Evaluation of Oropharyngeal pH-Monitoring in the Assessment of Laryngopharyngeal Reflux
Abstract
1. Introduction
2. Materials and Methods
2.1. Oropharyngeal pH-Monitoring (PHM)
2.2. Horvath Score
2.3. Statistics
3. Results
3.1. Clinical Data
3.2. Oropharyngeal pH-Monitoring
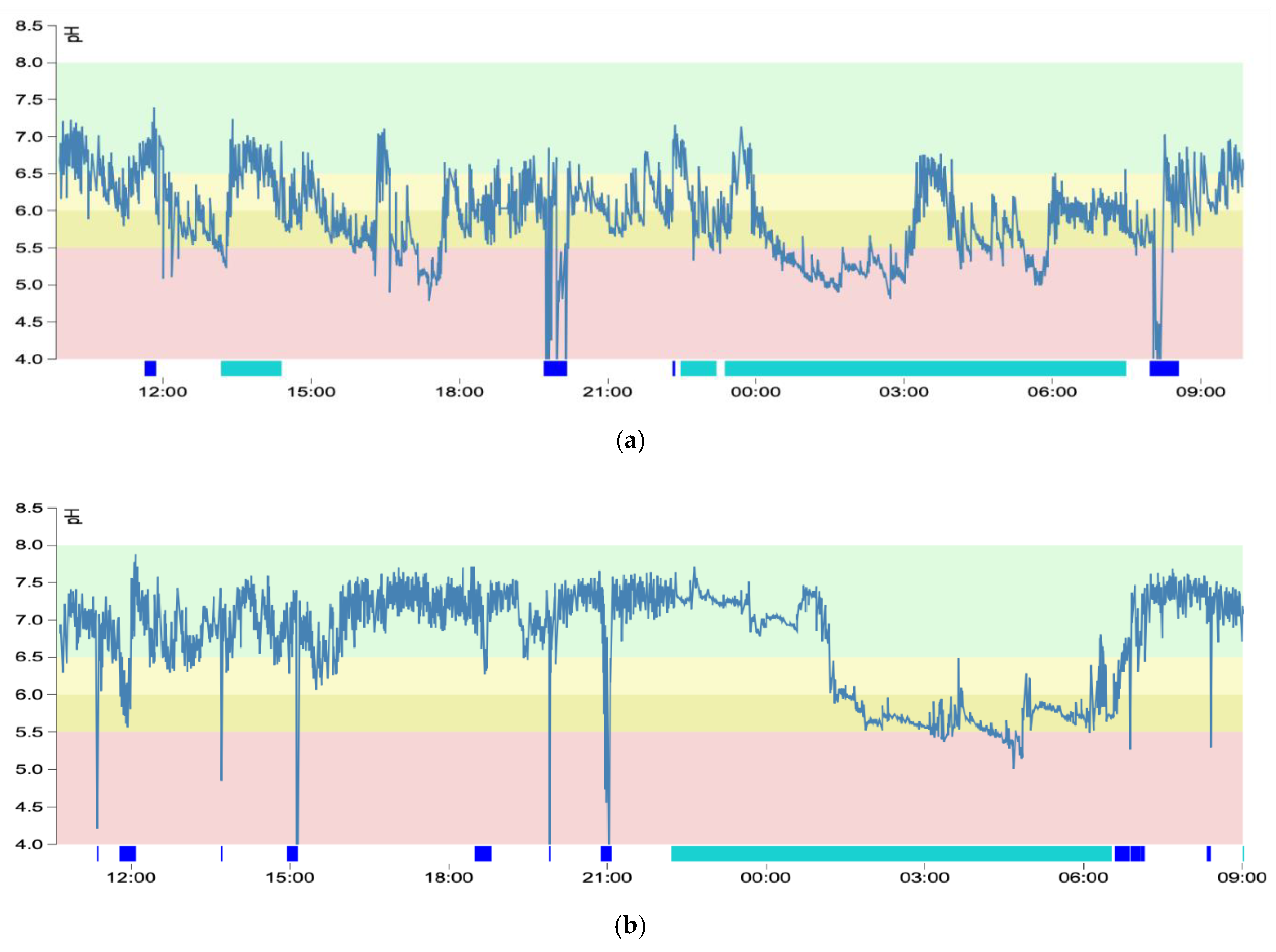
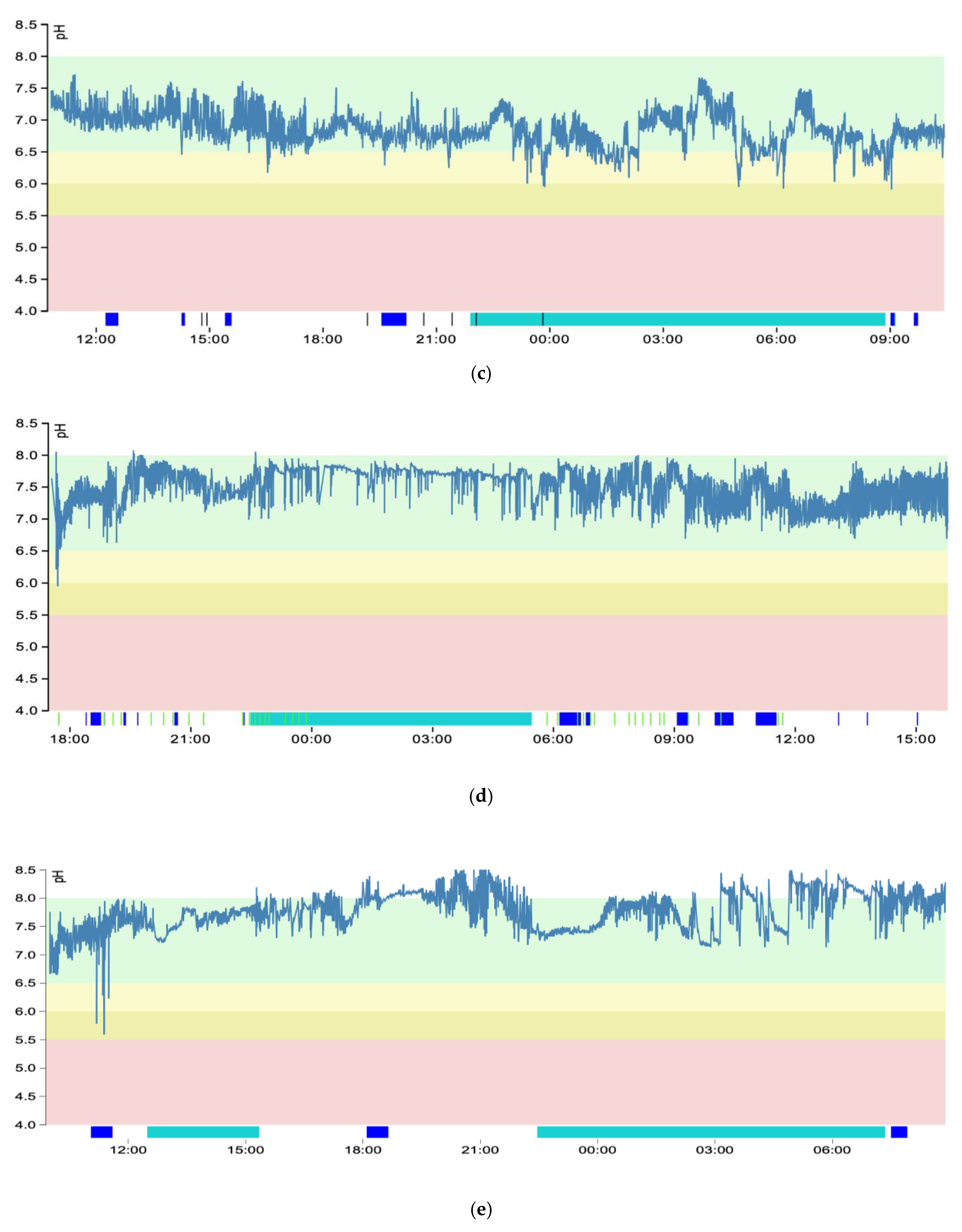
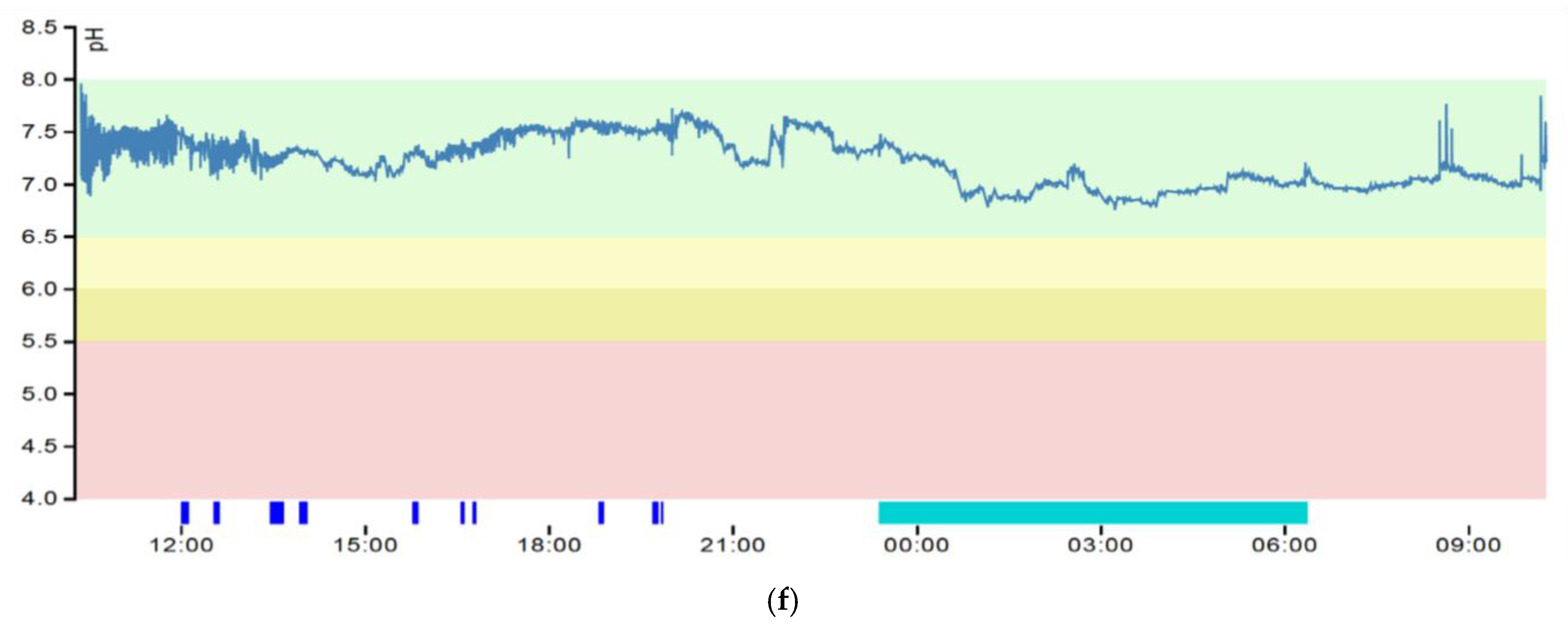
3.3. pH Graphs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koufman, J.A.; Aviv, J.E.; Casiano, R.R.; Shaw, G.Y. Laryngopharyngeal Reflux: Position Statement of the Committee on Speech, Voice, and Swallowing Disorders of the American Academy of Otolaryngology-Head and Neck Surgery. YMHN 2016, 127, 32–35. [Google Scholar] [CrossRef]
- Shaker, R.; Babaei, A.; Naini, S.R. Prevention of esophagopharyngeal reflux by augmenting the upper esophageal sphincter pressure barrier. Laryngoscope 2014, 124, 2268–2274. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Wu, M.; Zhao, J.; Guo, H. The role of nonacid reflux in laryngopharyngeal reflux diseases. Eur. Arch. Oto-Rhino-Laryngology 2020, 277, 2813–2819. [Google Scholar]
- Lenderking, W.R.; Hillson, E.; Crawley, J.A.; Moore, D.; Berzon, R.; Pashos, C.L. The clinical characteristics and impact of laryngopharyngeal reflux disease on health-related quality of life. Value Health 2003, 6, 560–565. [Google Scholar] [CrossRef]
- Madani, A.; Sowerby, L.; Gregor, J.C.; Wong, E.; Fung, K. Detecting the other reflux disease: Laryngopharyngeal reflux is a disorder, unlike GERD, that few patients (and too few physicians) are familiar with. Misdiagnosis is common--unless you know what to look for. J. Fam. Pract. 2010, 59, 102–108. [Google Scholar]
