Mitochondrial Dysfunction in Atrial Fibrillation—Mechanisms and Pharmacological Interventions
Abstract
1. Introduction
2. General Mechanisms of AF
3. Role of Mitochondria in the Physiology of the Heart
4. Involvement of Mitochondrial Dysfunction in the Pathogenesis of AF
4.1. Mitochondrial Ultrastructural Abnormalities
4.2. Disturbed Mitochondrial Biogenesis
4.3. Mitochondria-Related Oxidative Stress
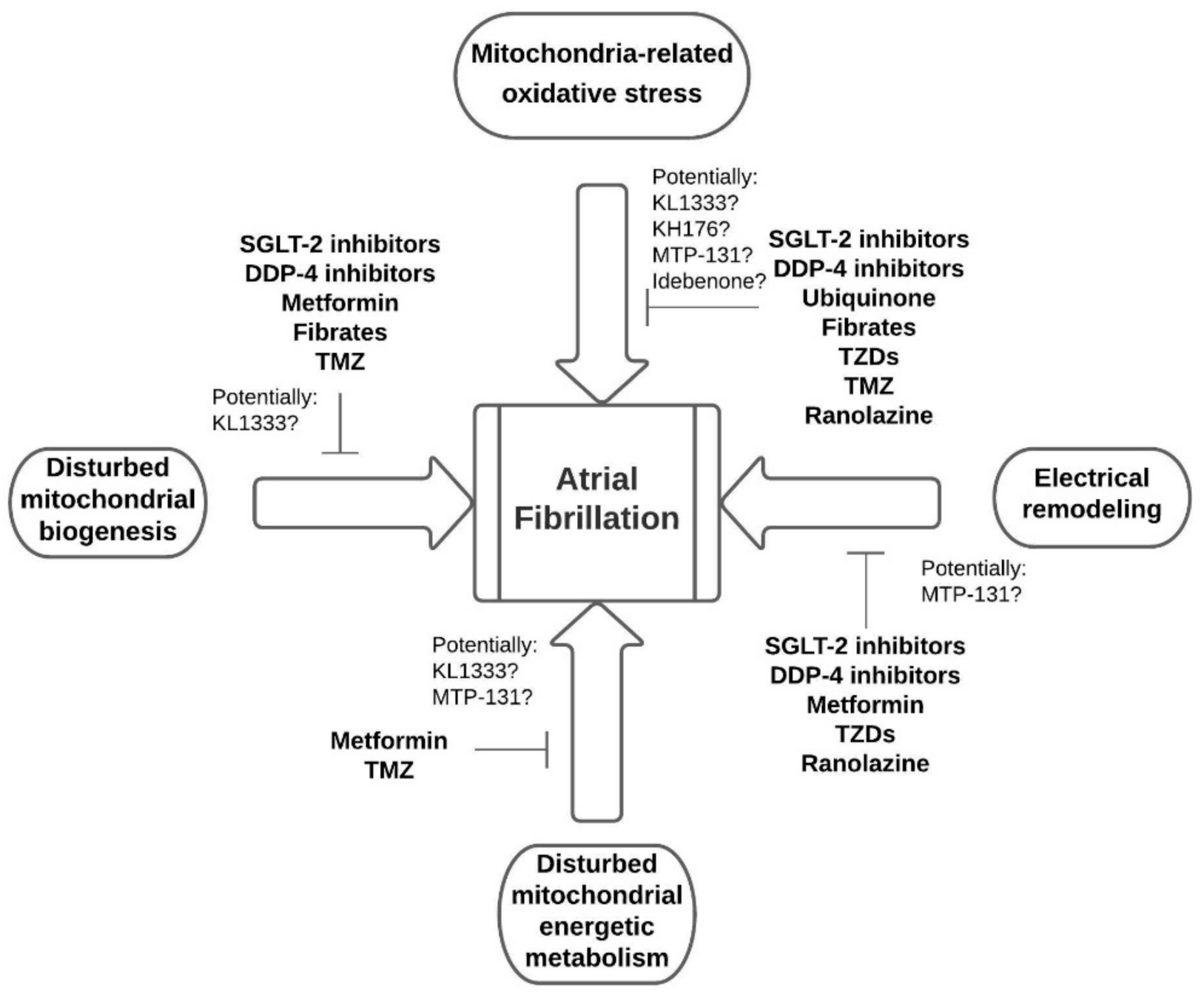
5. Pharmacological Interventions Improving Mitochondrial Function in AF
5.1. The Dipeptidyl Peptidase-4 (DDP-4) Inhibitors
5.2. Selective Inhibitors of the Sodium-Glucose Co-Transporter 2
5.3. Ubiquinone
5.4. Metformin
5.5. Thiazolidinediones
5.6. Fibrates
5.7. Trimetazidine
5.8. Ranolazine
5.9. Experimental Treatments Targeting Mitochondria
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zoni-Berisso, M.; Lercari, F.; Carazza, T.; Domenicucci, S. Epidemiology of atrial fibrillation: European perspective. Clin. Epidemiol. 2014, 6, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Kjerpeseth, L.J.; Igland, J.; Selmer, R.; Ellekjær, H.; Tveit, A.; Berge, T.; Kalstø, S.M.; Christophersen, I.E.; Myrstad, M.; Skovlund, E.; et al. Prevalence and incidence rates of atrial fibrillation in Norway 2004–2014. Heart 2021, 107, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.A.; Chamberlain, A.M.; Blankenship, J.C.; Hylek, E.M.; Voyce, S. Trends in Atrial Fibrillation Incidence Rates Within an Integrated Health Care Delivery System, 2006 to 2018. JAMA Netw. Open 2020, 3, e2014874. [Google Scholar] [CrossRef] [PubMed]
- Olesen, M.S.; Andreasen, L.; Jabbari, J.; Refsgaard, L.; Haunsø, S.; Olesen, S.-P.; Nielsen, J.B.; Schmitt, N.; Svendsen, J.H. Very early-onset lone atrial fibrillation patients have a high prevalence of rare variants in genes previously associated with atrial fibrillation. Hear. Rhythm. 2014, 11, 246–251. [Google Scholar] [CrossRef]
- Staerk, L.; Sherer, J.A.; Ko, D.; Benjamin, E.J.; Helm, R.H. Atrial Fibrillation. Circ. Res. 2017, 120, 1501–1517. [Google Scholar] [CrossRef]
- Mandalenakis, Z.; Rosengren, A.; Lappas, G.; Eriksson, P.; Gilljam, T.; Hansson, P.-O.; Skoglund, K.; Fedchenko, M.; Dellborg, M. Atrial Fibrillation Burden in Young Patients with Congenital Heart Disease. Circulation 2018, 137, 928–937. [Google Scholar] [CrossRef]
- Saguner, A.M.; Maurer, T.; Wissner, E.; Santoro, F.; Lemes, C.; Mathew, S.; Sohns, C.; Heeger, C.H.; Reißmann, B.; Riedl, J.; et al. Catheter ablation of atrial fibrillation in very young adults: A 5-year follow-up study. Europace 2016, 20, 58–64. [Google Scholar] [CrossRef]
- Lau, D.H.; Schotten, U.; Mahajan, R.; Antic, N.A.; Hatem, S.N.; Pathak, R.K.; Hendriks, J.; Kalman, J.M.; Sanders, P. Novel mechanisms in the pathogenesis of atrial fibrillation: Practical applications. Eur. Hear. J. 2015, 37, 1573–1581. [Google Scholar] [CrossRef]
- Platonov, P.G.; Mitrofanova, L.B.; Orshanskaya, V.; Ho, S.Y. Structural Abnormalities in Atrial Walls Are Associated with Presence and Persistency of Atrial Fibrillation But Not With Age. J. Am. Coll. Cardiol. 2011, 58, 2225–2232. [Google Scholar] [CrossRef]
- Wolowacz, S.E.; Samuel, M.; Brennan, V.K.; Jasso-Mosqueda, J.G.; Van Gelder, I.C. The cost of illness of atrial fibrillation: A systematic review of the recent literature. Europace 2011, 13, 1375–1385. [Google Scholar] [CrossRef]
- Steffel, J.; Verhamme, P.; Potpara, T.S.; Albaladejo, P.; Antz, M.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur. Heart J. 2018, 39, 1330–1393. [Google Scholar] [CrossRef]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur J. Cardiothorac. Surg. 2016, 50, e1–e88. [Google Scholar] [CrossRef]
- Thiedemann, K.U.; Ferrans, V.J. Left atrial ultrastructure in mitral valvular disease. Am. J. Pathol. 1977, 89, 575–604. [Google Scholar] [CrossRef]
- Harada, M.; Tadevosyan, A.; Qi, X.; Xiao, J.; Liu, T.; Voigt, N.; Karck, M.; Kamler, M.; Kodama, I.; Murohara, T.; et al. Atrial Fibrillation Activates AMP-Dependent Protein Kinase and its Regulation of Cellular Calcium Handling: Potential Role in Metabolic Adaptation and Prevention of Progression. J. Am. Coll. Cardiol. 2015, 66, 47–58. [Google Scholar] [CrossRef]
- Molvin, J.; Jujic, A.; Melander, O.; Pareek, M.; Råstam, L.; Lindblad, U.; Daka, B.; Leosdottir, M.; Nilsson, P.; Olsen, M.; et al. Exploration of pathophysiological pathways for incident atrial fibrillation using a multiplex proteomic chip. Open Heart 2020, 7, e001190. [Google Scholar] [CrossRef]
- Kornej, J.; Büttner, P.; Hammer, E.; Engelmann, B.; Dinov, B.; Sommer, P.; Husser, D.; Hindricks, G.; Völker, U.; Bollmann, A. Circulating proteomic patterns in AF related left atrial remodeling indicate involvement of coagulation and complement cascade. PLoS ONE 2018, 13, e0198461. [Google Scholar] [CrossRef]
