Mini-Invasive, Ultrasound Guided Repair of the Achilles Tendon Rupture—A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Methods
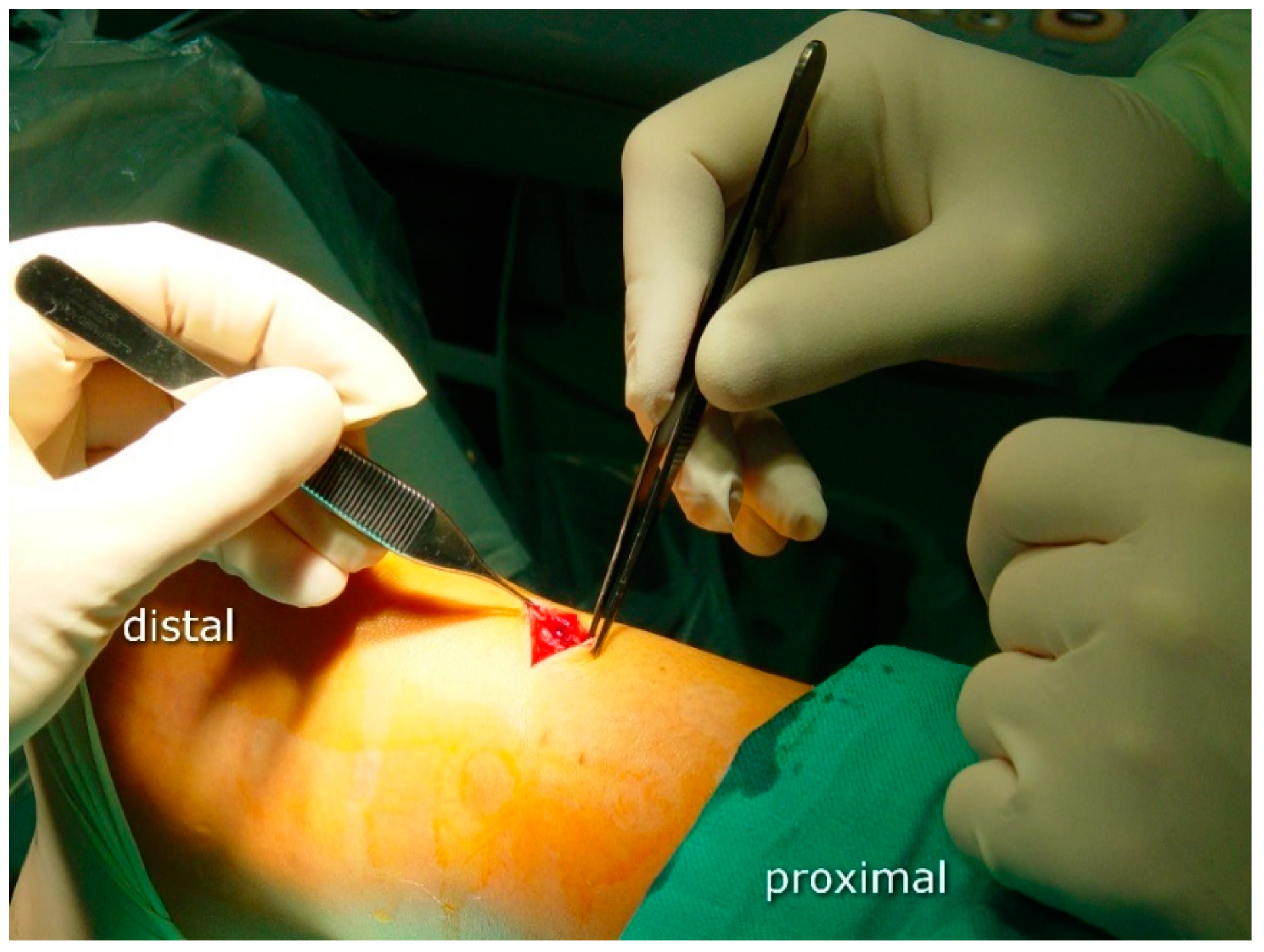
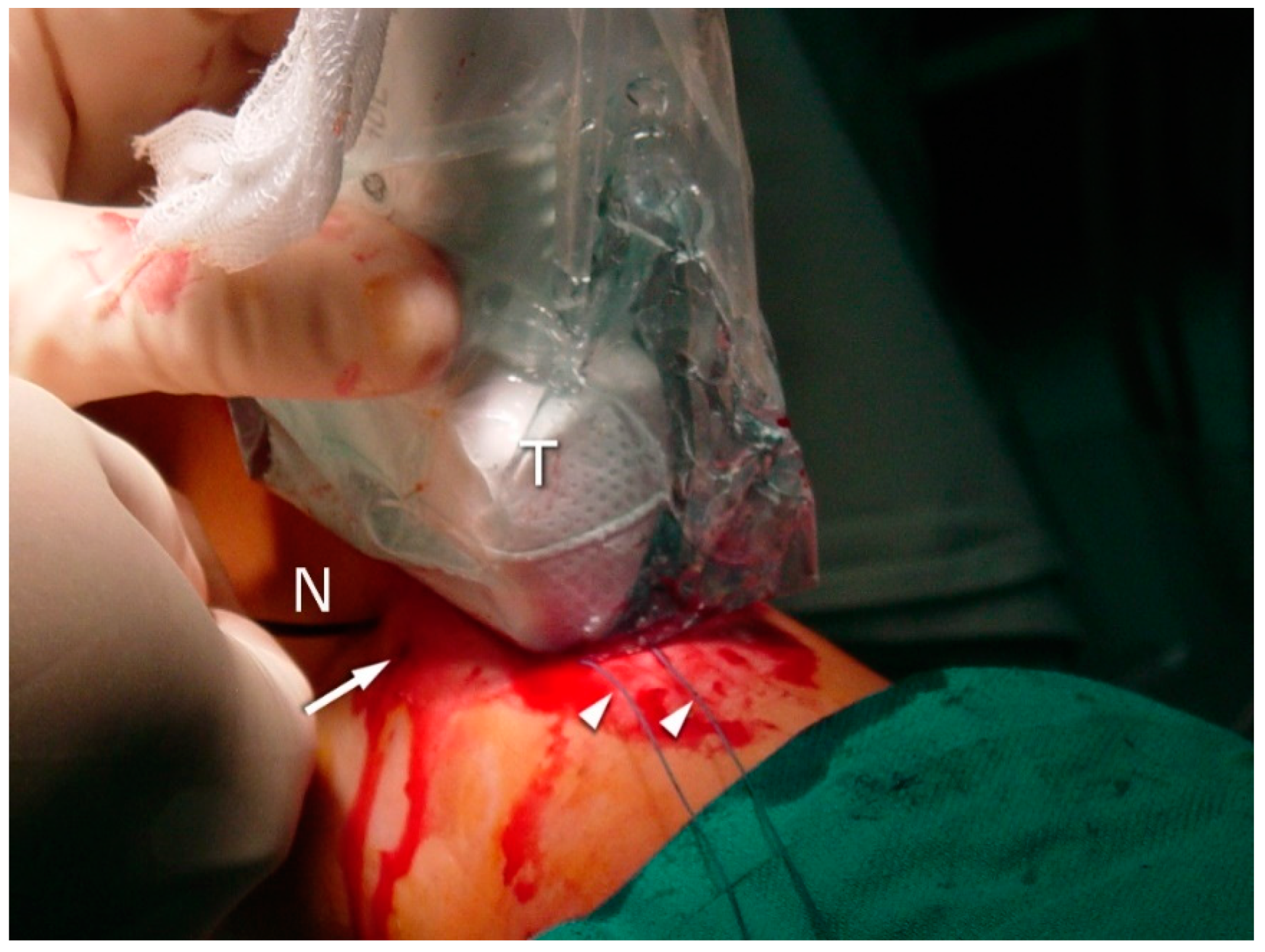
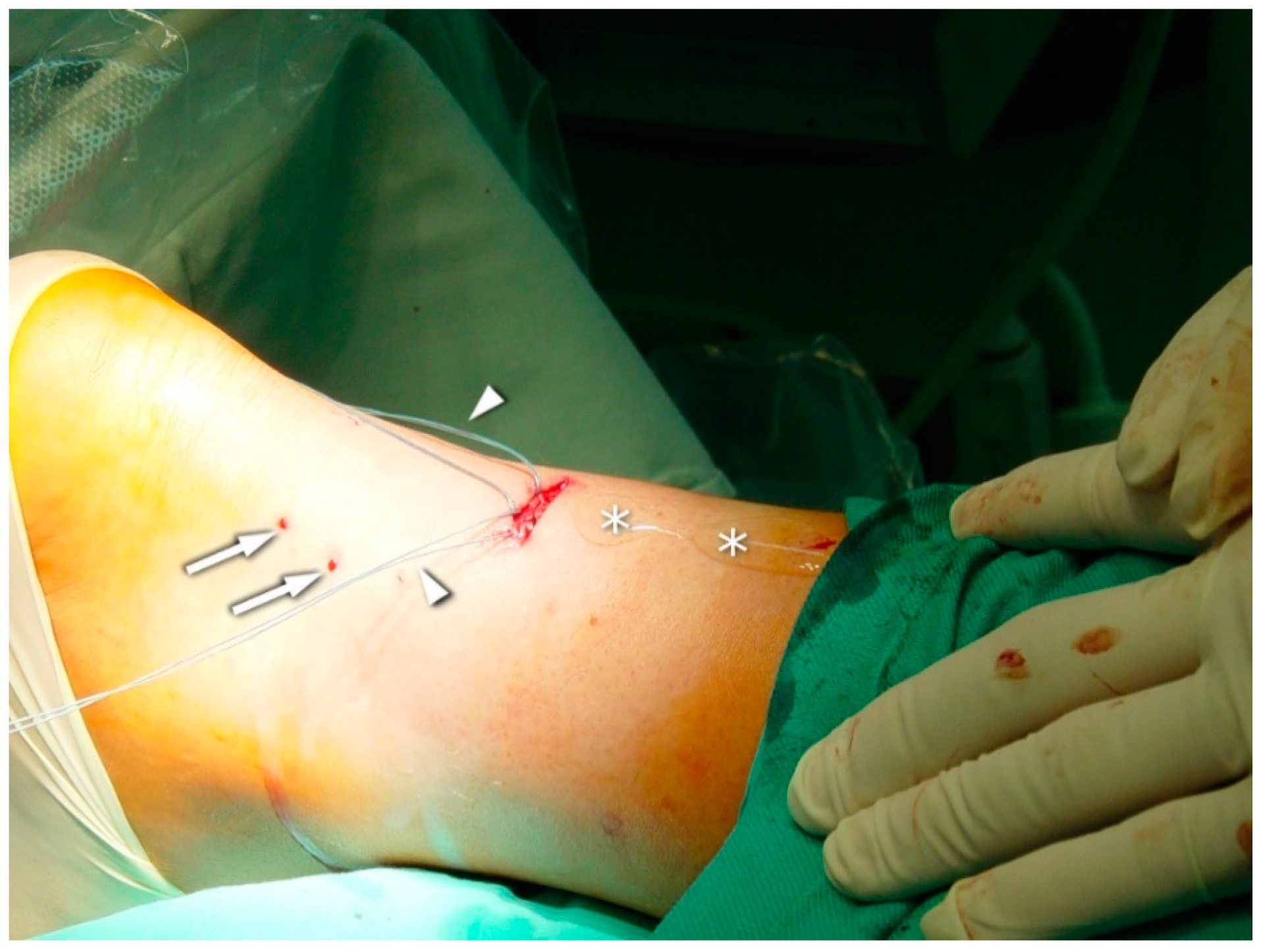
2.3. Surgical Procedure
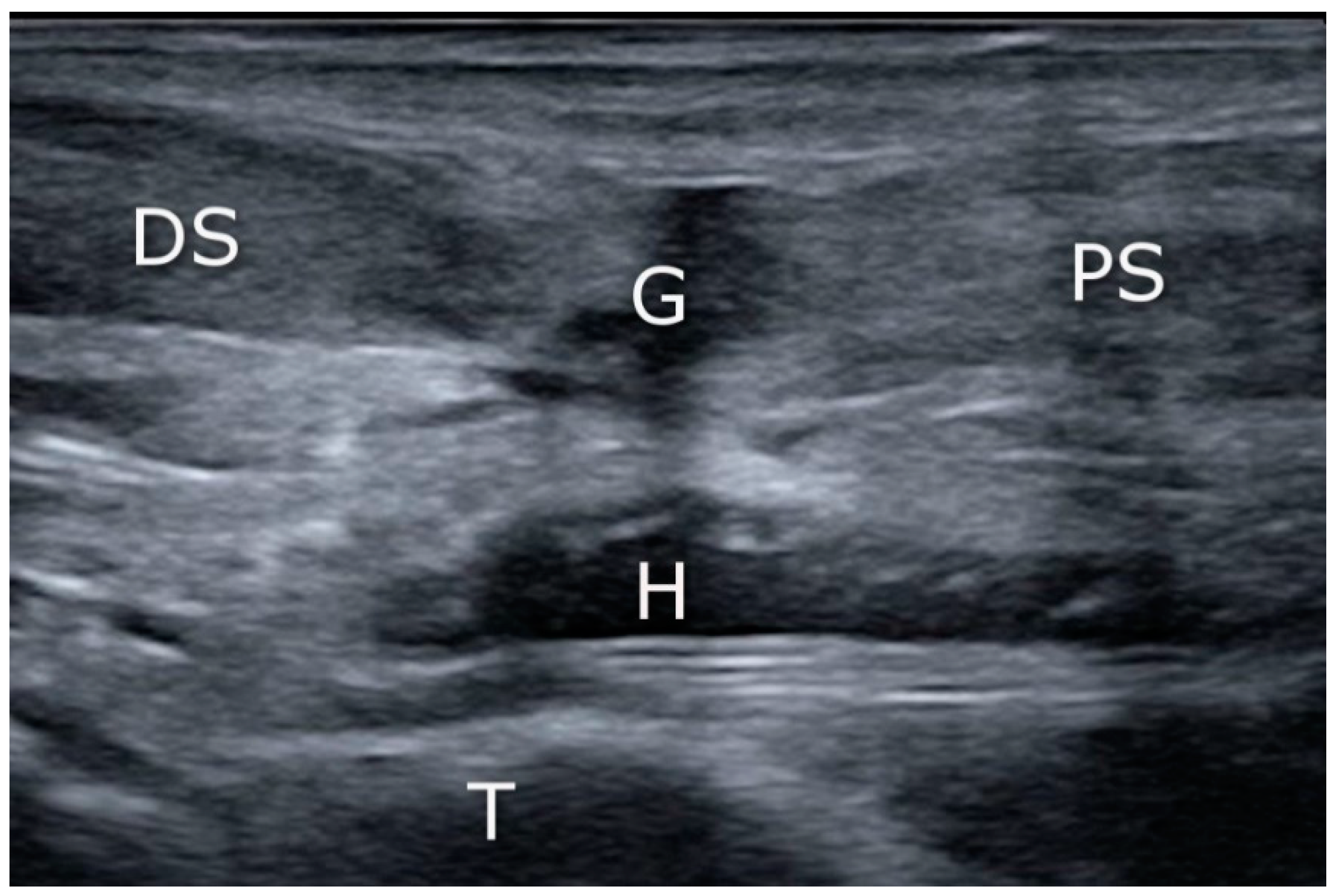
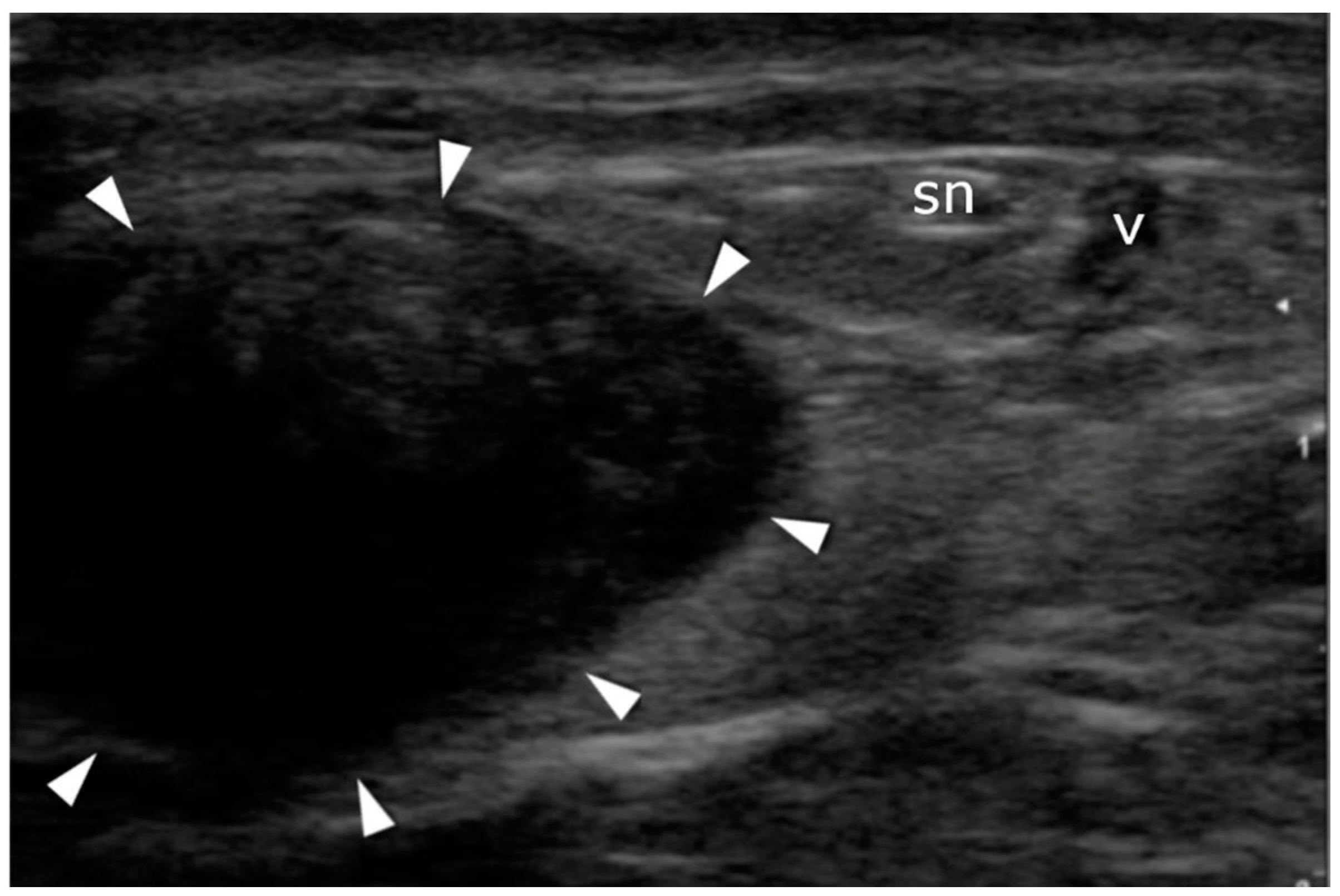
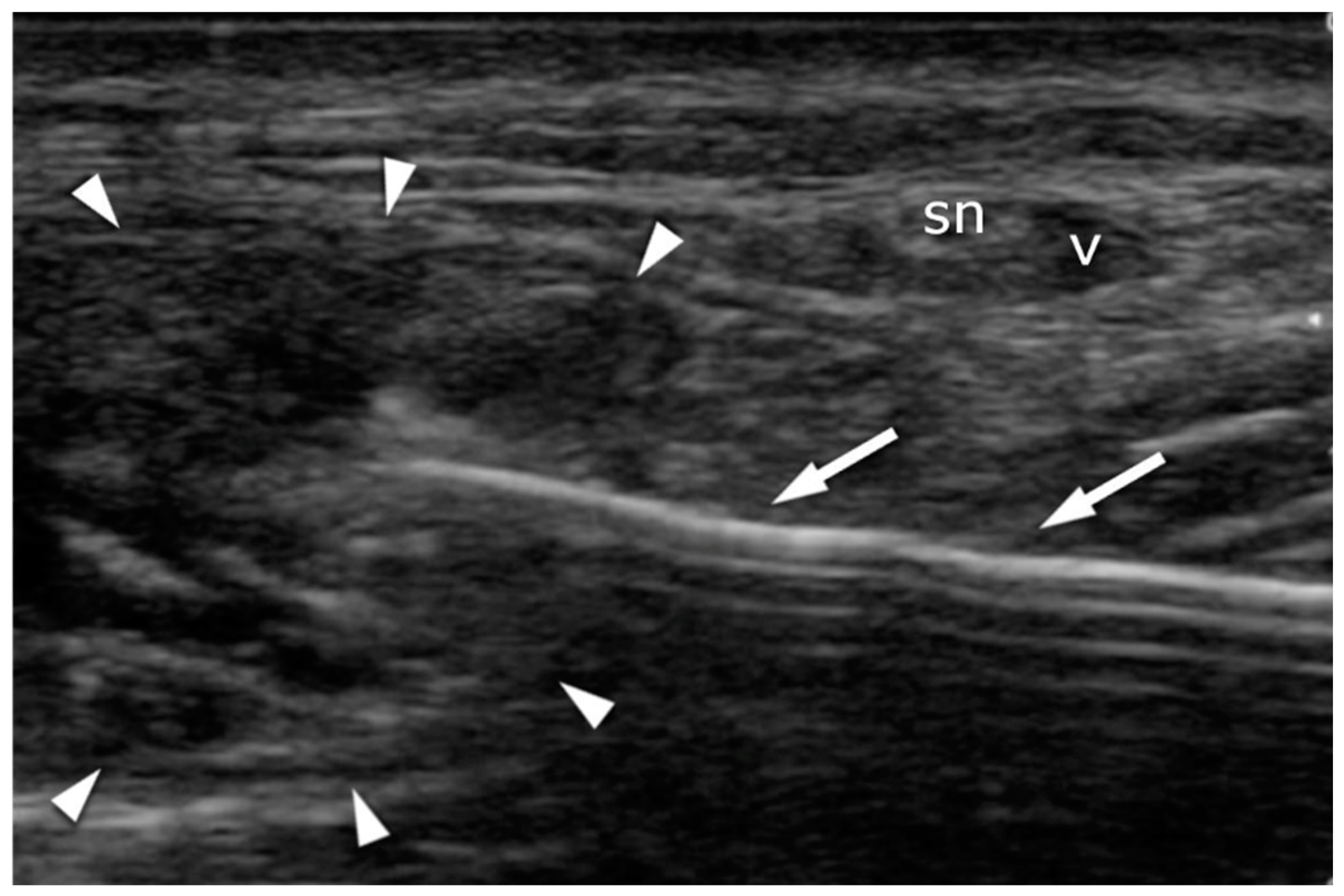
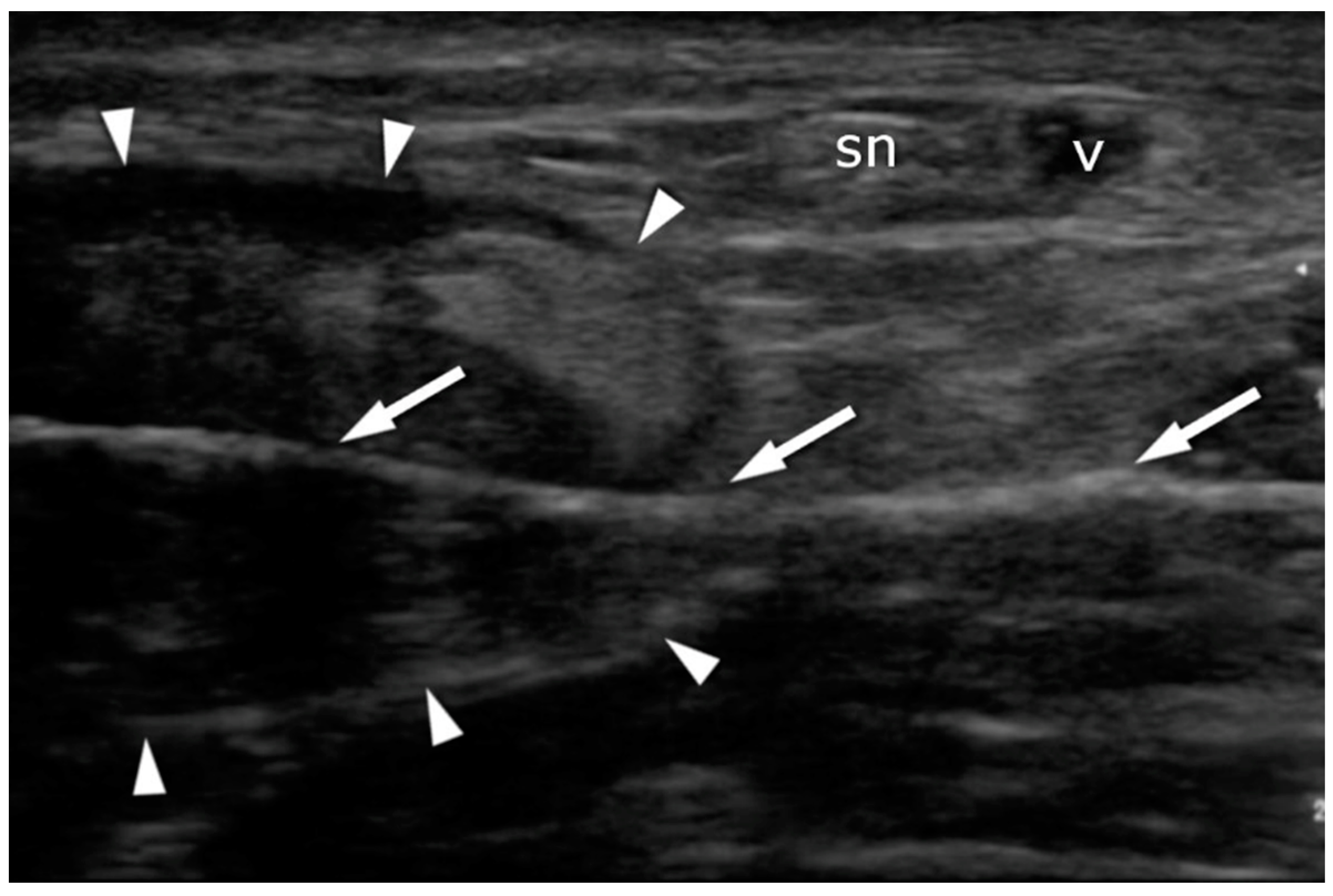
2.4. Ultrasound Imaging in the US-Guided Group
2.5. Aftercare
2.6. Statistical Analyses
3. Results
3.1. FAOQ
3.2. Re-Ruptures
3.3. Sural Nerve Complications
3.4. US Achilles Assessment
3.5. US Sural Nerve Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Brien, M. The anatomy of the Achilles tendon. Foot Ankle Clin. 2005, 10, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Maffulli, N. Biology of tendon injury: Healing, modeling and remodeling. J. Musculoskelet Neuronal. Interact. 2006, 6, 181–190. [Google Scholar]
- Longo, U.G.; Ronga, M.; Maffulli, N. Achilles tendinopathy. Sports Med. Arthrosc. Rev. 2009, 17, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Kaux, J.F.; Forthomme, B.; Goff, C.L.; Crielaard, J.M.; Croisier, J.L. Current opinions on tendinopathy. J. Sports Sci. Med. 2011, 10, 238–253. [Google Scholar] [PubMed]
