Congenital Obstructive Müllerian Anomaly: The Pitfalls of a Magnetic Resonance Imaging-Based Diagnosis and the Importance of Intraoperative Biopsy
Abstract
1. Introduction
2. Materials and Methods
2.1. MRI Assessment
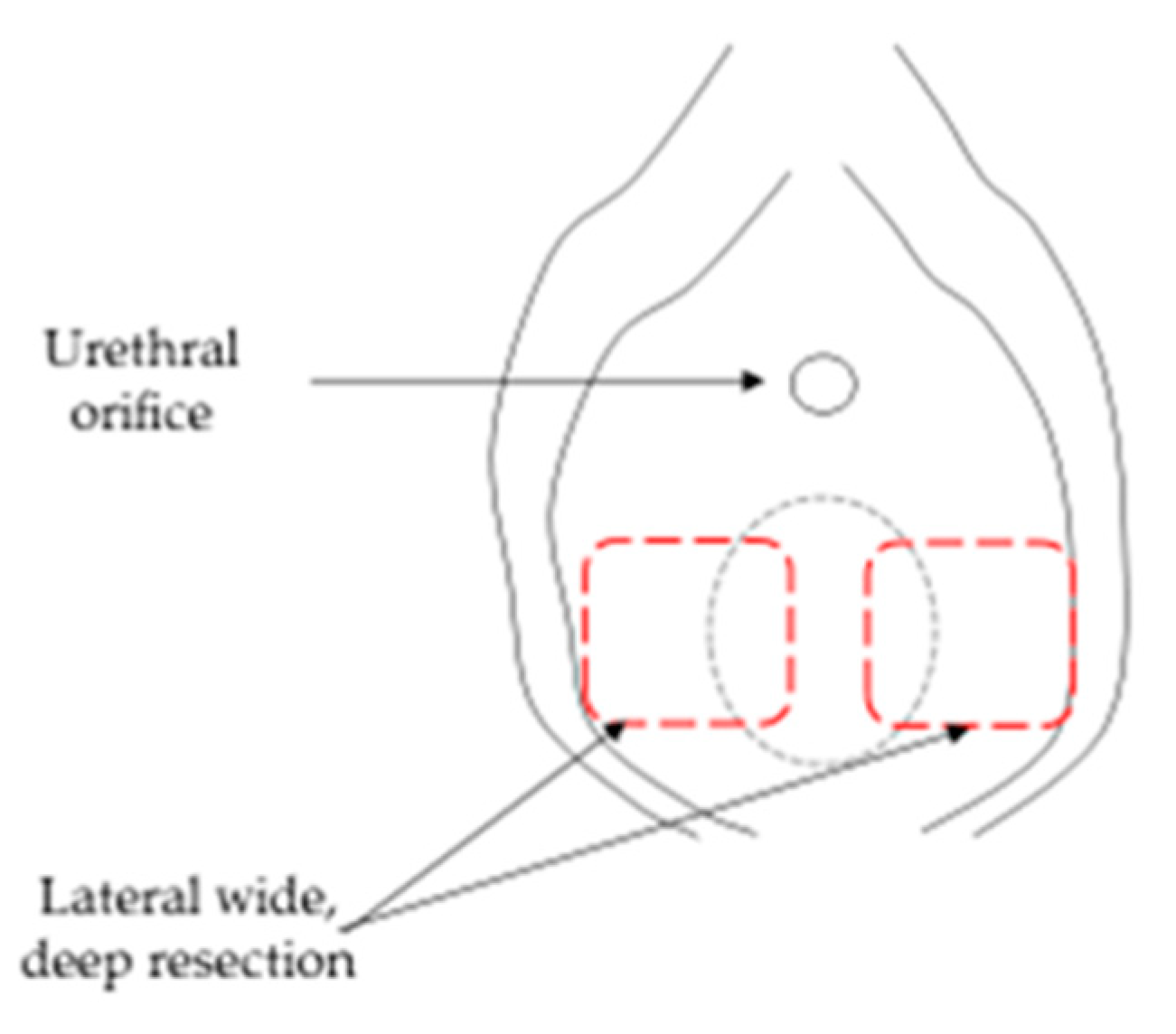
2.2. Surgical Assessment
2.3. Statistical Analysis
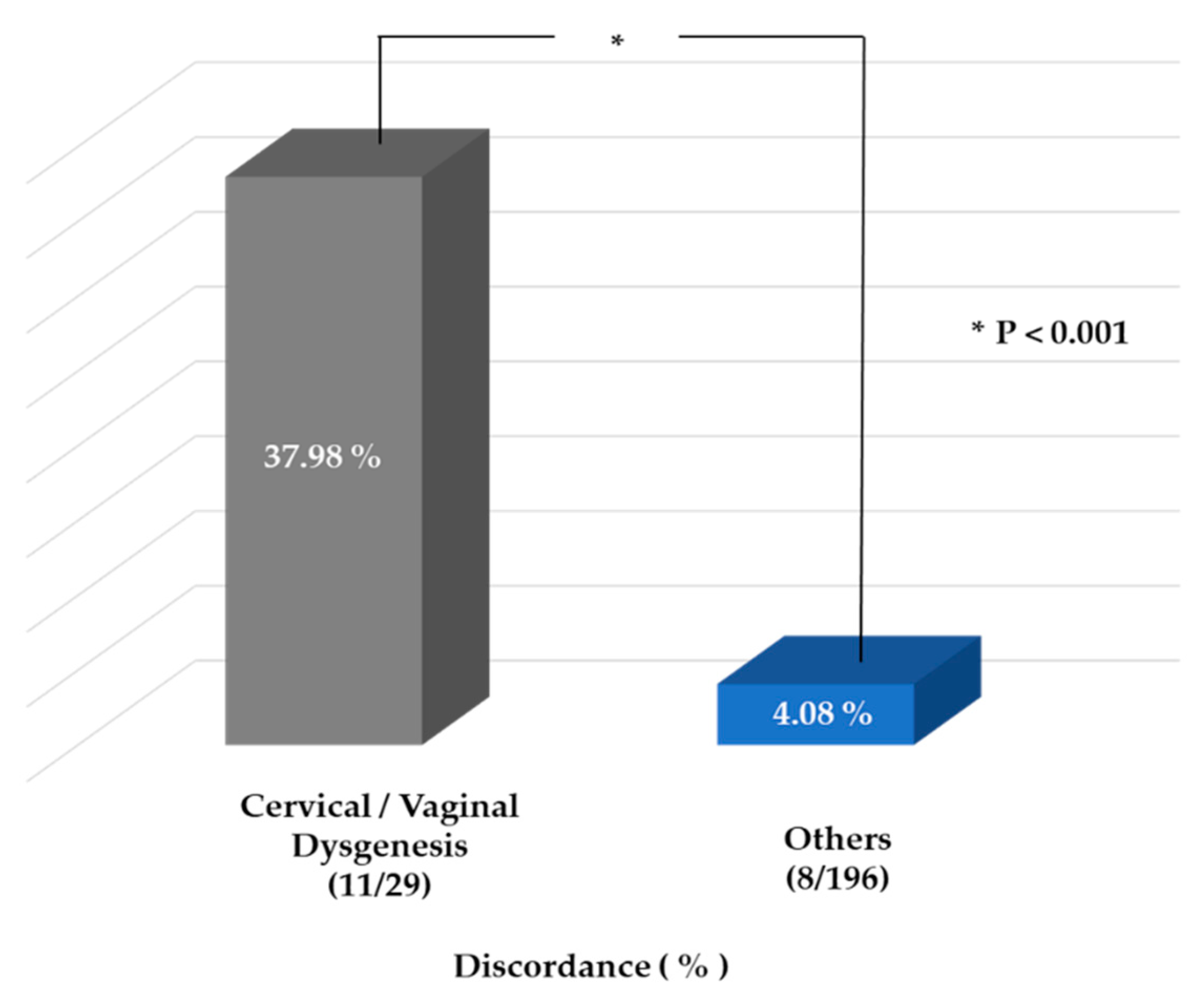
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saravelos, S.H.; Cocksedge, K.A.; Li, T.C. Prevalence and diagnosis of congenital uterine anomalies in women with reproductive failure: A critical appraisal. Hum. Reprod. Updat. 2008, 14, 415–429. [Google Scholar] [CrossRef]
- Acien, P. Incidence of Müllerian defects in fertile and infertile women. Hum. Reprod. 1997, 12, 1372–1376. [Google Scholar] [CrossRef]
- Acién, P.; Acién, M.; Sánchez-Ferrer, M. Complex malformations of the female genital tract. New types and revision of classification. Hum. Reprod. 2004, 19, 2377–2384. [Google Scholar] [CrossRef]
- Grimbizis, G.F.; Sardo, A.D.S.; Saravelos, S.H.; Gordts, S.; Exacoustos, C.; Van Schoubroeck, D.; Bermejo, C.; Amso, N.N.; Nargund, G.; Timmerman, D.; et al. The Thessaloniki ESHRE/ESGE consensus on diagnosis of female genital anomalies. Hum. Reprod. 2015, 31, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, J.E.; Millar, D.M.; Quint, E.H. Obstructive Reproductive Tract Anomalies. J. Pediatr. Adolesc. Gynecol. 2014, 27, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, V.Y.; Miller, J.; Klein, N.A.; Soules, M.R. Congenital cervical atresia: Report of seven cases and review of the literature. Am. J. Obstet. Gynecol. 1997, 177, 1419–1425. [Google Scholar] [CrossRef]
- Lekovich, J.; Pfeifer, S.M. Cervical Agenesis. Congenit. Müllerian Anom. 2016, 2016, 55–63. [Google Scholar] [CrossRef]
