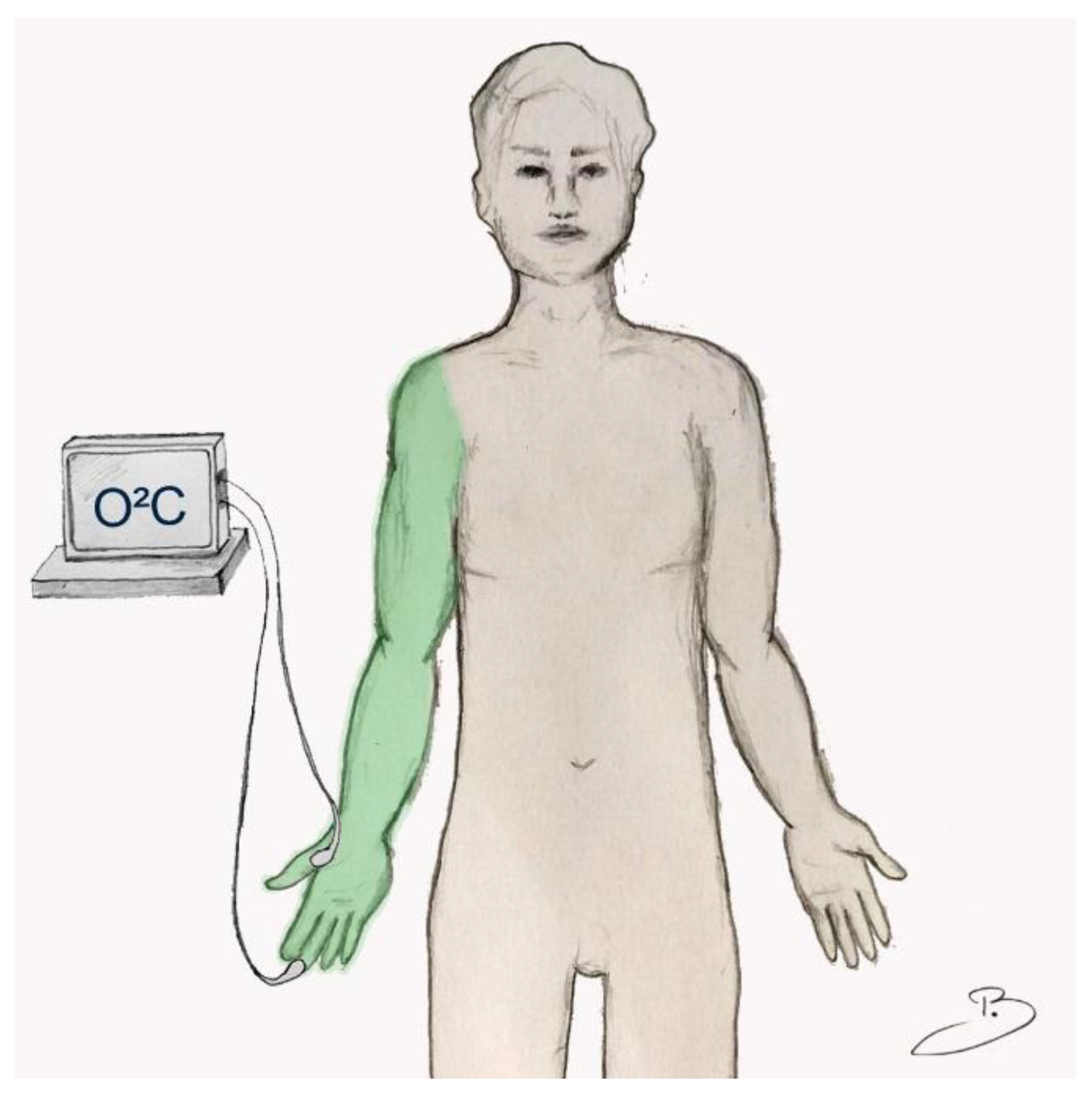
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Microcirculatory Measurements
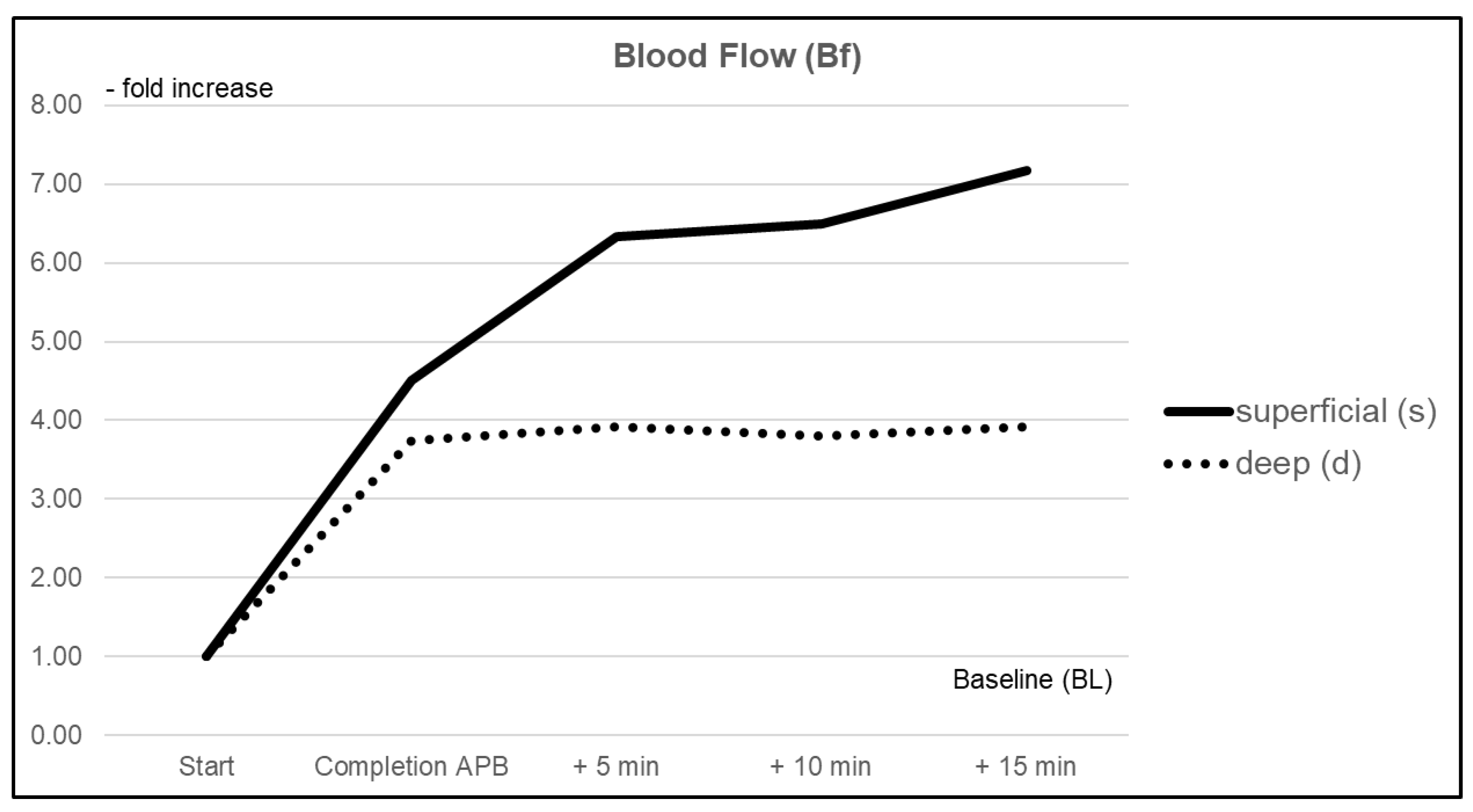
3.1.1. Blood Flow (Bf)
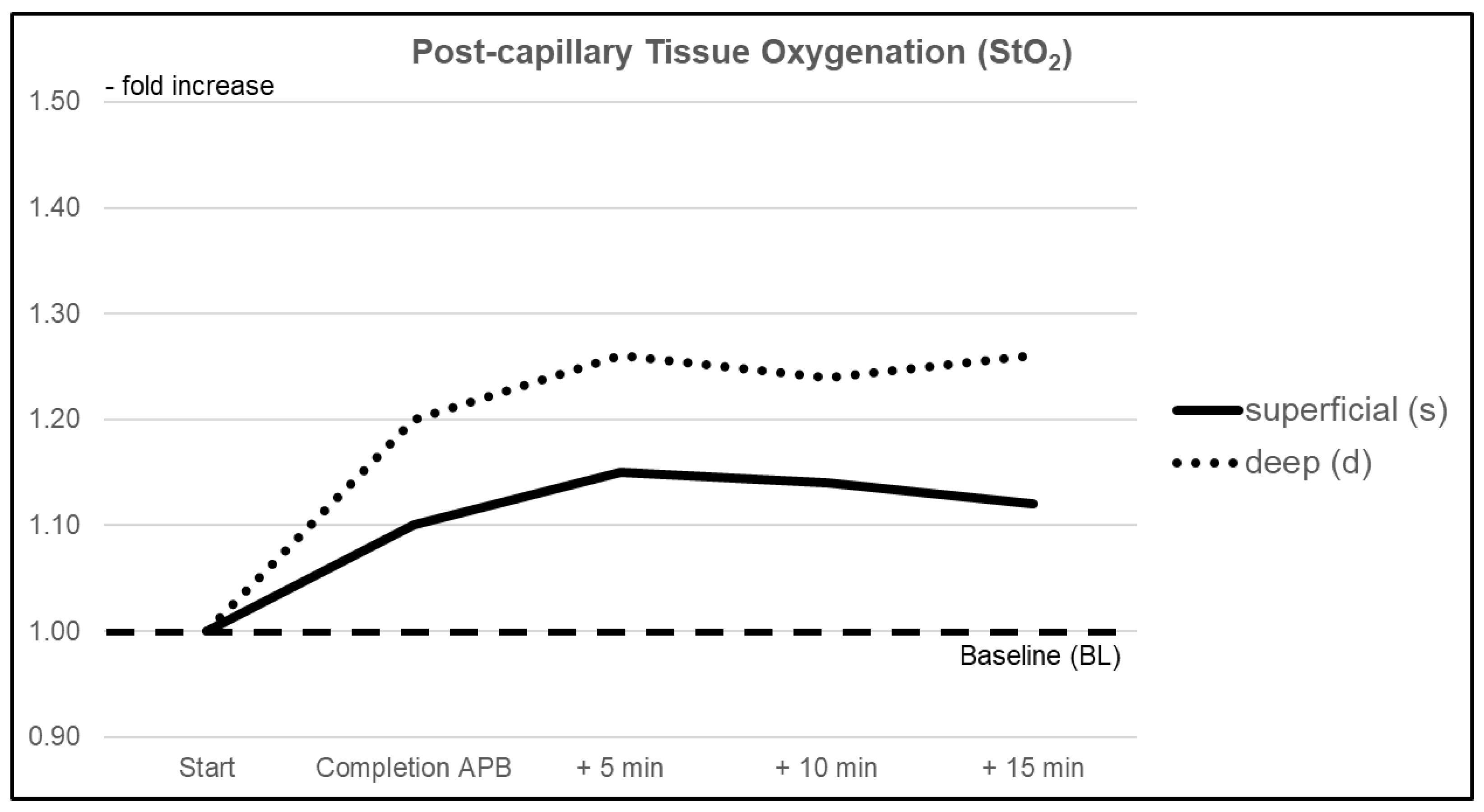
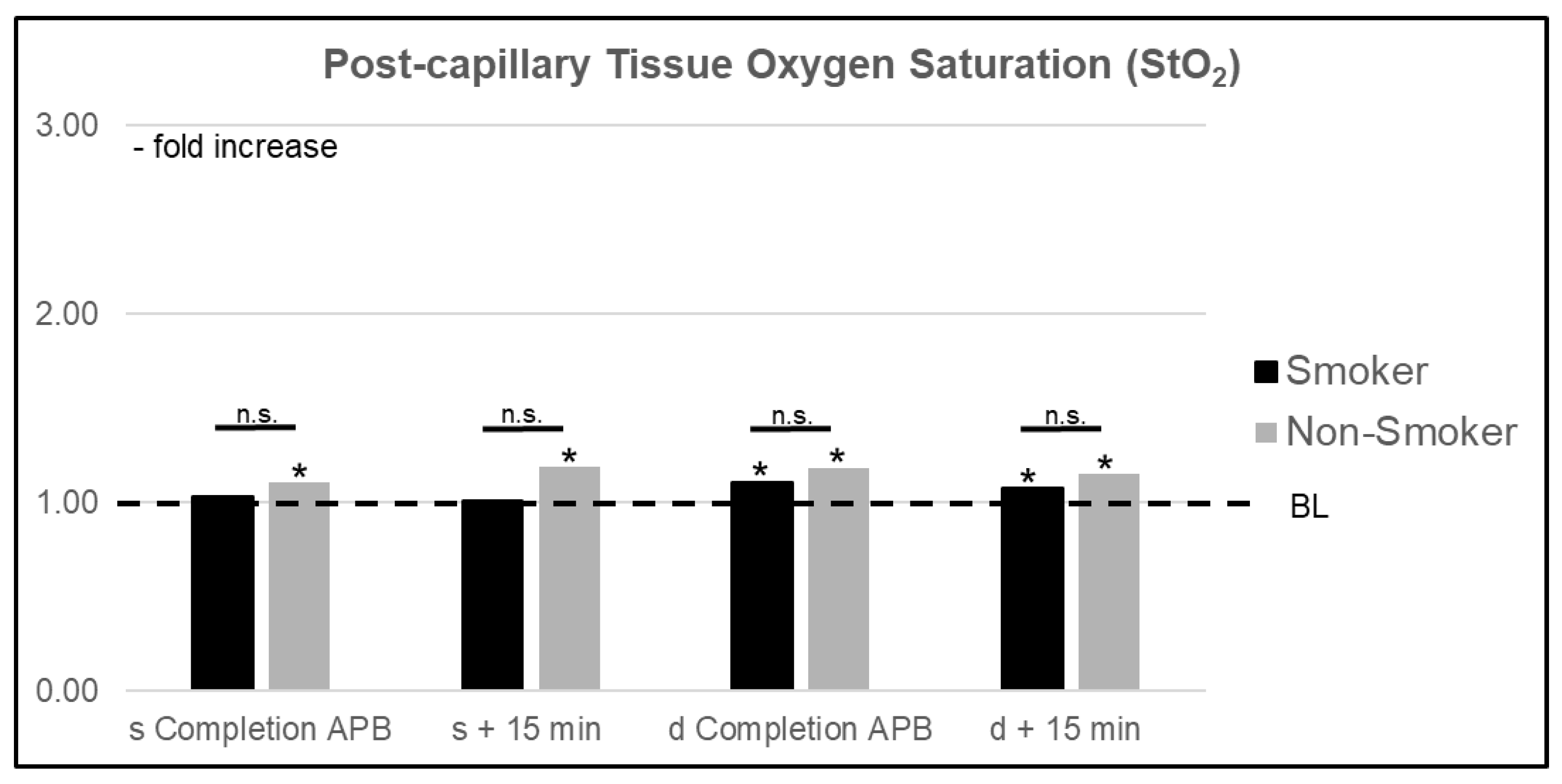
3.1.2. Postcapillary Tissue Oxygen Saturation (StO2)
3.1.3. Relative Hemoglobin Content (rHb)
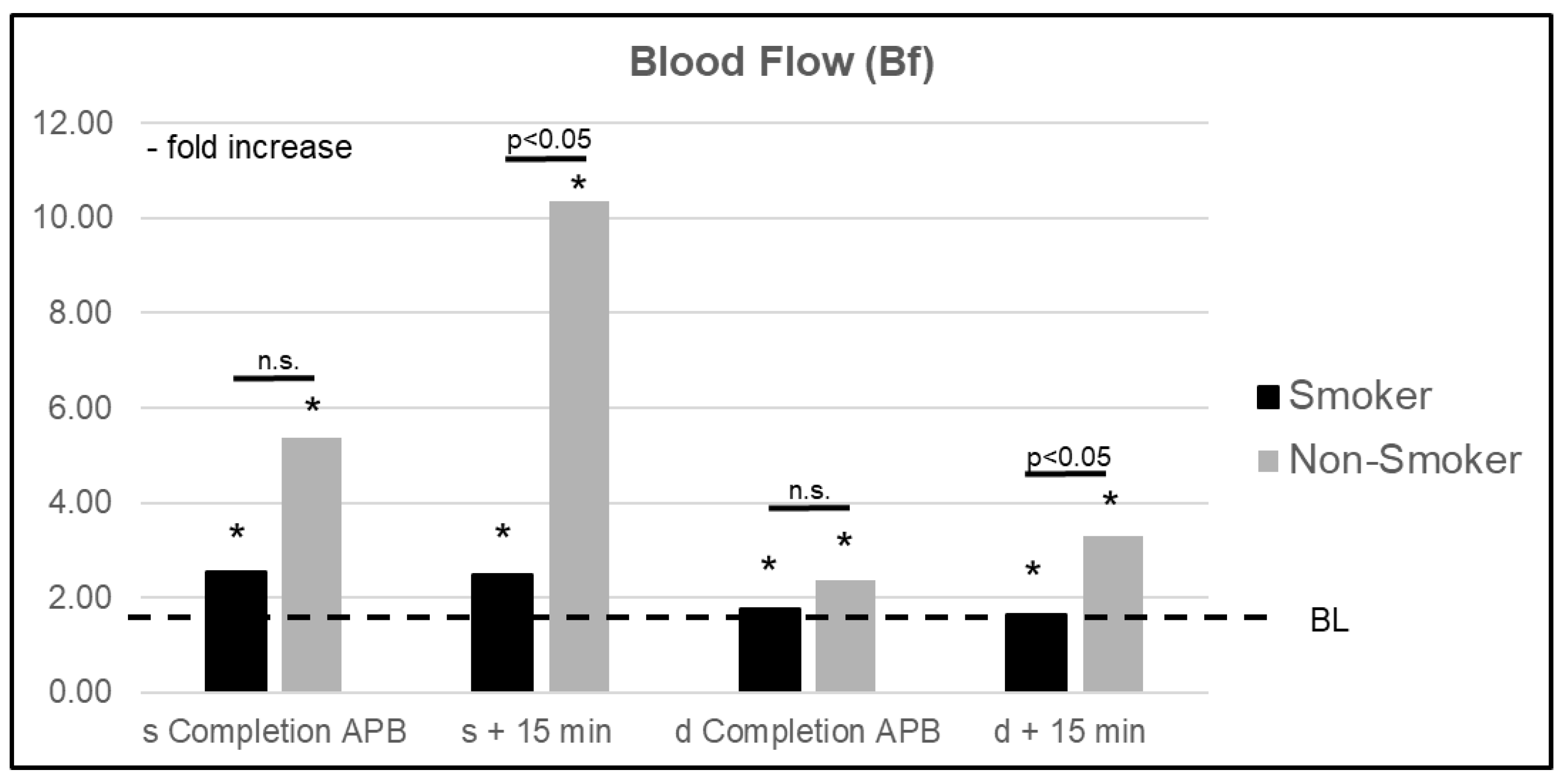
3.2. Microcirculatory Analysis: Smoker vs. Nonsmoker
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Albrecht, E.; Chin, K.J. Advances in regional anaesthesia and acute pain management: A narrative review. Anaesthesia 2020, 75, e101–e110. [Google Scholar] [CrossRef] [PubMed]
- Kremer, T.; Bauer, M.; Zahn, P.; Wallner, C.; Fuchs, P.; Horch, R.E.; Schaefer, D.J.; Bader, R.D.; Lenhardt, M.; Reichert, B.; et al. [Perioperative Management in Microsurgery—Consensus Statement of the German Speaking Society for Microsurgery of Peripheral Nerves and Vessels]. Handchir Mikrochir Plast. Chir 2016, 48, 205–211. [Google Scholar] [PubMed]
- Kremer, T.; Bauer, M.; Zahn, P.; Wallner, C.; Fuchs, P.; Horch, R.E.; Schaefer, D.J.; Bader, R.D.; Lenhardt, M.; Reichert, B.; et al. Perioperatives Management in der Mikrochirurgie–Konsensus-Statement der Deutschsprachigen Arbeitsgemeinschaft für Mikrochirurgie der peripheren Nerven und Gefäße. Handchir Mikrochir Plast. Chir 2020, 52, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Manoj, K.K.; Xiang, L.; Wing, H.K.; Warwick, D.N.K. Regional hemodynamic changes after an axillary brachial plexus block: A pulsed-wave Doppler ultrasound study. Reg. Anesth. Pain Med. 2012, 37, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Wenger, A.; Amr, A.; Schaller, H.-E.; Rothenberger, J. Skin Perfusion Changes within 12 h after Axillary Plexus Block. Eur. Surg. Res. 2017, 58, 227–234. [Google Scholar] [CrossRef]
- Wenger, A.; Rothenberger, J.; Hakim-Meibodi, L.-E.; Notheisen, T.; Schaller, H.-E. Quantification of the vasodilatory effect of axillary plexus block. A prospective controlled study. J. Surg. Res. 2017, 212, 153–158. [Google Scholar] [CrossRef]
- Su, H.-H.; Lui, P.-W.; Yu, C.-L.; Liew, C.-S.; Lin, C.-H.; Lin, Y.-T.; Chang, C.-H.; Yang, M.-W. The effects of continuous axillary brachial plexus block with ropivacaine infusion on skin temperature and survival of crushed fingers after microsurgical replantation. Chang. Gung Med. J. 2005, 28, 567–574. [Google Scholar]
- Bjorklund, K.A.; Venkatramani, H.; Venkateshwaran, G.; Boopathi, V.; Sabapathy, S.R. Regional anesthesia alone for pediatric free flaps. J. Plast. Reconstr. Aesthetic Surg. 2015, 68, 705–708. [Google Scholar] [CrossRef]
- Berger, A.; Tizian, C.; Zenz, M. Continuous Plexus Blockade for Improved Circulation in Microvascular Surgery. Ann. Plast. Surg. 1985, 14, 16–19. [Google Scholar] [CrossRef]