- Ko, D.; Benson, M.D.; Ngo, D.; Yang, Q.; Larson, M.G.; Wang, T.J.; Trinquart, L.; McManus, D.D.; Lubitz, S.A.; Ellinor, P.T.; et al. Proteomics Profiling and Risk of New-Onset Atrial Fibrillation: Framingham Heart Study. J. Am. Heart Assoc. 2019, 8, e010976. [Google Scholar] [CrossRef]
- Scholman, K.T.; Meijborg, V.M.F.; Galvez-Monton, C.; Lodder, E.M.; Boukens, B.J. From Genome-Wide Association Studies to Cardiac Electrophysiology: Through the Maze of Biological Complexity. Front. Physiol. 2020, 11, 557. [Google Scholar] [CrossRef]
- Pandit, S.V.; Jalife, J. Rotors and the dynamics of cardiac fibrillation. Circ. Res. 2013, 112, 849–862. [Google Scholar] [CrossRef]
- Khan, R. Identifying and understanding the role of pulmonary vein activity in atrial fibrillation. Cardiovasc. Res. 2004, 64, 387–394. [Google Scholar] [CrossRef]
- Voigt, N.; Heijman, J.; Wang, Q.; Chiang, D.Y.; Li, N.; Karck, M.; Wehrens, X.H.T.; Nattel, S.; Dobrev, D. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation 2014, 129, 145–156. [Google Scholar] [CrossRef]
- Pellman, J.; Sheikh, F. Atrial fibrillation: Mechanisms, therapeutics, and future directions. Compr. Physiol. 2015, 5, 649–665. [Google Scholar] [CrossRef]
- Schnabel, R.B.; Sullivan, L.M.; Levy, D.; Pencina, M.J.; Massaro, J.M.; D’Agostino, R.B., Sr.; Newton-Cheh, C.; Yamamoto, J.F.; Magnani, J.W.; Tadros, T.M.; et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): A community-based cohort study. Lancet 2009, 373, 739–745. [Google Scholar] [CrossRef]
- Wasmer, K.; Eckardt, L.; Breithardt, G. Predisposing factors for atrial fibrillation in the elderly. J. Geriatr. Cardiol. 2017, 14, 179–184. [Google Scholar] [CrossRef]
- Tomaszuk-Kazberuk, A.; Koziński, M.; Kuźma, Ł.; Bujno, E.; Łopatowska, P.; Rogalska, E.; Dobrzycki, S.; Sobkowicz, B.; Lip, G.Y.H. Atrial fibrillation is more frequently associated with nonobstructive coronary lesions: The Bialystok Coronary Project. Pol. Arch. Intern. Med. 2020, 130, 1029–1036. [Google Scholar] [CrossRef]
- Vlachos, K.; Letsas, K.P.; Korantzopoulos, P.; Liu, T.; Georgopoulos, S.; Bakalakos, A.; Karamichalakis, N.; Xydonas, S.; Efremidis, M.; Sideris, A. Prediction of atrial fibrillation development and progression: Current perspectives. World J. Cardiol. 2016, 8, 267–276. [Google Scholar] [CrossRef]
- Pinnell, J.; Turner, S.; Howell, S. Cardiac muscle physiology. Contin. Educ. Anaesth. Crit. Care Pain 2007, 7, 85–88. [Google Scholar] [CrossRef]
- Maack, C.; O’Rourke, B. Excitation-contraction coupling and mitochondrial energetics. Basic Res. Cardiol. 2007, 102, 369–392. [Google Scholar] [CrossRef]
- Kohlhaas, M.; Nickel, A.G.; Maack, C. Mitochondrial energetics and calcium coupling in the heart. J. Physiol. 2017, 595, 3753–3763. [Google Scholar] [CrossRef]
- Harada, M.; Melka, J.; Sobue, Y.; Nattel, S. Metabolic Considerations in Atrial Fibrillation-Mechanistic Insights and Therapeutic Opportunities. Circ. J. 2017, 81, 1749–1757. [Google Scholar] [CrossRef]
- Hassanpour, S.H.; Dehghani, M.A.; Karami, S.Z. Study of respiratory chain dysfunction in heart disease. J. Cardiovasc. Thorac. Res. 2018, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.C. Is MCU dispensable for normal heart function? J. Mol. Cell. Cardiol. 2020, 143, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Wiersma, M.; van Marion, D.M.S.; Wust, R.C.I.; Houtkooper, R.H.; Zhang, D.; Groot, N.M.S.; Henning, R.H.; Brundel, B. Mitochondrial Dysfunction Underlies Cardiomyocyte Remodeling in Experimental and Clinical Atrial Fibrillation. Cells 2019, 8, 1202. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bai, F.; Liu, N.; Ouyang, F.; Liu, Q. The Warburg effect: A new insight into atrial fibrillation. Clin. Chim. Acta 2019, 499, 4–12. [Google Scholar] [CrossRef]
- Hu, H.J.; Zhang, C.; Tang, Z.H.; Qu, S.L.; Jiang, Z.S. Regulating the Warburg effect on metabolic stress and myocardial fibrosis remodeling and atrial intracardiac waveform activity induced by atrial fibrillation. Biochem. Biophys. Res. Commun. 2019, 516, 653–660. [Google Scholar] [CrossRef]
- Yoshida, H.; Bao, L.; Kefaloyianni, E.; Taskin, E.; Okorie, U.; Hong, M.; Dhar-Chowdhury, P.; Kaneko, M.; Coetzee, W.A. AMP-activated protein kinase connects cellular energy metabolism to KATP channel function. J. Mol. Cell. Cardiol 2012, 52, 410–418. [Google Scholar] [CrossRef]
- Liu, M.; Liu, H.; Dudley, S.C., Jr. Reactive oxygen species originating from mitochondria regulate the cardiac sodium channel. Circ. Res. 2010, 107, 967–974. [Google Scholar] [CrossRef]
- Karam, B.S.; Chavez-Moreno, A.; Koh, W.; Akar, J.G.; Akar, F.G. Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Cardiovasc. Diabetol. 2017, 16, 120. [Google Scholar] [CrossRef]