- Grimbizis, G.F.; Tsalikis, T.; Mikos, T.; Papadopoulos, N.; Tarlatzis, B.C.; Bontis, J.N. Successful end-to-end cervico-cervical anastomo-sis in a patient with congenital cervical fragmentation: Case report. Hum. Reprod. 2004, 19, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Emans, S.; Laufer, M.; Laufer, E. Goldstein’s Pediatric and Adolescent Gynecology; Lippincott Williams & Wilkins Philadelphia: Philadelphia, PA, USA, 2012. [Google Scholar]
- Shifren, J.; Schiff, I. Reproductive endocrinology. In Berek and Novak’s Gynecology, 15th ed.; Wolters Kluwer Health: Philadelphia, PA, USA, 2012; pp. 1233–1249. [Google Scholar]
- Song, X.; Zhu, L.; Ding, J.; Xu, T.; Lang, J. Clinical characteristics of congenital cervical atresia and associated endometriosis among 96 patients. Int. J. Gynecol. Obstet. 2016, 134, 252–255. [Google Scholar] [CrossRef]
- Kang, J.; Zhu, L.; Zhang, Y.; Ma, C.; Ma, Y. Postoperative Pelvic Abscess after Cervicovaginal Canalization for Congenital Cervi-cal and Vaginal Agenesis: A Report of 4 Cases. J. Pediatr. Adolesc. Gynecol. 2020, 33, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Niver, D.H.; Barrette, G.; Jewelewicz, R. Congenital Atresia of the Uterine Cervix and Vagina: Three Cases. Fertil. Steril. 1980, 33, 25–29. [Google Scholar] [CrossRef]
- Casey, A.C.; Laufer, M.R. Cervical agenesis: Septic death after surgery. Obstet. Gynecol. 1997, 90, 706–707. [Google Scholar]
- Jeon, G.H.; Kim, S.; Chae, H.; Kim, C.H.; Kang, B.M. Simple uterovaginal anastomosis for cervicovaginal atresia diagnosed by mag-netic resonance imaging: A report of two cases. J. Obstet. Gynaecol. Res. 2016, 42, 738–742. [Google Scholar] [CrossRef]
- Padmawar, A.; Syed, R.; Naval, S. Laparoscopic Uterovaginal Anastomosis for Cervical Agenesis: A Case Report. J. Minim. Invasive Gynecol. 2018, 25, 334–335. [Google Scholar] [CrossRef]