- Kamani, T.; Penney, S.; Mitra, I.; Pothula, V. The prevalence of laryngopharyngeal reflux in the English population. Eur. Arch. Oto-Rhino-Laryngology 2012, 269, 2219–2225. [Google Scholar] [CrossRef]
- Lechien, J.R.; Mouawad, F.; Mortuaire, G.; Remacle, M.; Bobin, F.; Huet, K.; Nacci, A.; Barillari, M.R.; Crevier-Buchman, L.; Hans, S.; et al. Awareness of European Otolaryngologists and General Practitioners Toward Laryngopharyngeal Reflux. Ann. Otol. Rhinol. Laryngol. 2019, 128, 1030–1040. [Google Scholar] [CrossRef]
- Lin, R.J.; Sridharan, S.; Smith, L.J.; Young, V.N.; Rosen, C.A. Weaning of proton pump inhibitors in patients with suspected laryngopharyngeal reflux disease. Laryngoscope 2017, 128, 133–137. [Google Scholar] [CrossRef]
- Desjardin, M.; Roman, S.; des Varannes, S.B.; Gourcerol, G.; Coffin, B.; Ropert, A.; Mion, F.; Zerbib, F. Pharyngeal pH alone is not reliable for the detection of pharyngeal reflux events: A study with oesophageal and pharyngeal pH-impedance monitoring. United Eur. Gastroenterol. J. 2013, 1, 438–444. [Google Scholar] [CrossRef]
- Ayazi, S.; Lipham, J.C.; Hagen, J.A.; Tang, A.L.; Zehetner, J.; Leers, J.M.; Oezcelik, A.; Abate, E.; Banki, F.; DeMeester, S.R.; et al. A New Technique for Measurement of Pharyngeal pH: Normal Values and Discriminating pH Threshold. J. Gastrointest. Surg. 2009, 13, 1422–1429. [Google Scholar] [CrossRef]