- Shao, Q.; Meng, L.; Lee, S.; Tse, G.; Gong, M.; Zhang, Z.; Zhao, J.; Zhao, Y.; Li, G.; Liu, T. Empagliflozin, a sodium glucose co-transporter-2 inhibitor, alleviates atrial remodeling and improves mitochondrial function in high-fat diet/streptozotocin-induced diabetic rats. Cardiovasc. Diabetol. 2019, 18, 165. [Google Scholar] [CrossRef]
- Cogliati, S.; Enriquez, J.A.; Scorrano, L. Mitochondrial Cristae: Where Beauty Meets Functionality. Trends Biochem. Sci. 2016, 41, 261–273. [Google Scholar] [CrossRef]
- Ausma, J.; Litjens, N.; Lenders, M.H.; Duimel, H.; Mast, F.; Wouters, L.; Ramaekers, F.; Allessie, M.; Borgers, M. Time course of atrial fibrillation-induced cellular structural remodeling in atria of the goat. J. Mol. Cell. Cardiol. 2001, 33, 2083–2094. [Google Scholar] [CrossRef]
- Ozcan, C.; Li, Z.; Kim, G.; Jeevanandam, V.; Uriel, N. Molecular Mechanism of the Association Between Atrial Fibrillation and Heart Failure Includes Energy Metabolic Dysregulation Due to Mitochondrial Dysfunction. J. Card. Fail. 2019, 25, 911–920. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, G.; Hote, M.; Devagourou, V.; Kesari, V.; Arava, S.; Airan, B.; Ray, R. Light and electron microscopic features of surgically excised left atrial appendage in rheumatic heart disease patients with atrial fibrillation and sinus rhythm. Cardiovasc. Pathol. 2014, 23, 319–326. [Google Scholar] [CrossRef]
- Bukowska, A.; Schild, L.; Keilhoff, G.; Hirte, D.; Neumann, M.; Gardemann, A.; Neumann, K.H.; Rohl, F.W.; Huth, C.; Goette, A.; et al. Mitochondrial dysfunction and redox signaling in atrial tachyarrhythmia. Exp. Biol. Med. (Maywood) 2008, 233, 558–574. [Google Scholar] [CrossRef]
- Xie, W.; Santulli, G.; Reiken, S.R.; Yuan, Q.; Osborne, B.W.; Chen, B.X.; Marks, A.R. Mitochondrial oxidative stress promotes atrial fibrillation. Sci. Rep. 2015, 5, 11427. [Google Scholar] [CrossRef]
- Yang, K.C.; Bonini, M.G.; Dudley, S.C., Jr. Mitochondria and arrhythmias. Free Radic. Biol. Med. 2014, 71, 351–361. [Google Scholar] [CrossRef]
- Bouchez, C.; Devin, A. Mitochondrial Biogenesis and Mitochondrial Reactive Oxygen Species (ROS): A Complex Relationship Regulated by the cAMP/PKA Signaling Pathway. Cells 2019, 8, 287. [Google Scholar] [CrossRef]
- Villena, J.A. New insights into PGC-1 coactivators: Redefining their role in the regulation of mitochondrial function and beyond. FEBS J. 2015, 282, 647–672. [Google Scholar] [CrossRef]
- Jornayvaz, F.R.; Shulman, G.I. Regulation of mitochondrial biogenesis. Essays Biochem. 2010, 47, 69–84. [Google Scholar] [CrossRef]
- Scarpulla, R.C. Nucleus-encoded regulators of mitochondrial function: Integration of respiratory chain expression, nutrient sensing and metabolic stress. Biochim. Biophys. Acta 2012, 1819, 1088–1097. [Google Scholar] [CrossRef]
- Rimbaud, S.; Garnier, A.; Ventura-Clapier, R. Mitochondrial biogenesis in cardiac pathophysiology. Pharmacol. Rep. 2009, 61, 131–138. [Google Scholar] [CrossRef]
- Dong, J.; Zhao, J.; Zhang, M.; Liu, G.; Wang, X.; Liu, Y.; Yang, N.; Liu, Y.; Zhao, G.; Sun, J.; et al. beta3-Adrenoceptor Impairs Mitochondrial Biogenesis and Energy Metabolism During Rapid Atrial Pacing-Induced Atrial Fibrillation. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 114–126. [Google Scholar] [CrossRef]
- Jeganathan, J.; Saraf, R.; Mahmood, F.; Pal, A.; Bhasin, M.K.; Huang, T.; Mittel, A.; Knio, Z.; Simons, R.; Khabbaz, K.; et al. Mitochondrial Dysfunction in Atrial Tissue of Patients Developing Postoperative Atrial Fibrillation. Ann. Thorac. Surg. 2017, 104, 1547–1555. [Google Scholar] [CrossRef]
- Kowaltowski, A.J.; de Souza-Pinto, N.C.; Castilho, R.F.; Vercesi, A.E. Mitochondria and reactive oxygen species. Free Radic. Biol. Med. 2009, 47, 333–343. [Google Scholar] [CrossRef]
- Montaigne, D.; Marechal, X.; Lefebvre, P.; Modine, T.; Fayad, G.; Dehondt, H.; Hurt, C.; Coisne, A.; Koussa, M.; Remy-Jouet, I.; et al. Mitochondrial dysfunction as an arrhythmogenic substrate: A translational proof-of-concept study in patients with metabolic syndrome in whom post-operative atrial fibrillation develops. J. Am. Coll. Cardiol. 2013, 62, 1466–1473. [Google Scholar] [CrossRef]
- Korantzopoulos, P.; Kolettis, T.M.; Galaris, D.; Goudevenos, J.A. The role of oxidative stress in the pathogenesis and perpetuation of atrial fibrillation. Int. J. Cardiol. 2007, 115, 135–143. [Google Scholar] [CrossRef]
- Mihm, M.J.; Yu, F.; Carnes, C.A.; Reiser, P.J.; McCarthy, P.M.; Van Wagoner, D.R.; Bauer, J.A. Impaired myofibrillar energetics and oxidative injury during human atrial fibrillation. Circulation 2001, 104, 174–180. [Google Scholar] [CrossRef]
- Youn, J.Y.; Zhang, J.; Zhang, Y.; Chen, H.; Liu, D.; Ping, P.; Weiss, J.N.; Cai, H. Oxidative stress in atrial fibrillation: An emerging role of NADPH oxidase. J. Mol. Cell. Cardiol. 2013, 62, 72–79. [Google Scholar] [CrossRef]