- Rees, J.D.; Wilson, A.M.; Wolman, R.L. Current concepts in the management of tendon disorders. Rheumatology 2006, 45, 508–521. [Google Scholar] [CrossRef] [PubMed]
- Abate, M.; Silbernagel, K.G.; Siljeholm, C.; Di Iorio, A.; De Amicis, D.; Salini, V.; Werner, S.; Paganelli, R. Pathogenesis of tendinopathies: Inflammation or degeneration? Arthritis Res. Ther. 2009, 11, 235. [Google Scholar] [CrossRef]
- Krahe, M.A.; Berlet, G.C. Achilles tendon ruptures, re rupture with revision surgery, tendinosis, and insertional disease. Foot Ankle Clin. 2009, 14, 247–275. [Google Scholar] [CrossRef]
- Park, S.-H.; Lee, H.S.; Young, K.W.; Seo, S.G. Treatment of Acute Achilles Tendon Rupture. Clin. Orthop. Surg. 2020, 12, 1–8. [Google Scholar] [CrossRef]
- Kvist, M. Achilles tendon injuries in athletes. Sports Med. 1994, 18, 173–201. [Google Scholar] [CrossRef]
- Carmont, M.R.; Rossi, R.; Scheffler, S.; Mei-Dan, O.; Beaufils, P. Percutaneous & Mini Invasive Achilles tendon repair. Sports Med. Arthrosc. Rehabil. Ther. Technol. 2011, 3, 28. [Google Scholar] [CrossRef]