- Koshima, I.; Yamamoto, T.; Narushima, M.; Mihara, M.; Iida, T. Perforator Flaps and Supermicrosurgery. Clin. Plast. Surg. 2010, 37, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Harder, Y.; Amon, M.; Laschke, M.W.; Schramm, R.; Rücker, M.; Wettstein, R.; Bastiaanse, J.; Frick, A.; Machens, H.G.; Küntscher, M.; et al. An old dream revitalised: Preconditioning strategies to protect surgical flaps from critical ischaemia and is-chaemia-reperfusion injury. J. Plast Reconstr. Aesthet. Surg. 2008, 61, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Yoshimatsu, H.; Hayashi, A.; Yamamoto, T.; Visconti, G.; Karakawa, R.; Fuse, Y.; Iida, T. Visualization of the “Intradermal Plexus” Using Ultrasonography in the Dermis Flap: A Step beyond Perforator Flaps. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2411. [Google Scholar] [CrossRef] [PubMed]
- Narushima, M.; Yamasoba, T.; Iida, T.; Yamamoto, T.; Yoshimatsu, H.; Hara, H.; Oshima, A.; Todokoro, T.; Kikuchi, K.; Araki, J.; et al. Pure skin perforator flap for microtia and congenital aural atresia using supermicrosurgical techniques. J. Plast. Reconstr. Aesthetic Surg. 2011, 64, 1580–1584. [Google Scholar] [CrossRef] [PubMed]
- Bierbach, B.; Scheewe, J.; Derfuss, T.; Krug, A.; Schramm, R.; Dahm, M.; Kuroczynski, W.; Kempski, O.; Horstick, G. Continuous Regional Myocardial Blood Flow Measurement: Validation of a Near-Infrared Laser Doppler Device in a Porcine Model. Microcirculation 2012, 19, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Krug, A. Microcirculation and oxygen supply of tissue: Method of so-called 02C. Phlebologie 2006, 35, 300–312. [Google Scholar]
