Impact of Cardiovascular Risk Factors on the Occurrence of Cardiovascular Events in Antineutrophil Cytoplasmic Antibody (ANCA)-Associated Vasculitides
Abstract
Significance and Innovations
- Despite improvement in the prognosis of ANCA-associated vasculitides (AAVs), increased mortality, mainly from a cardiovascular origin, persists. This 3-year cohort study revealed that the occurrence of MACEs (major cardiovascular events) in AAVs was associated with older age, the presence of a history of CVD (cardiovascular diseases), dyslipidemia, hypertension, and a sedentary lifestyle.
- The implementation of a screening and management program for modifiable CVRFs (cardiovascular risk factors), particularly hypertension, a sedentary lifestyle, and dyslipidemia, may be beneficial for AAV patients in order to reduce their cardiovascular risk.
- While a tight control of AAV inflammation is required to prevent CVD, traditional CVRFs should not be overlooked. The specific management of cardiovascular risk should combine the control of AAV disease activity and traditional CVRFs.
1. Introduction
2. Methods
2.1. Study Population and Setting
2.2. Data Collection at Baseline
2.3. Outcomes and Follow-Up
2.4. Statistics
3. Results
3.1. Study Population
3.2. Outcomes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walton, E.W. Giant-Cell Granuloma of the Respiratory Tract (Wegener’s Granulomatosis). Br. Med. J. 1958, 2, 265–270. [Google Scholar] [CrossRef]
- Eriksson, P.; Jacobsson, L.; Lindell, Å.; Nilsson, J.-Å.; Skogh, T. Improved Outcome in Wegener’s Granulomatosis and Microscopic Polyangiitis? A Retrospective Analysis of 95 Cases in Two Cohorts. J. Intern. Med. 2009, 265, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.A.; Dehghan, N.; Chen, W.; Xie, H.; Esdaile, J.M.; Avina-Zubieta, J.A. Mortality in ANCA-Associated Vasculitis: A Meta-Analysis of Observational Studies. Ann. Rheum. Dis. 2017, 76, 1566–1574. [Google Scholar] [CrossRef]
- Tan, J.A.; Choi, H.K.; Xie, H.; Sayre, E.C.; Esdaile, J.M.; Aviña-Zubieta, J.A. All-Cause and Cause-Specific Mortality in Patients with Granulomatosis with Polyangiitis: A Population-Based Study. Arthritis Care Res. 2019, 71, 155–163. [Google Scholar] [CrossRef]
- Wallace, Z.S.; Lu, N.; Miloslavsky, E.; Unizony, S.; Stone, J.H.; Choi, H.K. Nationwide Trends in Hospitalizations and In-Hospital Mortality in Granulomatosis with Polyangiitis (Wegener’s). Arthritis Care Res. 2017, 69, 915–921. [Google Scholar] [CrossRef]
- Seo, P.; Min, Y.-I.; Holbrook, J.T.; Hoffman, G.S.; Merkel, P.A.; Spiera, R.; Davis, J.C.; Ytterberg, S.R.; Clair, E.W.S.; McCune, W.J.; et al. Damage Caused by Wegener’s Granulomatosis and Its Treatment: Prospective Data from the Wegener’s Granulomatosis Etanercept Trial (WGET). Arthritis Rheum. 2005, 52, 2168–2178. [Google Scholar] [CrossRef] [PubMed]
- Faurschou, M.; Ahlström, M.G.; Lindhardsen, J.; Baslund, B.; Obel, N. Impact of Pre-Existing Co-Morbidities on Mortality in Granulomatosis with Polyangiitis: A Cohort Study. Rheumatology 2016, 55, 649–653. [Google Scholar] [CrossRef][Green Version]
- Hissaria, P.; Cai, F.Z.J.; Ahern, M.; Smith, M.; Gillis, D.; Roberts-Thomson, P. Wegener’s Granulomatosis: Epidemiological and Clinical Features in a South Australian Study. Intern. Med. J. 2008, 38, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Suppiah, R.; Judge, A.; Batra, R.; Flossmann, O.; Harper, L.; Höglund, P.; Javaid, M.K.; Jayne, D.; Mukhtyar, C.; Westman, K.; et al. A Model to Predict Cardiovascular Events in Patients with Newly Diagnosed Wegener’s Granulomatosis and Microscopic Polyangiitis. Arthritis Care Res. 2011, 63, 588–596. [Google Scholar] [CrossRef]