- Emelyanova, L.; Ashary, Z.; Cosic, M.; Negmadjanov, U.; Ross, G.; Rizvi, F.; Olet, S.; Kress, D.; Sra, J.; Tajik, A.J.; et al. Selective downregulation of mitochondrial electron transport chain activity and increased oxidative stress in human atrial fibrillation. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H54–H63. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Xie, W.; Betzenhauser, M.; Reiken, S.; Chen, B.X.; Wronska, A.; Marks, A.R. Calcium leak through ryanodine receptors leads to atrial fibrillation in 3 mouse models of catecholaminergic polymorphic ventricular tachycardia. Circ. Res. 2012, 111, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Rusciano, M.R.; Sommariva, E.; Douin-Echinard, V.; Ciccarelli, M.; Poggio, P.; Maione, A.S. CaMKII Activity in the Inflammatory Response of Cardiac Diseases. Int. J. Mol. Sci. 2019, 20, 4374. [Google Scholar] [CrossRef] [PubMed]
- Suetomi, T.; Miyamoto, S.; Brown, J.H. Inflammation in nonischemic heart disease: Initiation by cardiomyocyte CaMKII and NLRP3 inflammasome signaling. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H877–H890. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Li, G.; Liu, C.; Li, J.; Wang, X.; Cheng, L.; Liu, T. Probucol prevents atrial remodeling by inhibiting oxidative stress and TNF-alpha/NF-kappaB/TGF-beta signal transduction pathway in alloxan-induced diabetic rabbits. J. Cardiovasc. Electrophysiol. 2015, 26, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; Pfenniger, A.; Hoffman, J.; Zhang, W.; Ng, J.; Burrell, A.; Johnson, D.A.; Gussak, G.; Waugh, T.; Bull, S.; et al. Attenuation of Oxidative Injury With Targeted Expression of NADPH Oxidase 2 Short Hairpin RNA Prevents Onset and Maintenance of Electrical Remodeling in the Canine Atrium: A Novel Gene Therapy Approach to Atrial Fibrillation. Circulation 2020, 142, 1261–1278. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, J.; Wang, C.; Zhang, Q.; Xu, Y.; Liu, H.; Xiang, X.; Ma, J. DPP-4 inhibitor anagliptin protects against hypoxia-induced cytotoxicity in cardiac H9C2 cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3823–3831. [Google Scholar] [CrossRef]
- Yamamoto, T.; Shimano, M.; Inden, Y.; Takefuji, M.; Yanagisawa, S.; Yoshida, N.; Tsuji, Y.; Hirai, M.; Murohara, T. Alogliptin, a dipeptidyl peptidase-4 inhibitor, regulates the atrial arrhythmogenic substrate in rabbits. Heart Rhythm 2015, 12, 1362–1369. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Yang, Y.; Suo, Y.; Liu, R.; Qiu, J.; Zhao, Y.; Jiang, N.; Liu, C.; Tse, G.; et al. Alogliptin prevents diastolic dysfunction and preserves left ventricular mitochondrial function in diabetic rabbits. Cardiovasc. Diabetol. 2018, 17, 160. [Google Scholar] [CrossRef]
- Igarashi, T.; Niwano, S.; Niwano, H.; Yoshizawa, T.; Nakamura, H.; Fukaya, H.; Fujiishi, T.; Ishizue, N.; Satoh, A.; Kishihara, J.; et al. Linagliptin prevents atrial electrical and structural remodeling in a canine model of atrial fibrillation. Heart Vessels 2018, 33, 1258–1265. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Zhao, Y.; Jiang, N.; Qiu, J.; Yang, Y.; Li, J.; Liang, X.; Wang, X.; Tse, G.; et al. Alogliptin, a Dipeptidyl Peptidase-4 Inhibitor, Alleviates Atrial Remodeling and Improves Mitochondrial Function and Biogenesis in Diabetic Rabbits. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Chang, C.Y.; Yeh, Y.H.; Chan, Y.H.; Liu, J.R.; Chang, S.H.; Lee, H.F.; Wu, L.S.; Yen, K.C.; Kuo, C.T.; See, L.C. Dipeptidyl peptidase-4 inhibitor decreases the risk of atrial fibrillation in patients with type 2 diabetes: A nationwide cohort study in Taiwan. Cardiovasc. Diabetol. 2017, 16, 159. [Google Scholar] [CrossRef]
- Fitchett, D.; Zinman, B.; Wanner, C.; Lachin, J.M.; Hantel, S.; Salsali, A.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Inzucchi, S.E.; et al. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: Results of the EMPA-REG OUTCOME(R) trial. Eur. Heart J. 2016, 37, 1526–1534. [Google Scholar] [CrossRef]
- Kosiborod, M.; Cavender, M.A.; Fu, A.Z.; Wilding, J.P.; Khunti, K.; Holl, R.W.; Norhammar, A.; Birkeland, K.I.; Jorgensen, M.E.; Thuresson, M.; et al. Lower Risk of Heart Failure and Death in Patients Initiated on Sodium-Glucose Cotransporter-2 Inhibitors Versus Other Glucose-Lowering Drugs: The CVD-REAL Study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors). Circulation 2017, 136, 249–259. [Google Scholar] [CrossRef]
- Batzias, K.; Antonopoulos, A.S.; Oikonomou, E.; Siasos, G.; Bletsa, E.; Stampouloglou, P.K.; Mistakidi, C.V.; Noutsou, M.; Katsiki, N.; Karopoulos, P.; et al. Effects of Newer Antidiabetic Drugs on Endothelial Function and Arterial Stiffness: A Systematic Review and Meta-Analysis. J. Diabetes Res. 2018, 2018, 1232583. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, S.; Zhu, P.; Hu, S.; Chen, Y.; Ren, J. Empagliflozin rescues diabetic myocardial microvascular injury via AMPK-mediated inhibition of mitochondrial fission. Redox Biol. 2018, 15, 335–346. [Google Scholar] [CrossRef]