- Zhang, X.; Qiu, J.; Ding, Y.; Sun, L.; Hua, K. Single port laparoscopy combined with vaginal cervicovaginal reconstruction in a patient with congenital atresia of the cervix. Fertil. Steril. 2020, 113, 681–682. [Google Scholar] [CrossRef] [PubMed]
- Deffarges, J.; Haddad, B.; Musset, R.; Paniel, B. Utero-vaginal anastomosis in women with uterine cervix atresia: Long-term fol-low-up and reproductive performance. A study of 18 cases. Hum. Reprod. 2001, 16, 1722–1725. [Google Scholar] [CrossRef] [PubMed]
- Mikos, T.; Gordts, S.; Grimbizis, G.F. Current knowledge about the management of congenital cervical malformations: A literature review. Fertil. Steril. 2020, 113, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Fedele, L.; Portuese, A.; Bianchi, S.; Zanconato, G.; Raffaelli, R. Transrectal ultrasonography in the assessment of congenital vagi-nal canalization defects. Hum. Reprod. 1999, 14, 359–362. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wildenberg, J.C.; Yam, B.L.; Langer, J.E.; Jones, L.P. US of the Nongravid Cervix with Multimodality Imaging Correlation: Normal Appearance, Pathologic Conditions, and Diagnostic Pitfalls. Radiogr. 2016, 36, 596–617. [Google Scholar] [CrossRef]
- Troiano, R.N.; McCarthy, S.M. Müllerian Duct Anomalies: Imaging and Clinical Issues. Radiology 2004, 233, 19–34. [Google Scholar] [CrossRef]
- Santos, X.; Krishnamurthy, R.; Bercaw-Pratt, J.; Dietrich, J. The utility of ultrasound and magnetic resonance imaging versus sur-gery for the characterization of Müllerian anomalies in the pediatric and adolescent population. J. Pediatr. Adolesc. Gynecol. 2012, 25, 181–184. [Google Scholar] [CrossRef]