- Chheda, N.N.; Seybt, M.W.; Schade, R.R.; Postma, G.N. Normal values for pharyngeal pH monitoring. Ann. Otol. Rhinol. Laryngol. 2009, 118, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Muddana, S.; Slaughter, J.C.; Casey, S.; Hill, E.; Farrokhi, F.; Garrett, C.G.; Vaezi, M.F. A new pH catheter for laryngopharyngeal reflux: Normal values. Laryngoscope 2009, 119, 1639–1643. [Google Scholar] [CrossRef] [PubMed]
- Horvath, L.; Hagmann, P.; Burri, E.; Kraft, M. A Novel Scoring System for Evaluating Laryngopharyngeal Reflux. Clin. Otolaryngol. 2021, 46, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Belafsky, P.C.; Postma, G.N.; Koufman, J.A. Validity and Reliability of the Reflux Symptom Index (RSI). J. Voice 2002, 16, 274–277. [Google Scholar] [CrossRef]
- Belafsky, P.C.; Postma, G.N.; Koufman, J.A. The validity and reliability of the reflux finding score (RFS). Laryngoscope 2001, 111, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Worrell, S.G.; DeMeester, S.R.; Greene, C.L.; Oh, D.S.; Hagen, J.A. Pharyngeal pH monitoring better predicts a successful outcome for extraesophageal reflux symptoms after antireflux surgery. Surg. Endosc. 2013, 27, 4113–4118. [Google Scholar] [CrossRef]
- Wiener, G.J.; Tsukashima, R.; Kelly, C.; Wolf, E.; Schmeltzer, M.; Bankert, C.; Fisk, L.; Vaezi, M. Oropharyngeal pH Monitoring for the Detection of Liquid and Aerosolized Supraesophageal Gastric Reflux. J. Voice 2009, 23, 498–504. [Google Scholar] [CrossRef]