- Braverman, I.M. The Cutaneous Microcirculation. J. Investig. Dermatol. Symp. Proc. 2000, 5, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Wong, B.J.; Hollowed, C.G. Current concepts of active vasodilation in human skin. Temperature 2017, 4, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Roddie, I.C.; Shepherd, J.T.; Whelan, R.F. The contribution of constrictor and dilator nerves to the skin vasodilatation during body heating. J. Physiol. 1957, 136, 489–497. [Google Scholar] [CrossRef]
- Johansson, K.; Eriksson, M.; Wahlqvist, I.; Mühlen, B.V.Z.; Lind, L. Effects of blockade of alpha- and beta-adrenoceptors and neuropeptide Y(1) receptors, as well as brachial plexus blockade, on endothelium-dependent vasodilation in the human forearm. Clin. Exp. Pharmacol. Physiol. 2002, 29, 603–607. [Google Scholar] [CrossRef]
- Yen, A.; Braverman, I.M. Ultrastructure Of The Human Dermal Microcirculation: The Horizontal Plexus Of The Papillary Dermis. J. Investig. Dermatol. 1976, 66, 131–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braverman, I.M.; Yen, A. Ultrastructure of the human dermal microcirculation. II. The capillary loops of the dermal papillae. J. Investig. Dermatol. 