- Uthman, L.; Baartscheer, A.; Bleijlevens, B.; Schumacher, C.A.; Fiolet, J.W.T.; Koeman, A.; Jancev, M.; Hollmann, M.W.; Weber, N.C.; Coronel, R.; et al. Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: Inhibition of Na(+)/H(+) exchanger, lowering of cytosolic Na(+) and vasodilation. Diabetologia 2018, 61, 722–726. [Google Scholar] [CrossRef]
- Bay, J.; Kohlhaas, M.; Maack, C. Intracellular Na(+) and cardiac metabolism. J. Mol. Cell. Cardiol. 2013, 61, 20–27. [Google Scholar] [CrossRef]
- Kohlhaas, M.; Liu, T.; Knopp, A.; Zeller, T.; Ong, M.F.; Bohm, M.; O’Rourke, B.; Maack, C. Elevated cytosolic Na+ increases mitochondrial formation of reactive oxygen species in failing cardiac myocytes. Circulation 2010, 121, 1606–1613. [Google Scholar] [CrossRef]
- Ye, Y.; Jia, X.; Bajaj, M.; Birnbaum, Y. Dapagliflozin Attenuates Na(+)/H(+) Exchanger-1 in Cardiofibroblasts via AMPK Activation. Cardiovasc. Drugs Ther. 2018, 32, 553–558. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Xue, M.; Li, X.; Han, F.; Liu, X.; Xu, L.; Lu, Y.; Cheng, Y.; Li, T.; et al. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc. Diabetol. 2019, 18, 15. [Google Scholar] [CrossRef]
- Bohm, M.; Slawik, J.; Brueckmann, M.; Mattheus, M.; George, J.T.; Ofstad, A.P.; Inzucchi, S.E.; Fitchett, D.; Anker, S.D.; Marx, N.; et al. Efficacy of empagliflozin on heart failure and renal outcomes in patients with atrial fibrillation: Data from the EMPA-REG OUTCOME trial. Eur. J. Heart Fail. 2020, 22, 126–135. [Google Scholar] [CrossRef]
- Okunrintemi, V.; Mishriky, B.M.; Powell, J.R.; Cummings, D.M. Sodium-glucose co-transporter-2 inhibitors and atrial fibrillation in the cardiovascular and renal outcome trials. Diabetes Obes. Metab. 2021, 23, 276–280. [Google Scholar] [CrossRef]
- Ling, A.W.; Chan, C.C.; Chen, S.W.; Kao, Y.W.; Huang, C.Y.; Chan, Y.H.; Chu, P.H. The risk of new-onset atrial fibrillation in patients with type 2 diabetes mellitus treated with sodium glucose cotransporter 2 inhibitors versus dipeptidyl peptidase-4 inhibitors. Cardiovasc. Diabetol. 2020, 19, 188. [Google Scholar] [CrossRef] [PubMed]
- Zelniker, T.A.; Bonaca, M.P.; Furtado, R.H.M.; Mosenzon, O.; Kuder, J.F.; Murphy, S.A.; Bhatt, D.L.; Leiter, L.A.; McGuire, D.K.; Wilding, J.P.H.; et al. Effect of Dapagliflozin on Atrial Fibrillation in Patients with Type 2 Diabetes Mellitus. Circulation 2020, 141, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Martelli, A.; Testai, L.; Colletti, A.; Cicero, A.F.G. Coenzyme Q10: Clinical Applications in Cardiovascular Diseases. Antioxidants 2020, 9, 341. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Fonarow, G.C.; Butler, J.; Ezekowitz, J.A.; Felker, G.M. Coenzyme Q10 and Heart Failure: A State-of-the-Art Review. Circ. Heart Fail. 2016, 9, e002639. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, A.L.; Rosenfeldt, F.; Filipiak, K.J. Effect of coenzyme Q10 in Europeans with chronic heart failure: A sub-group analysis of the Q-SYMBIO randomized double-blind trial. Cardiol. J. 2019, 26, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeldt, F.; Marasco, S.; Lyon, W.; Wowk, M.; Sheeran, F.; Bailey, M.; Esmore, D.; Davis, B.; Pick, A.; Rabinov, M.; et al. Coenzyme Q10 therapy before cardiac surgery improves mitochondrial function and in vitro contractility of myocardial tissue. J. Thorac. Cardiovasc. Surg. 2005, 129, 25–32. [Google Scholar] [CrossRef]
- Zhao, Q.; Kebbati, A.H.; Zhang, Y.; Tang, Y.; Okello, E.; Huang, C. Effect of coenzyme Q10 on the incidence of atrial fibrillation in patients with heart failure. J. Investig. Med. 2015, 63, 735–739. [Google Scholar] [CrossRef]
- Moludi, J.; Keshavarz, S.; Mohammad Javad, H.-a.; Rahimi Frooshani, A.; Sadeghpour, A.; Salarkia, S.; Gholizadeh, F. Coenzyme Q10 effect in prevention of atrial fibrillation after Coronary Artery Bypass Graft: Double-blind randomized clinical trial. Tehran Univ. Med. J. 2015, 73, 79–85. [Google Scholar]
- De Frutos, F.; Gea, A.; Hernandez-Estefania, R.; Rabago, G. Prophylactic treatment with coenzyme Q10 in patients undergoing cardiac surgery: Could an antioxidant reduce complications? A systematic review and meta-analysis. Interact. Cardiovasc. Thorac. Surg. 2015, 20, 254–259. [Google Scholar] [CrossRef]
- Sun, D.; Yang, F. Metformin improves cardiac function in mice with heart failure after myocardial infarction by regulating mitochondrial energy metabolism. Biochem. Biophys. Res. Commun. 2017, 486, 329–335. [Google Scholar] [CrossRef]
- Liu, Y.; Bai, F.; Liu, N.; Zhang, B.; Qin, F.; Tu, T.; Li, B.; Li, J.; Ma, Y.; Ouyang, F.; et al. Metformin improves lipid metabolism and reverses the Warburg effect in a canine model of chronic atrial fibrillation. BMC Cardiovasc. Disord. 2020, 20, 50. [Google Scholar] [CrossRef]