- Vailati, C.; Mazzoleni, G.; Bondi, S.; Bussi, M.; Testoni, P.A.; Passaretti, S. Oropharyngeal pH Monitoring for Laryngopharyngeal Reflux: Is It a Reliable Test Before Therapy? J. Voice 2013, 27, 84–89. [Google Scholar] [CrossRef]
- Cumpston, E.C.; Blumin, J.H.; Bock, J.M. Dual pH with Multichannel Intraluminal Impedance Testing in the Evaluation of Subjective Laryngopharyngeal Reflux Symptoms. Otolaryngol. Head Neck Surg. 2016, 155, 1014–1020. [Google Scholar] [CrossRef]
- Gyawali, C.P.; Kahrilas, P.J.; Savarino, E.; Zerbib, F.; Mion, F.; Smout, A.J.P.M.; Vaezi, M.; Sifrim, D.; Fox, M.R.; Vela, M.F.; et al. Modern diagnosis of GERD: The Lyon Consensus. Gut 2018, 67, 1351–1362. [Google Scholar] [CrossRef]
- Borges, L.F.; Chan, W.W.; Carroll, T.L. Dual pH Probes Without Proximal Esophageal and Pharyngeal Impedance May Be Deficient in Diagnosing LPR. J. Voice 2019, 33, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Andrews, T.M.; Orobello, N. Histologic versus pH probe results in pediatric laryngopharyngeal reflux. Int. J. Pediatric Otorhinolaryngol. 2013, 77, 813–816. [Google Scholar] [CrossRef]
- Golub, J.S.; Johns, M.M.; Lim, J.H.; DelGaudio, J.M.; Klein, A.M. Comparison of an oropharyngeal pH probe and a standard dual pH probe for diagnosis of laryngopharyngeal reflux. Ann. Otol. Rhinol. Laryngol. 2009, 118, 1–5. [Google Scholar] [CrossRef]
- Fuchs, H.F.; Müller, D.T.; Berlth, F.; Maus, M.K.; Fuchs, C.; Dübbers, M.; Schröder, W.; Bruns, C.J.; Leers, J.M. Simultaneous laryngopharyngeal pH monitoring (Restech) and conventional esophageal pH monitoring—correlation using a large patient cohort of more than 100 patients with suspected gastroesophageal reflux disease. Dis. Esophagus 2018, 31, 1753–1758. [Google Scholar] [CrossRef] [PubMed]