- Mueller, G.C.; Hussain, H.K.; Smith, Y.R.; Quint, E.H.; Carlos, R.C.; Johnson, T.D.; DeLancey, J.O. Müllerian Duct Anomalies: Comparison of MRI Diagnosis and Clinical Diagnosis. Am. J. Roentgenol. 2007, 189, 1294–1302. [Google Scholar] [CrossRef]
- Rock, J.A.; Roberts, C.P.; Jones, H.W. Congenital anomalies of the uterine cervix: Lessons from 30 cases managed clinically by a common protocol. Fertil. Steril. 2010, 94, 1858–1863. [Google Scholar] [CrossRef]
- Buttram, V.C.; Gomel, V.; Siegler, A.; DeCherney, A.; Gibbons, W.; March, C. The American Fertility Society classifications of adnex-al adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, Müllerian anomalies and intrauterine adhesions. Fertil. Steril. 1988, 49, 944–955. [Google Scholar]
- Miller, R.J.; Breech, L.L. Surgical Correction of Vaginal Anomalies. Clin. Obstet. Gynecol. 2008, 51, 223–236. [Google Scholar] [CrossRef]
- Willemsen, W.N.; Kluivers, K.B. Long-term results of vaginal construction with the use of Frank dilation and a peritoneal graft (Davydov procedure) in patients with Mayer-Rokitansky-Küster syndrome. Fertil. Steril. 2015, 103, 220–227.e1. [Google Scholar] [CrossRef] [PubMed]
- Callens, N.; De Cuypere, G.; De Sutter, P.; Monstrey, S.; Weyers, S.; Hoebeke, P.; Cools, M. An update on surgical and non-surgical treatments for vaginal hypoplasia. Hum. Reprod. Updat. 2014, 20, 775–801. [Google Scholar] [CrossRef]
- Banister, J.B.; McIndoe, A.H. Congenital Absence of the Vagina, Treated by Means of an Indwelling Skin-Graft; SAGE Publications: Southend Oaks, CA, USA, 1938; Volume 31, pp. 1055–1056. [Google Scholar]
- Davydov, S.N. Colpopoeisis from the peritoneum of the uterorectal space. Akusherstvo Ginekol. 1969, 45, 55–57. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 21 May 2021).