- Chang, S.H.; Wu, L.S.; Chiou, M.J.; Liu, J.R.; Yu, K.H.; Kuo, C.F.; Wen, M.S.; Chen, W.J.; Yeh, Y.H.; See, L.C. Association of metformin with lower atrial fibrillation risk among patients with type 2 diabetes mellitus: A population-based dynamic cohort and in vitro studies. Cardiovasc. Diabetol. 2014, 13, 123. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Bai, F.; Ma, Y.; Liu, N.; Liu, Y.; Wang, Y.; Liu, Q. Metformin therapy confers cardioprotection against the remodeling of gap junction in tachycardia-induced atrial fibrillation dog model. Life Sci. 2020, 254, 117759. [Google Scholar] [CrossRef]
- Barreto-Torres, G.; Parodi-Rullan, R.; Javadov, S. The role of PPARalpha in metformin-induced attenuation of mitochondrial dysfunction in acute cardiac ischemia/reperfusion in rats. Int. J. Mol. Sci. 2012, 13, 7694–7709. [Google Scholar] [CrossRef]
- Liu, C.; Liu, R.; Fu, H.; Li, J.; Wang, X.; Cheng, L.; Korantzopoulos, P.; Tse, G.; Li, G.; Liu, T. Pioglitazone attenuates atrial remodeling and vulnerability to atrial fibrillation in alloxan-induced diabetic rabbits. Cardiovasc. Ther. 2017, 35. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Murakoshi, N.; Igarashi, M.; Hirayama, A.; Ito, Y.; Seo, Y.; Tada, H.; Aonuma, K. PPAR-gamma activator pioglitazone prevents age-related atrial fibrillation susceptibility by improving antioxidant capacity and reducing apoptosis in a rat model. J. Cardiovasc. Electrophysiol. 2012, 23, 209–217. [Google Scholar] [CrossRef]
- Pallisgaard, J.L.; Lindhardt, T.B.; Staerk, L.; Olesen, J.B.; Torp-Pedersen, C.; Hansen, M.L.; Gislason, G.H. Thiazolidinediones are associated with a decreased risk of atrial fibrillation compared with other antidiabetic treatment: A nationwide cohort study. Eur. Heart J. Cardiovasc. Pharmacother. 2017, 3, 140–146. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Korantzopoulos, P.; Letsas, K.P.; Tse, G.; Gong, M.; Meng, L.; Li, G.; Liu, T. Thiazolidinedione use and atrial fibrillation in diabetic patients: A meta-analysis. BMC Cardiovasc. Disord. 2017, 17, 96. [Google Scholar] [CrossRef]
- Pallisgaard, J.L.; Brooks, M.M.; Chaitman, B.R.; Boothroyd, D.B.; Perez, M.; Hlatky, M.A.; Bypass Angioplasty Revascularization Investigation 2 Diabetes Study, G. Thiazolidinediones and Risk of Atrial Fibrillation Among Patients with Diabetes and Coronary Disease. Am. J. Med. 2018, 131, 805–812. [Google Scholar] [CrossRef]
- Gu, J.; Hu, W.; Song, Z.P.; Liu, X.; Zhang, D.D. PPARgamma agonist use and recurrence of atrial fibrillation after successful electrical cardioversion. Hellenic. J. Cardiol. 2017, 58, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Staels, B.; Dallongeville, J.; Auwerx, J.; Schoonjans, K.; Leitersdorf, E.; Fruchart, J.C. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation 1998, 98, 2088–2093. [Google Scholar] [CrossRef] [PubMed]
- Kar, D.; Bandyopadhyay, A. Targeting Peroxisome Proliferator Activated Receptor alpha (PPAR alpha) for the Prevention of Mitochondrial Impairment and Hypertrophy in Cardiomyocytes. Cell. Physiol. Biochem. 2018, 49, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Z.; Hou, T.T.; Yuan, Y.; Hang, P.Z.; Zhao, J.J.; Sun, L.; Zhao, G.Q.; Zhao, J.; Dong, J.M.; Wang, X.B.; et al. Fenofibrate inhibits atrial metabolic remodelling in atrial fibrillation through PPAR-alpha/sirtuin 1/PGC-1alpha pathway. Br. J. Pharmacol. 2016, 173, 1095–1109. [Google Scholar] [CrossRef]
- Augustyniak, J.; Lenart, J.; Gaj, P.; Kolanowska, M.; Jazdzewski, K.; Stepien, P.P.; Buzanska, L. Bezafibrate Upregulates Mitochondrial Biogenesis and Influence Neural Differentiation of Human-Induced Pluripotent Stem Cells. Mol. Neurobiol. 2019, 56, 4346–4363. [Google Scholar] [CrossRef]
- Hanna, I.R.; Heeke, B.; Bush, H.; Brosius, L.; King-Hageman, D.; Dudley, S.C., Jr.; Beshai, J.F.; Langberg, J.J. Lipid-lowering drug use is associated with reduced prevalence of atrial fibrillation in patients with left ventricular systolic dysfunction. Heart Rhythm 2006, 3, 881–886. [Google Scholar] [CrossRef]
- Cavar, M.; Ljubkovic, M.; Bulat, C.; Bakovic, D.; Fabijanic, D.; Kraljevic, J.; Karanovic, N.; Dujic, Z.; Lavie, C.J.; Wisloff, U.; et al. Trimetazidine does not alter metabolic substrate oxidation in cardiac mitochondria of target patient population. Br. J. Pharmacol. 2016, 173, 1529–1540. [Google Scholar] [CrossRef]
- MacInnes, A.; Fairman, D.A.; Binding, P.; Rhodes, J.; Wyatt, M.J.; Phelan, A.; Haddock, P.S.; Karran, E.H. The antianginal agent trimetazidine does not exert its functional benefit via inhibition of mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ. Res. 2003, 93, e26–e32. [Google Scholar] [CrossRef]
- Monteiro, P.; Duarte, A.I.; Goncalves, L.M.; Moreno, A.; Providencia, L.A. Protective effect of trimetazidine on myocardial mitochondrial function in an ex-vivo model of global myocardial ischemia. Eur. J. Pharmacol. 2004, 503, 123–128. [Google Scholar] [CrossRef]