- Gang, W.; Changmin, Q.; Lei, W.; Hongdan, L.; Haolun, H.; Bingxin, X.; Ying, Z.; Baowei, L.; Yiyan, Z.; Zhezhe, S.; et al. Utility of 24-hour pharyngeal pH monitoring and clinical feature in laryngopharyngeal reflux disease. Acta Oto-Laryngologica 2019, 139, 299–303. [Google Scholar]
| Horvath Score | Severity | Pathologic RSI | Pathologic RFS | Pathologic PHM | Positive Ryan | Pathologic TNE | Total |
|---|---|---|---|---|---|---|---|
| 4–5 | Severe LPR | 79 (80%) | 57 (58%) | 99 (100%) | 94 (95%) | 97 (98%) | 99 (55%) |
| 2–3 | Nonsevere LPR | 52 (76%) | 15 (22%) | 65 (96%) | 6 (9%) | 62 (91%) | 68 (38%) |
| Moderate | 20 (69%) | 7 (24%) | 27 (93%) | 5 (17%) | 25 (86%) | 29 (16%) | |
| Mild | 16 (70%) | 3 (13%) | 23 (100%) | 1 (4%) | 21 (91%) | 23 (13%) | |
| Neutral | 9 (100%) | 3 (33%) | 8 (89%) | 0 (0%) | 9 (100%) | 9 (5%) | |
| Alkaline | 7 (100%) | 2 (29%) | 7 (100%) | 0 (0%) | 7 (100%) | 7 (4%) | |
| 0–1 | No LPR | 6 (46%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (15%) | 13 (7%) |
| PHM | Severe LPR | Moderate LPR | Mild LPR | Neutral LPR | Alkaline LPR | No LPR |
|---|---|---|---|---|---|---|
| Initial | 100 (55%) | 28 (16%) | 23 (13%) | 9 (5%) | 7 (4%) | 13 (7%) |
| Final | 99 (55%) | 29 (16%) | 23 (13%) | 9 (5%) | 7 (4%) | 13 (7%) |
| Method | True Positive | False Positive | False Negative | True Negative | Total |
|---|---|---|---|---|---|