- Pellerito, J.; McCarthy, S.; Doyle, M.; Glickman, M.; DeCherney, A. Diagnosis of uterine anomalies: Relative accuracy of MR imag-ing, endovaginal sonography, and hysterosalpingography. Radiology 1992, 183, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Pompili, G.; Munari, A.; Franceschelli, G.; Flor, N.; Meroni, R.; Frontino, G.; Fedele, L.; Cornalba, G. Magnetic resonance imaging in the preoperative assessment of Mayer-Rokitansky-Kuster-Hauser syndrome. La Radiol. Medica 2009, 114, 811–826. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, Q.; Sun, Z.J.; Jiang, B.; Hou, B.; Lu, J.J.; Zhu, L.; Feng, F.; Jin, Z.Y.; Lang, J.H. Value of MRI in the pre-operative diagnosis and classification of oblique vaginal septum syndrome. Zhonghua Fu Chan Ke Za Zhi 2018, 53, 534–539. [Google Scholar]
- Lang, I.M.; Babyn, P.; Oliver, G.D. MR imaging of paediatric uterovaginal anomalies. Pediatr. Radiol. 1999, 29, 163–170. [Google Scholar] [CrossRef]
- Soares, S.R.; dos Reis, M.M.B.B.; Camargos, A.F. Diagnostic accuracy of sonohysterography, transvaginal sonography, and hyster-osalpingography in patients with uterine cavity diseases. Fertil. Steril. 2000, 73, 406–411. [Google Scholar] [CrossRef]
- Lermann, J.; Mueller, A.; Wiesinger, E.; Häberle, L.; Brucker, S.; Wallwiener, D.; Dittrich, R.; Renner, S.; Beckmann, M.W.; Oppelt, P.G. Comparison of different diagnostic procedures for the staging of malformations associated with Mayer-Rokitansky-Küster-Hauser syndrome. Fertil. Steril. 2011, 96, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Kimble, R.; Molloy, G.; Sutton, B. Partial cervical agenesis and complete vaginal atresia. J. Pediatr. Adolesc. Gynecol. 2016, 29, e43–e47. [Google Scholar] [CrossRef] [PubMed]
- El Saman, A.M. Endoscopically monitored canalization for treatment of congenital cervical atresia: The least invasive approach. Fertil. Steril. 2010, 94, 313–316. [Google Scholar] [CrossRef]
- Frank, R.T. The formation of an artificial vagina without operation. Am. J. Obstet. Gynecol. 1938, 35, 1053–1055. [Google Scholar] [CrossRef]
- McIndoe, A. The treatment of congenital absence and obliterative conditions of the vagina. Br. J. Plast. Surg. 1950, 2, 254–267. [Google Scholar]
- Garcia, J.; Jones, H.W., Jr. The split thickness graft technic for vaginal agenesis. Obstet. Gynecol. 1977, 49, 328–332. [Google Scholar]
- Fedele, L.; Bianchi, S.; Frontino, G.; Berlanda, N.; Montefusco, S.; Borruto, F. Laparoscopically assisted uterovestibular anastomosis in patients with uterine cervix atresia and vaginal aplasia. Fertil. Steril. 2008, 89, 212–216. [Google Scholar] [CrossRef]

| Diagnosis | Cases (%) |
|---|---|
| MRKH (with cervical dysgenesis) | 75 (33.3) |
| Anomalies of the hymen and lower one-third of the vagina Imperforate hymen Distal vaginal atresia Transverse vaginal septum Imperforate hymen or transverse vaginal septum * | 15 (6.7) 10 (4.4) 2 (0.9) 2 |
| Hypoplastic uterus (POI, Kallmann syndrome) | 9 (4.0) |
| Arcuate uterus | 5 (2.2) |
| Septate uterus | 4 (1.8) |
| Unicornuate uterus | 8 (3.6) |