1977, 68, 44–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellogg, D.L.; Johnson, J.M.; Kosiba, W.A. Selective abolition of adrenergic vasoconstrictor responses in skin by local iontophoresis of bretylium. Am. J. Physiol. Circ. Physiol. 1989, 257, H1599–H1606. [Google Scholar] [CrossRef] [PubMed]
- Pellaton, C.; Kubli, S.; Feihl, F.; Waeber, B. Blunted vasodilatory responses in the cutaneous microcirculation of cigarette smokers. Am. Heart J. 2002, 144, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Leow, Y.H.; Maibach, H.I. Cigarette smoking, cutaneous vasculature, and tissue oxygen. Clin. Dermatol. 1998, 16, 579–584. [Google Scholar] [CrossRef]
- Ambrose, A.J.; Barua, R.S. The pathophysiology of cigarette smoking and cardiovascular disease: An update. J. Am. Coll. Cardiol. 2004, 43, 1731–1737. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Ye, Q.; Wu, D.; Li, J.; Yu, J. Dose-response studies of Ropivacaine in blood flow of upper extremity after supraclavicular block: A double-blind randomized controlled study. BMC Anesthesiol. 2017, 17, 161. [Google Scholar] [CrossRef]
- Weisberger, J.S.; Oleck, N.C.; Ayyala, H.S.; Dalena, M.M.; Lee, E.S. Utility of Regional Anesthesia in Extremity Reconstruction. J. Reconstr. Microsurg. 2019, 36, 053–058. [Google Scholar] [CrossRef]