- Shi, W.; Shangguan, W.; Zhang, Y.; Li, C.; Li, G. Effects of trimetazidine on mitochondrial respiratory function, biosynthesis, and fission/fusion in rats with acute myocardial ischemia. Anatol. J. Cardiol. 2017, 18, 175–181. [Google Scholar] [CrossRef]
- Dehina, L.; Vaillant, F.; Tabib, A.; Bui-Xuan, B.; Chevalier, P.; Dizerens, N.; Bui-Xuan, C.; Descotes, J.; Blanc-Guillemaud, V.; Lerond, L.; et al. Trimetazidine demonstrated cardioprotective effects through mitochondrial pathway in a model of acute coronary ischemia. Naunyn Schmiedeberg’s Arch. Pharmacol. 2013, 386, 205–215. [Google Scholar] [CrossRef]
- Gunes, Y.; Tuncer, M.; Guntekin, U.; Akdag, S.; Gumrukcuoglu, H.A. The effects of trimetazidine on p-wave duration and dispersion in heart failure patients. Pacing Clin. Electrophysiol. 2009, 32, 239–244. [Google Scholar] [CrossRef]
- Zhang, J.; He, S.; Wang, X.; Wang, D. Effect of trimetazidine on heart rate variability in elderly patients with acute coronary syndrome. Pak. J. Med. Sci. 2016, 32, 75–78. [Google Scholar] [CrossRef][Green Version]
- Cera, M.; Salerno, A.; Fragasso, G.; Montanaro, C.; Gardini, C.; Marinosci, G.; Arioli, F.; Spoladore, R.; Facchini, A.; Godino, C.; et al. Beneficial electrophysiological effects of trimetazidine in patients with postischemic chronic heart failure. J. Cardiovasc. Pharmacol. Ther. 2010, 15, 24–30. [Google Scholar] [CrossRef]
- Li, Z.; Chaolan, L.; Chengcheng, W.; Xi, H.; Yingying, W.; Jiaqiu, L.; Wei, H. GW28-e0789 Trimetazidine decreases inducibility and duration of atrial fibrillation in a dog model of congestive heart failure. J. Am. Coll. Cardiol. 2017, 70, C29. [Google Scholar] [CrossRef]
- Han, W.; Yang, S.S.; Wei, N.; Huo, H.; Li, W.M.; Zhou, H.Y.; Zhou, G.; Cao, Y.; Dong, G.; Fu, S.B. Effects of chronic trimetazidine treatment on atrial energy metabolism in a canine model of chronic atrial fibrillation. Zhonghua Xin Xue Guan Bing Za Zhi 2008, 36, 556–559. [Google Scholar]
- Han, W.; Li, W.M.; Zhou, H.Y.; Huo, H.; Wei, N.; Dong, G.; Cao, Y.; Zhou, G.; Yang, S.S. Effects of trimetazidine on atrial structural remodeling and platelet activation in dogs with atrial fibrillation. Chin. Med. J. 2009, 122, 2180–2183. [Google Scholar] [PubMed]
- Francis, J.; Antzelevitch, C. Ranolazine as Antiarrhythmic Agent. BMH Med. J. 2019, 56, 58–64. [Google Scholar]
- Guerra, F.; Romandini, A.; Barbarossa, A.; Belardinelli, L.; Capucci, A. Ranolazine for rhythm control in atrial fibrillation: A systematic review and meta-analysis. Int. J. Cardiol. 2017, 227, 284–291. [Google Scholar] [CrossRef]
- Gong, M.; Zhang, Z.; Fragakis, N.; Korantzopoulos, P.; Letsas, K.P.; Li, G.; Yan, G.X.; Liu, T. Role of ranolazine in the prevention and treatment of atrial fibrillation: A meta-analysis of randomized clinical trials. Heart Rhythm 2017, 14, 3–11. [Google Scholar] [CrossRef]
- Frommeyer, G.; Schmidt, M.; Clauß, C.; Kaese, S.; Stypmann, J.; Pott, C.; Eckardt, L.; Milberg, P. Further insights into the underlying electrophysiological mechanisms for reduction of atrial fibrillation by ranolazine in an experimental model of chronic heart failure. Eur. J. Heart Fail. 2012, 14, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Geng, N.; Chen, Y.; Ren, L.; Liu, X.; Wan, J.; Guo, S.; Wang, S. Ranolazine improves oxidative stress and mitochondrial function in the atrium of acetylcholine-CaCl2 induced atrial fibrillation rats. Life Sci. 2016, 156, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Chatfield, K.C.; Sparagna, G.C.; Chau, S.; Phillips, E.K.; Ambardekar, A.V.; Aftab, M.; Mitchell, M.B.; Sucharov, C.C.; Miyamoto, S.D.; Stauffer, B.L. Elamipretide Improves Mitochondrial Function in the Failing Human Heart. JACC Basic Transl. Sci. 2019, 4, 147–157. [Google Scholar] [CrossRef]
- Sabbah, H.N.; Gupta, R.C.; Kohli, S.; Wang, M.; Hachem, S.; Zhang, K. Chronic Therapy with Elamipretide (MTP-131), a Novel Mitochondria-Targeting Peptide, Improves Left Ventricular and Mitochondrial Function in Dogs With Advanced Heart Failure. Circ. Heart Fail. 2016, 9, e002206. [Google Scholar] [CrossRef]
- Daubert, M.A.; Yow, E.; Dunn, G.; Marchev, S.; Barnhart, H.; Douglas, P.S.; O’Connor, C.; Goldstein, S.; Udelson, J.E.; Sabbah, H.N. Novel Mitochondria-Targeting Peptide in Heart Failure Treatment: A Randomized, Placebo-Controlled Trial of Elamipretide. Circ. Heart Fail. 2017, 10. [Google Scholar] [CrossRef]
- Butler, J.; Khan, M.S.; Anker, S.D.; Fonarow, G.C.; Kim, R.J.; Nodari, S.; O’Connor, C.M.; Pieske, B.; Pieske-Kraigher, E.; Sabbah, H.N.; et al. Effects of Elamipretide on Left Ventricular Function in Patients With Heart Failure With Reduced Ejection Fraction: The PROGRESS-HF Phase 2 Trial. J. Card. Fail. 2020, 26, 429–437. [Google Scholar] [CrossRef]