| Bicornuate uterus | 13 (5.8) |
| Uterine didelphys (include OHVIRA) | 42 (18.7) |
| DSD (AIS, Swyer syndrome) | 26 (11.6) |
| Mixed gonadal dysgenesis | 4 (1.8) |
| Gonadal dysgenesis | 4 (1.8) |
| 5α-reductase deficiency | 3 (1.3) |
| Others † | 5 (2.2) |
| Total | 225 (100) |
| Patient No. | MRI Diagnosis | Age | Final Diagnosis |
|---|---|---|---|
| 1 | Imperforate hymen | 12y | Cervical anomaly with a vaginal septum |
| 2 | Septate vagina | 15y | Cervical cyst |
| 3 | Uterine and gonadal aplasia | 17y | Premature ovarian failure |
| 4 | Degenerative myoma in the uterus | 14y | Uterine didelphys with non-communicating horn |
| 5 | Hysterectomy state | 14y | MRKH |
| 6 | True hermaphroditism | 16y | MRKH |
| 7 | MRKH | 15y | 46, XY DSD male pseudohermaphroditism |
| 8 | Vaginal stenosis | 16y | Cervical agenesis with transverse vaginal septum |
| 9 | Lower vaginal agenesis | 12y | Cervical dysgenesis |
| 10 | MRKH | 15y | 46, XY DSD male pseudohermaphroditism |
| 11 | Imperforate hymen | 22y | Cervicovaginal agenesis |
| 12 | True hermaphroditism | 17y | MRKH (Bx:No testicular tissue) |
| 13 | Hypoplastic or aplastic vagina with hematocolpometra | 13y | Cervical dysgenesis |
| 14 | Stricture of the lower vagina | 13y | Cervical dysgenesis |
| 15 | Vaginal stenosis | 13y | Cervical dysgenesis with vaginal agenesis |
| 16 | Bicornuate uterus with an imperforate hymen | 14y | Bicornuate uterus with cervical anomaly |
| 17 | Uterine didelphys with a left hemi-vaginal septum | 15y | Uterine didelphys with left cervical agenesis |
| 18 | Vaginal atresia | 12y | Vaginal atresia with cervical agenesis |
| 19 | Obstructive hemivagina with ipsilateral renal anomaly | 12y | Uterine didelphys with unilateral cervical dysgenesis, vaginal agenesis, renal agenesis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.Y.; Nam, G.; Lee, S.R.; Kim, S.H.; Chae, H.D.; Kang, B.M. Congenital Obstructive Müllerian Anomaly: The Pitfalls of a Magnetic Resonance Imaging-Based Diagnosis and the Importance of Intraoperative Biopsy. J. Clin. Med. 2021, 10, 2414. https://doi.org/10.3390/jcm10112414
Kim DY, Nam G, Lee SR, Kim SH, Chae HD, Kang BM. Congenital Obstructive Müllerian Anomaly: The Pitfalls of a Magnetic Resonance Imaging-Based Diagnosis and the Importance of Intraoperative Biopsy. Journal of Clinical Medicine. 2021; 10(11):2414. https://doi.org/10.3390/jcm10112414
Chicago/Turabian StyleKim, Do Young, Gina Nam, Sa Ra Lee, Sung Hoon Kim, Hee Dong Chae, and Byung Moon Kang. 2021. "Congenital Obstructive Müllerian Anomaly: The Pitfalls of a Magnetic Resonance Imaging-Based Diagnosis and the Importance of Intraoperative Biopsy" Journal of Clinical Medicine 10, no. 11: 2414. https://doi.org/10.3390/jcm10112414
APA StyleKim, D. Y., Nam, G., Lee, S. R., Kim, S. H., Chae, H. D., & Kang, B. M. (2021). Congenital Obstructive Müllerian Anomaly: The Pitfalls of a Magnetic Resonance Imaging-Based Diagnosis and the Importance of Intraoperative Biopsy. Journal of Clinical Medicine, 10(11), 2414. https://doi.org/10.3390/jcm10112414