- Patel, M.S.; Kadakia, A.R. Minimally Invasive Treatments of Acute Achilles Tendon Ruptures. Foot Ankle Clin. 2019, 24, 399–424. [Google Scholar] [CrossRef]
- Wong, J.; Barrass, V.; Maffulli, N. Quantitative review of operative and nonoperative management of achilles tendon ruptures. Am. J. Sports Med. 2002, 30, 565–575. [Google Scholar] [CrossRef]
- Van Maele, M.; Misselyn, D.; Metsemakers, W.J.; Sermon, A.; Nijs, S.; Hoekstra, H. Is open acute Achilles tendon rupture repair still justified? A single center experience and critical appraisal of the literature. Injury 2018, 49, 1947–1952. [Google Scholar] [CrossRef]
- Maffulli, N.; Longo, U.G.; Spiezia, F.; Denaro, V. Minimally invasive surgery for Achilles tendon pathologies. Open Access J. Sports Med. 2010, 1, 95–103. [Google Scholar] [CrossRef]
- Carmont, M.R.; Maffulli, N. Modified percutaneous repair of ruptured Achilles tendon. Knee Surg. Sports Traumatol. Arthrosc. 2008, 16, 199–203. [Google Scholar] [CrossRef]
- Majewski, M.; Rohrbach, M.; Czaja, S.; Ochsner, P. Avoiding sural nerve injuries during percutaneous Achilles tendon repair. Am. J. Sports Med. 2006, 34, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Buchgraber, A.; Pässler, H.H. Percutaneous