- Lai, Q.; Ma, T.-T.; Li, Z.; Chang, D.; Zhao, M.-H.; Chen, M. Predictors for Mortality in Patients with Antineutrophil Cytoplasmic Autoantibody-Associated Vasculitis: A Study of 398 Chinese Patients. J. Rheumatol. 2014, 41, 1849–1855. [Google Scholar] [CrossRef]
- Flossmann, O.; Berden, A.; de Groot, K.; Hagen, C.; Harper, L.; Heijl, C.; Höglund, P.; Jayne, D.; Luqmani, R.; Mahr, A.; et al. Long-Term Patient Survival in ANCA-Associated Vasculitis. Ann. Rheum. Dis. 2011, 70, 488–494. [Google Scholar] [CrossRef]
- Mercuzot, C.; Letertre, S.; Daien, C.I.; Zerkowski, L.; Guilpain, P.; Terrier, B.; Fesler, P.; Roubille, C. Comorbidities and Health-Related Quality of Life in Patients with Antineutrophil Cytoplasmic Antibody (ANCA)—Associated Vasculitis. Autoimmun. Rev. 2021, 20, 102708. [Google Scholar] [CrossRef]
- Mourguet, M.; Chauveau, D.; Faguer, S.; Ruidavets, J.B.; Béjot, Y.; Ribes, D.; Huart, A.; Alric, L.; Balardy, L.; Astudillo, L.; et al. Increased Ischemic Stroke, Acute Coronary Artery Disease and Mortality in Patients with Granulomatosis with Polyangiitis and Microscopic Polyangiitis. J. Autoimmun. 2019, 96, 134–141. [Google Scholar] [CrossRef]
- Faurschou, M.; Mellemkjaer, L.; Sorensen, I.J.; Thomsen, B.S.; Dreyer, L.; Baslund, B. Increased Morbidity from Ischemic Heart Disease in Patients with Wegener’s Granulomatosis. Arthritis Rheum. 2009, 60, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Zöller, B.; Li, X.; Sundquist, J.; Sundquist, K. Risk of Subsequent Coronary Heart Disease in Patients Hospitalized for Immune-Mediated Diseases: A Nationwide Follow-Up Study from Sweden. PLoS ONE 2012, 7, e33442. [Google Scholar] [CrossRef] [PubMed]
- Aviña-Zubieta, J.A.; Mai, A.; Amiri, N.; Dehghan, N.; Tan, J.A.; Sayre, E.C.; Choi, H.K. Risk of Myocardial Infarction and Stroke in Patients with Granulomatosis With Polyangiitis (Wegener’s): A Population-Based Study. Arthritis Rheumatol. 2016, 68, 2752–2759. [Google Scholar] [CrossRef] [PubMed]
- Houben, E.; Penne, E.L.; Voskuyl, A.E.; van der Heijden, J.W.; Otten, R.H.J.; Boers, M.; Hoekstra, T. Cardiovascular Events in Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis: A Meta-Analysis of Observational Studies. Rheumatology 2018, 57, 555–562. [Google Scholar] [CrossRef]
- Berti, A.; Matteson, E.L.; Crowson, C.S.; Specks, U.; Cornec, D. Risk of Cardiovascular Disease and Venous Thromboembolism Among Patients With Incident ANCA-Associated Vasculitis: A 20 Year Population-Based Cohort Study. Mayo Clin. Proc. 2018, 93, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Frangou, E.; Vassilopoulos, D.; Boletis, J.; Boumpas, D.T. An Emerging Role of Neutrophils and NETosis in Chronic Inflammation and Fibrosis in Systemic Lupus Erythematosus (SLE) and ANCA-Associated Vasculitides (AAV): Implications for the Pathogenesis and Treatment. Autoimmun. Rev. 2019, 18, 751–760. [Google Scholar] [CrossRef]
- Hajj-Ali, R.; Major, J.; Langford, C.; Hoffman, G.; Clark, T.; Zhang, L.; Sun, Z.; Silverstein, R. The Interface of Inflammation and Subclinical Atherosclerosis in Granulomatosis with Polyangiitis (Wegener’s): A Preliminary Study. Transl. Res. J. Lab. Clin. Med. 2015, 166, 366–374. [Google Scholar] [CrossRef]
- Pacholczak, R.; Bazan-Socha, S.; Iwaniec, T.; Zaręba, L.; Kielczewski, S.; Walocha, J.A.; Musiał, J.; Dropiński, J. Endothelial Dysfunction in Patients with Granulomatosis with Polyangiitis: A Case–Control Study. Rheumatol. Int. 2018, 38, 1521–1530. [Google Scholar] [CrossRef]
- Pacholczak, R.; Bazan-Socha, S.; Iwaniec, T.; Zaręba, L.; Kielczewski, S.; Walocha, J.A.; Musiał, J.; Dropiński, J. Endothelial Dysfunction in Patients with Eosinophilic Granulomatosis with Polyangiitis. Clin. Rheumatol. 2019, 38, 417–424. [Google Scholar] [CrossRef] [PubMed]