- Scott, G.R.; Rothkopf, D.M.; Walton, R.L. Efficacy of Epidural Anesthesia in Free Flaps to the Lower Extremity. Plast. Reconstr. Surg. 1993, 91, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Erni, D.; Banic, A.; Signer, C.; Sigurdsson, G.H. Effects of epidural anaesthesia on microcirculatory blood flow in free flaps in patients under general anaesthesia. Eur. J. Anaesthesiol. 1999, 16, 692–698. [Google Scholar] [CrossRef]

| Relative Change (CI) | p (s vs. d) | ||
|---|---|---|---|
| Superficial (s) | Deep (d) | ||
| Blood Flow (Bf) | |||
| Completion of APB | 4.51 (2.349–6.676) | 3.74 (0.568–6.920) | 0.037 |
| +5 min | 6.33 (4.079–8.588) | 3.92 (1.401–6.446) | 0.002 |
| +10 min | 6.49 (4.169–8.809) | 3.80 (1.369–6.229) | 0.001 |
| +15 min | 7.17 (4.122–10.211) | 3.92 (1.346–6.497) | 0.001 |
| Post-capillary Tissue Oxygen Saturation (StO2) | |||
| Completion of APB | 1.10 (1.023–1.167) | 1.20 (0.987–1.406) | 0.428 |
| +5 min | 1.15 (1.049–1.245) | 1.26 (1.051–1.469) | 0.299 |
| +10 min | 1.14 (1.030–1.246) | 1.24 (1.076–1.399) | 0.102 |
| +15 min | 1.12 (1.008–1.236) | 1.26 (1.054–1.475) | 0.102 |
| Relative Hemoglobin Content (rHb) | |||
| Completion of APB | 1.06 (0.984–1.138) | 1.21 (1.072–1.344) | 0.349 |
| +5 min | 1.13 (1.075–1.178) | 1.37 (1.192–1.553) | 0.060 |
| +10 min | 1.14 (1.088–1.184) | 1.35 (1.191–1.515) | 0.023 |
| +15 min | 1.10 (1.043–1.166) | 1.40 (1.236–1.558) | <0.001 |
| Relative Change (CI) | ||||||
|---|---|---|---|---|---|---|
| Superficial | p s vs. ns | Deep | p s vs. ns | |||
| Smoker (s) | Non-Smoker (ns) | Smoker (s) | Non-Smoker (ns) | |||