repair of Achilles tendon rupture. Immobilization versus functional postoperative treatment. Clin. Orthop. Relat. Res. 1997, 341, 113–122. [Google Scholar] [CrossRef]
- Cetti, R.; Christensen, S.E.; Ejsted, R.; Jensen, N.M.; Jorgensen, U. Operative versus nonoperative treatment of Achilles tendon rupture. A prospective randomized study and review of the literature. Am. J. Sports. Med. 1993, 21, 791–799. [Google Scholar] [CrossRef]
- Khan, R.J.; Fick, D.; Keogh, A.; Crawford, J.; Brammar, T.; Parker, M. Treatment of acute achilles tendon ruptures. A meta-analysis of randomized, controlled trials. J. Bone Jt. Surg. Am. 2005, 87, 2202–2210. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Dalal, R.; Waseem, M. Percutaneous vs. open repair of the ruptured Achilles tendon--a prospective randomized controlled study. Foot Ankle Int. 2001, 22, 559–568. [Google Scholar] [CrossRef]
- Möller, M.; Movin, T.; Granhed, H.; Lind, K.; Faxén, E.; Karlsson, J. Acute rupture of tendon Achillis. A prospective randomised study of comparison between surgical and non-surgical treatment. J. Bone Jt. Surg. 2001, 83, 843–848. [Google Scholar] [CrossRef]