| RSI | 131 | 6 | 36 | 7 | 180 |
| RFS | 69 | 3 | 64 | 44 | 180 |
| PHM | 94 | 6 | 5 | 75 | 180 |
| TNE | 159 | 2 | 5 | 14 | 180 |
| Method | Sensitivity | Specificity | Accuracy | Pos. Pred. Value | Neg. Pred. Value |
|---|---|---|---|---|---|
| RSI | 78%† (p = 0.000) | 54%† (p = 0.001) | 77%† (p = 0.000) | 96% (p = 0.392) | 16% † (p = 0.000) |
| RFS | 52%† (p = 0.000) | 94% (p = 0.566) | 63%† (p = 0.000) | 96% (p = 0.434) | 41% † (p = 0.000) |
| PHM | 95% | 93% | 94% | 94% | 94% |
| TNE | 97% (p = 0.306) | 88% (p = 0.492) | 96% (p = 0.235) | 99%* (p = 0.038) | 74% * (p = 0.021) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horvath, L.; Hagmann, P.; Burri, E.; Kraft, M. Evaluation of Oropharyngeal pH-Monitoring in the Assessment of Laryngopharyngeal Reflux. J. Clin. Med. 2021, 10, 2409. https://doi.org/10.3390/jcm10112409
Horvath L, Hagmann P, Burri E, Kraft M. Evaluation of Oropharyngeal pH-Monitoring in the Assessment of Laryngopharyngeal Reflux. Journal of Clinical Medicine. 2021; 10(11):2409. https://doi.org/10.3390/jcm10112409
Chicago/Turabian StyleHorvath, Lukas, Patricia Hagmann, Emanuel Burri, and Marcel Kraft. 2021. "Evaluation of Oropharyngeal pH-Monitoring in the Assessment of Laryngopharyngeal Reflux" Journal of Clinical Medicine 10, no. 11: 2409. https://doi.org/10.3390/jcm10112409
APA StyleHorvath, L., Hagmann, P., Burri, E., & Kraft, M. (2021). Evaluation of Oropharyngeal pH-Monitoring in the Assessment of Laryngopharyngeal Reflux. Journal of Clinical Medicine, 10(11), 2409. https://doi.org/10.3390/jcm10112409