| Blood Flow (Bf) | ||||||
| Completion of APB | 2.54 (1.324–3.750) | 5.38 (1.929–8.841) | 0.245 | 1.77 (1.059–2.474) | 2.37 (1.597–3.140) | 0.394 |
| +5 min | 2.76 (1.837–3.679) | 8.69 (5.231–12-149) | 0.011 | 1.72 (1.181–2.257) | 3.30 (2.580–4.015) | 0.005 |
| +10 min | 2.51 (1.698–3.331) | 9.17 (5.673–12.659) | 0.012 | 1.62 (1.073–2.166) | 3.24 (2.538–3.941) | 0.004 |
| +15 min | 2.49 (1.667–3.314) | 10.36 (5.597–15.118) | 0.006 | 1.65 (1.080–2.218) | 3.30 (2.604–6.497) | 0.003 |
| Post-capillary Tissue Oxygen Saturation (StO2) | ||||||
| Completion of APB | 1.03 (0.967–1.088) | 1.11 (1.013–1.213) | 0.347 | 1.11 (1.035–1.191) | 1.08 (1.080–1.159) | 0.556 |
| +5 min | 1.04 (0.962–1.115) | 1.21 (1.058–1.364) | 0.195 | 1.17 (1.035–1.314) | 1.15 (1.082–1.211) | 0.394 |
| +10 min | 1.01 (0.954–1.073) | 1.21 (1.042–1.388) | 0.088 | 1.19 (1.055–1.322) | 1.14 (1.078–1.207) | 0.777 |
| +15 min | 1.01 (0.941–1.072) | 1.19 (1.003–1.372) | 0.303 | 1.18 (1.041–1.328) | 1.15 (1.076–1.217) | 0.777 |
| Relative Hemoglobin Content (rHb) | ||||||
| Completion of APB | 0.97 (0.803–1.130) | 1.12 (1.080–1.159) | 0.038 | 1.30 (1.050-1.559) | 1.15 (1.004-1.287) | 0.586 |
| +5 min | 1.07 (0.983–1.158) | 1.16 (1.104–1.220) | 0.166 | 1.44 (1.125–1.750) | 1.34 (1.126–1.558) | 0.983 |
| +10 min | 1.07 (1.010–1.122) | 1.18 (1.118–1.246) | 0.021 | 1.47 (1.196–1.736) | 1.29 (1.093–1.487) | 0.444 |