- Alcelik, I.; Saeed, Z.M.; Haughton, B.A.; Shahid, R.; Alcelik, J.C.; Brogden, C.; Budgen, A. Achillon versus open surgery in acute Achilles tendon repair. Foot Ankle Surg. 2018, 24, 427–434. [Google Scholar] [CrossRef]
- Hsu, A.R.; Jones, C.P.; Cohen, B.E.; Davis, W.H.; Ellington, J.K.; Anderson, R.B. Clinical Outcomes and Complications of Percutaneous Achilles Repair System Versus Open Technique for Acute Achilles Tendon Ruptures. Foot Ankle Int. 2015, 36, 1279–1286. [Google Scholar] [CrossRef]
- Brown, M.N.; Pearce, B.S.; Vanetti, T.K. Sural Nerve Entrapment. In Peripheral Nerve Entrapments; Springer: Berlin/Heidelberg, Germany, 2016; pp. 795–810. [Google Scholar] [CrossRef]
- Ricci, S.; Moro, L.; Antonelli Incalzi, R. Ultrasound imaging of the sural nerve: Ultrasound anatomy and rationale for investigation. Eur. J. Vasc. Endovasc. Surg. 2010, 39, 636–641. [Google Scholar] [CrossRef]
- Giannetti, S.; Patricola, A.A.; Stancati, A.; Santucci, A. Intraoperative ultrasound assistance for percutaneous repair of the acute Achilles tendon rupture. Orthopedics 2014, 37, 820–824. [Google Scholar] [CrossRef] [PubMed]