- de Leeuw, K.; Sanders, J.-S.; Stegeman, C.; Smit, A.; Kallenberg, C.G.; Bijl, M. Accelerated Atherosclerosis in Patients with Wegener’s Granulomatosis. Ann. Rheum. Dis. 2005, 64, 753–759. [Google Scholar] [CrossRef] [PubMed]
- de Leeuw, K.; Bijzet, J.; van der Graaf, A.M.; Stegeman, C.A.; Smit, A.J.; Kallenberg, C.G.; Bijl, M. Patients with Wegener’s Granulomatosis: A Long-Term Follow-up Study. Clin. Exp. Rheumatol. 2010, 28, 18–23. [Google Scholar]
- Wei, L.; MacDonald, T.M.; Walker, B.R. Taking Glucocorticoids by Prescription Is Associated with Subsequent Cardiovascular Disease. Ann. Intern. Med. 2004, 141, 764–770. [Google Scholar] [CrossRef]
- Terrier, B.; Chironi, G.; Pagnoux, C.; Cohen, P.; Puéchal, X.; Simon, A.; Mouthon, L.; Guillevin, L. Factors Associated with Major Cardiovascular Events in Patients with Systemic Necrotizing Vasculitides: Results of a Longterm Followup Study. J. Rheumatol. 2014, 41, 723–729. [Google Scholar] [CrossRef]
- Kang, A.; Antonelou, M.; Wong, N.L.; Tanna, A.; Arulkumaran, N.; Tam, F.W.K.; Pusey, C.D. High Incidence of Arterial and Venous Thrombosis in Antineutrophil Cytoplasmic Antibody–Associated Vasculitis. J. Rheumatol. 2019, 46, 285–293. [Google Scholar] [CrossRef]
- Briot, K.; Dunogué, B.; Henriquez, S.; Etcheto, A.; Kolta, S.; Régent, A.; Cohen, P.; Berezne, A.; Le Jeunne, C.; Mouthon, L.; et al. Abdominal Adipose Tissue Predicts Major Cardiovascular Events in Systemic Necrotising Vasculitides. Clin. Exp. Rheumatol. 2019, 37 (Suppl. 117), 130–136. [Google Scholar] [PubMed]
- Leavitt, R.Y.; Fauci, A.S.; Bloch, D.A.; Michel, B.A.; Hunder, G.G.; Arend, W.P.; Calabrese, L.H.; Fries, J.F.; Lie, J.T.; Lightfoot, R.W. The American College of Rheumatology 1990 Criteria for the Classification of Wegener’s Granulomatosis. Arthritis Rheum. 1990, 33, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Masi, A.T.; Hunder, G.G.; Lie, J.T.; Michel, B.A.; Bloch, D.A.; Arend, W.P.; Calabrese, L.H.; Edworthy, S.M.; Fauci, A.S.; Leavitt, R.Y. The American College of Rheumatology 1990 Criteria for the Classification of Churg-Strauss Syndrome (Allergic Granulomatosis and Angiitis). Arthritis Rheum. 1990, 33, 1094–1100. [Google Scholar] [CrossRef]
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mukhtyar, C.; Lee, R.; Brown, D.; Carruthers, D.; Dasgupta, B.; Dubey, S.; Flossmann, O.; Hall, C.; Hollywood, J.; Jayne, D.; et al. Modification and Validation of the Birmingham Vasculitis Activity Score (Version 3). Ann. Rheum. Dis. 2009, 68, 1827–1832. [Google Scholar] [CrossRef] [PubMed]
- Exley, A.R.; Bacon, P.A.; Luqmani, R.A.; Kitas, G.D.; Gordon, C.; Savage, C.O.; Adu, D. Development and Initial Validation of the Vasculitis Damage Index for the Standardized Clinical Assessment of Damage in the Systemic Vasculitides. Arthritis Rheum. 1997, 40, 371–380. [Google Scholar] [CrossRef]
- Fries, J.F.; Spitz, P.; Kraines, R.G.; Holman, H.R. Measurement of Patient Outcome in Arthritis. Arthritis Rheum. 1980, 23, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E.; Gandek, B. Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. J. Clin. Epidemiol. 1998, 51, 903–912. [Google Scholar] [CrossRef]
- Iqubal, A.; Iqubal, M.K.; Sharma, S.; Ansari, M.A.; Najmi, A.K.; Ali, S.M.; Ali, J.; Haque, S.E. Molecular Mechanism Involved in Cyclophosphamide-Induced Cardiotoxicity: Old Drug with a New Vision. Life Sci. 2019, 218, 112–131. [Google Scholar] [CrossRef]
- Haris, Á.; Polner, K.; Arányi, J.; Braunitzer, H.; Kaszás, I.; Mucsi, I. Clinical Outcomes of ANCA-Associated Vasculitis in Elderly Patients. Int. Urol. Nephrol. 2014, 46, 1595–1600. [Google Scholar] [CrossRef]