| +15 min | 1.05 (0.964–1.134) | 1.14 (1.056–1.222) | 0.140 | 1.44 (1.174–1.698) | 1.39 (1.182–1.592) | 0.913 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosselmann, T.; Kolbenschlag, J.; Goertz, O.; Zahn, P.; Prantl, L.; Lehnhardt, M.; Behr, B.; Sogorski, A. Improvement of Superficial and Deep Cutaneous Microcirculation Due to Axillary Plexus Anesthesia Impaired by Smoking. J. Clin. Med. 2021, 10, 2114. https://doi.org/10.3390/jcm10102114
Bosselmann T, Kolbenschlag J, Goertz O, Zahn P, Prantl L, Lehnhardt M, Behr B, Sogorski A. Improvement of Superficial and Deep Cutaneous Microcirculation Due to Axillary Plexus Anesthesia Impaired by Smoking. Journal of Clinical Medicine. 2021; 10(10):2114. https://doi.org/10.3390/jcm10102114
Chicago/Turabian StyleBosselmann, Talia, Jonas Kolbenschlag, Ole Goertz, Peter Zahn, Lukas Prantl, Marcus Lehnhardt, Björn Behr, and Alexander Sogorski. 2021. "Improvement of Superficial and Deep Cutaneous Microcirculation Due to Axillary Plexus Anesthesia Impaired by Smoking" Journal of Clinical Medicine 10, no. 10: 2114. https://doi.org/10.3390/jcm10102114
APA StyleBosselmann, T., Kolbenschlag, J., Goertz, O., Zahn, P., Prantl, L., Lehnhardt, M., Behr, B., & Sogorski, A. (2021). Improvement of Superficial and Deep Cutaneous Microcirculation Due to Axillary Plexus Anesthesia Impaired by Smoking. Journal of Clinical Medicine, 10(10), 2114. https://doi.org/10.3390/jcm10102114