- Boszczyk, A.; Blonski, M.; Pomianowski, S. Translation, Cultural Adaptation and Validation of Polish Version of Foot and Ankle Outcomes Questionnaire. Ortop. Traumatol. Rehabil. 2015, 17, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.M.; Bannister, G.C. Percutaneous repair of the ruptured tendo Achillis. J. Bone Joint Surg. 1999, 81, 877–880. [Google Scholar] [CrossRef]
- Cretnik, A.; Kosanovic, M.; Smrkolj, V. Percutaneous versus open repair of the ruptured Achilles tendon: A comparative study. Am. J. Sports Med. 2005, 33, 1369–1379. [Google Scholar] [CrossRef]
- Cretnik, A.; Kosanovic, M.; Kosir, R. Long-Term Results with the Use of Modified Percutaneous Repair of the Ruptured Achilles Tendon Under Local Anaesthesia (15-Year Analysis with 270 Cases). J. Foot Ankle Surg. 2019, 58, 828–836. [Google Scholar] [CrossRef]
- Maes, R.; Copin, G.; Averous, C. Is percutaneous repair of the Achilles tendon a safe technique? A study of 124 cases. Acta Orthop. Belg. 2006, 72, 179–183. [Google Scholar]
- Lacoste, S.; Féron, J.M.; Cherrier, B. Percutaneous Tenolig® repair under intra-operative ultrasonography guidance in acute Achilles tendon rupture. Orthop. Traumatol. Surg. Res. 2014, 100, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Soubeyrand, M.; Serra-Tosio, G.; Campagna, R.; Molina, V.; Sitbon, P.; Biau, D.J. Intraoperative ultrasonography during percutaneous Achilles tendon repair. Foot Ankle Int. 2010, 31, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Blankstein, A.; Israeli, A.; Dudkiewicz, I.; Chechik, A.; Ganel, A. Percutaneous Achilles tendon repair combined with real-time sonography. Isr. Med. Assoc. J. 2007, 9, 83–85. [Google Scholar] [PubMed]
- Severyns, M.; Andriamananaivo, T.; Rollet, M.E.; Kajetanek, C.; Lopes, R.; Renard, G.; Noailles, T.; Odri, G.A.; Rouvillain, J.L. Acute Achilles Tendon Rupture: Ultrasonography and Endoscopy-Assisted Percutaneous Repair. Arthrosc. Tech. 2019, 8, e489–e493. [Google Scholar] [CrossRef] [PubMed]
| No. | US Guided Group | Non-Guided Group | |
|---|---|---|---|
| 20 | 15 | ||
| Age (years) | Range | 30–62 | 30–60 |
| Mean | 42 | 43 | |
| SD | 8.95 | 9.98 | |
| Follow-up (years) | Range | 3–13 | 5–10 |
| Mean | 8 | 8 | |
| SD | 3.32 | 1.51 | |
| FAOQ score (pts) | Range | 55–90 | 58–86 |
| Mean | 78 | 76 | |
| SD | 9.65 | 8.35 | |
| Complications | Re-ruptures | 2 | |
| SN lesions | 3 | ||