- Buyse, G.M.; Voit, T.; Schara, U.; Straathof, C.S.M.; D’Angelo, M.G.; Bernert, G.; Cuisset, J.-M.; Finkel, R.S.; Goemans, N.; McDonald, C.M.; et al. Efficacy of idebenone on respiratory function in patients with Duchenne muscular dystrophy not using glucocorticoids (DELOS): A double-blind randomised placebo-controlled phase 3 trial. Lancet 2015, 385, 1748–1757. [Google Scholar] [CrossRef]
- Lagedrost, S.J.; Sutton, M.S.; Cohen, M.S.; Satou, G.M.; Kaufman, B.D.; Perlman, S.L.; Rummey, C.; Meier, T.; Lynch, D.R. Idebenone in Friedreich ataxia cardiomyopathy-results from a 6-month phase III study (IONIA). Am. Heart J. 2011, 161, 639–645. [Google Scholar] [CrossRef]
- Seo, K.S.; Kim, J.H.; Min, K.N.; Moon, J.A.; Roh, T.C.; Lee, M.J.; Lee, K.W.; Min, J.E.; Lee, Y.M. KL1333, a Novel NAD(+) Modulator, Improves Energy Metabolism and Mitochondrial Dysfunction in MELAS Fibroblasts. Front. Neurol. 2018, 9, 552. [Google Scholar] [CrossRef]
- Beyrath, J.; Pellegrini, M.; Renkema, H.; Houben, L.; Pecheritsyna, S.; van Zandvoort, P.; van den Broek, P.; Bekel, A.; Eftekhari, P.; Smeitink, J.A.M. KH176 Safeguards Mitochondrial Diseased Cells from Redox Stress-Induced Cell Death by Interacting with the Thioredoxin System/Peroxiredoxin Enzyme Machinery. Sci. Rep. 2018, 8, 6577. [Google Scholar] [CrossRef]
- El-Hattab, A.W.; Zarante, A.M.; Almannai, M.; Scaglia, F. Therapies for mitochondrial diseases and current clinical trials. Mol. Genet. Metab. 2017, 122, 1–9. [Google Scholar] [CrossRef]
- Perry, J.B.; Davis, G.N.; Allen, M.E.; Makrecka-Kuka, M.; Dambrova, M.; Grange, R.W.; Shaikh, S.R.; Brown, D.A. Cardioprotective effects of idebenone do not involve ROS scavenging: Evidence for mitochondrial complex I bypass in ischemia/reperfusion injury. J. Mol. Cell. Cardiol. 2019, 135, 160–171. [Google Scholar] [CrossRef]
- Liu, Z.; Donahue, J.K. The Use of Gene Therapy for Ablation of Atrial Fibrillation. Arrhythm. Electrophysiol. Rev. 2014, 3, 139–144. [Google Scholar] [CrossRef]
- Bikou, O.; Thomas, D.; Trappe, K.; Lugenbiel, P.; Kelemen, K.; Koch, M.; Soucek, R.; Voss, F.; Becker, R.; Katus, H.A.; et al. Connexin 43 gene therapy prevents persistent atrial fibrillation in a porcine model. Cardiovasc. Res. 2011, 92, 218–225. [Google Scholar] [CrossRef]
- Trappe, K.; Thomas, D.; Bikou, O.; Kelemen, K.; Lugenbiel, P.; Voss, F.; Becker, R.; Katus, H.A.; Bauer, A. Suppression of persistent atrial fibrillation by genetic knockdown of caspase 3: A pre-clinical pilot study. Eur. Heart J. 2011, 34, 147–157. [Google Scholar] [CrossRef]

| Medication | Mechanism of Action | Target Diseases | Current Research Stage | References |
|---|---|---|---|---|
| Elamipretide (MTP-131) | Improves mitochondrial ultrastructure and bioenergetics | Primary Mitochondrial Myopathy | Experimental studies; phase 3 randomized, double-blind, placebo-controlled trial—terminated; phase 2 randimized trials in heart failure—completed | Clinical trial, identifier: NCT03323749 NCT02788747 NCT02814097 NCT02914665 |
| KL1333 | The safety and efficiency measurements in progress, with potential effects on NAD+/NADH, FGF21 and GDF15 concentrations | Primary mitochondrial disease | Phase Ia/Ib trial (recruiting) | Clinical trial, identifier: NCT03888716 |
| KH176 | ROS level reduction and cell protection against redox stress | Mitochondrial disease | Phase IIb open-label, multi-center trial (planned ending date: June 2021) | Clinical trial [107,108] identifier: NCT02544217; NCT02909400; NCT04165239 NCT04604548 |
| REN001 | Selective PPAR delta agonist | Primary Mitochondrial Myopathy | Finished phase I due to COVID-19 pandemic but with sufficient data gathered to achieve the study objective | Clinical trial, identifier: NCT03862846 |
| Idebenone | Antioxidant with ATP preserving properties: stimulates mitochondrial electron flux, increases respiratory function | Friedreich ataxia (FRDA) and Duchenne muscular dystrophy | Finished trial phase III in Duchenne muscular dystrophy and phase III study (IONIA) | [127,128] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muszyński, P.; Bonda, T.A. Mitochondrial Dysfunction in Atrial Fibrillation—Mechanisms and Pharmacological Interventions. J. Clin. Med. 2021, 10, 2385. https://doi.org/10.3390/jcm10112385
Muszyński P, Bonda TA. Mitochondrial Dysfunction in Atrial Fibrillation—Mechanisms and Pharmacological Interventions. Journal of Clinical Medicine. 2021; 10(11):2385. https://doi.org/10.3390/jcm10112385
Chicago/Turabian StyleMuszyński, Paweł, and Tomasz A. Bonda. 2021. "Mitochondrial Dysfunction in Atrial Fibrillation—Mechanisms and Pharmacological Interventions" Journal of Clinical Medicine 10, no. 11: 2385. https://doi.org/10.3390/jcm10112385
APA StyleMuszyński, P., & Bonda, T. A. (2021). Mitochondrial Dysfunction in Atrial Fibrillation—Mechanisms and Pharmacological Interventions. Journal of Clinical Medicine, 10(11), 2385. https://doi.org/10.3390/jcm10112385