| Infections | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paczesny, Ł.; Zabrzyński, J.; Domżalski, M.; Gagat, M.; Termanowski, M.; Szwedowski, D.; Łapaj, Ł.; Kruczyński, J. Mini-Invasive, Ultrasound Guided Repair of the Achilles Tendon Rupture—A Pilot Study. J. Clin. Med. 2021, 10, 2370. https://doi.org/10.3390/jcm10112370
Paczesny Ł, Zabrzyński J, Domżalski M, Gagat M, Termanowski M, Szwedowski D, Łapaj Ł, Kruczyński J. Mini-Invasive, Ultrasound Guided Repair of the Achilles Tendon Rupture—A Pilot Study. Journal of Clinical Medicine. 2021; 10(11):2370. https://doi.org/10.3390/jcm10112370
Chicago/Turabian StylePaczesny, Łukasz, Jan Zabrzyński, Marcin Domżalski, Maciej Gagat, Miron Termanowski, Dawid Szwedowski, Łukasz Łapaj, and Jacek Kruczyński. 2021. "Mini-Invasive, Ultrasound Guided Repair of the Achilles Tendon Rupture—A Pilot Study" Journal of Clinical Medicine 10, no. 11: 2370. https://doi.org/10.3390/jcm10112370
APA StylePaczesny, Ł., Zabrzyński, J., Domżalski, M., Gagat, M., Termanowski, M., Szwedowski, D., Łapaj, Ł., & Kruczyński, J. (2021). Mini-Invasive, Ultrasound Guided Repair of the Achilles Tendon Rupture—A Pilot Study. Journal of Clinical Medicine, 10(11), 2370. https://doi.org/10.3390/jcm10112370