- Li, L.; Neogi, T.; Jick, S. A Cohort Study of Comorbidity in Patients with Granulomatosis with Polyangiitis. Rheumatol. Oxf. Engl. 2018, 57, 291–299. [Google Scholar] [CrossRef]
- Englund, M.; Merkel, P.A.; Tomasson, G.; Segelmark, M.; Mohammad, A.J. Comorbidities in Patients with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis versus the General Population. J. Rheumatol. 2016, 43, 1553–1558. [Google Scholar] [CrossRef]
- Wallace, Z.S.; Fu, X.; Liao, K.; Kallenberg, C.G.M.; Langford, C.A.; Merkel, P.A.; Monach, P.; Seo, P.; Specks, U.; Spiera, R.; et al. Disease Activity, Antineutrophil Cytoplasmic Antibody Type, and Lipid Levels in Antineutrophil Cytoplasmic Antibody–Associated Vasculitis. Arthritis Rheumatol. 2019, 71, 1879–1887. [Google Scholar] [CrossRef]
- Neeland, I.J.; De Lemos, J.A. Time to Retire the BMI? J. Am. Coll. Cardiol. 2016, 68, 1522–1524. [Google Scholar] [CrossRef]
- Wildman, R.P.; Muntner, P.; Reynolds, K.; McGinn, A.P.; Rajpathak, S.; Wylie-Rosett, J.; Sowers, M.R. The Obese Without Cardiometabolic Risk Factor Clustering and the Normal Weight With Cardiometabolic Risk Factor Clustering. Arch. Intern. Med. 2008, 168, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Neeland, I.J.; Turer, A.T.; Ayers, C.R.; Berry, J.D.; Rohatgi, A.; Das, S.R.; Khera, A.; Vega, G.L.; McGuire, D.K.; Grundy, S.M.; et al. Body Fat Distribution and Incident Cardiovascular Disease in Obese Adults. J. Am. Coll. Cardiol. 2015, 65, 2150–2151. [Google Scholar] [CrossRef] [PubMed]
- Roubille, C.; Richer, V.; Starnino, T.; McCourt, C.; McFarlane, A.; Fleming, P.; Siu, S.; Kraft, J.; Lynde, C.; Pope, J.; et al. The Effects of Tumour Necrosis Factor Inhibitors, Methotrexate, Non-Steroidal Anti-Inflammatory Drugs and Corticosteroids on Cardiovascular Events in Rheumatoid Arthritis, Psoriasis and Psoriatic Arthritis: A Systematic Review and Meta-Analysis. Ann. Rheum. Dis. 2015, 74, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.; Merkel, P.A.; Mahr, A.; Jayne, D. The Effects of Duration of Glucocorticoid Therapy on Relapse Rate in Anti-Neutrophil Cytoplasm Antibody Associated Vasculitis: A Meta-Analysis. Arthritis Care Res. 2010, 62, 1166–1173. [Google Scholar] [CrossRef]
- Yusuf, S.; Hawken, S.; Ounpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of Potentially Modifiable Risk Factors Associated with Myocardial Infarction in 52 Countries (the INTERHEART Study): Case-Control Study. Lancet Lond. Engl. 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Roubille, F.; Kritikou, E.A.; Roubille, C.; Tardif, J.-C. Emerging Anti-Inflammatory Therapies for Atherosclerosis. Curr. Pharm. Des. 2013, 19, 5840–5849. [Google Scholar] [CrossRef]
- Bramlage, C.P.; Kröplin, J.; Wallbach, M.; Minguet, J.; Smith, K.H.; Lüders, S.; Schrader, J.; Patschan, S.; Gross, O.; Deutsch, C.; et al. Management of Cardiovascular Risk Factors in Patients with ANCA-Associated Vasculitis. J. Eval. Clin. Pract. 2017, 23, 747–754. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018, 36, 1953–2041. [Google Scholar] [CrossRef] [PubMed]
- Di Nora, C.; Cioffi, G.; Iorio, A.; Rivetti, L.; Poli, S.; Zambon, E.; Barbati, G.; Sinagra, G.; Di Lenarda, A. Systolic blood pressure target in systemic arterial hypertension: Is lower ever better? Results from a community-based Caucasian cohort. Eur. J. Intern. Med. 2018, 48, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Houben, E.; Mendel, A.; van der Heijden, J.W.; Simsek, S.; Bax, W.A.; Carette, S.; Voskuyl, A.E.; Pagnoux, C.; Penne, E.L. Prevalence and Management of Cardiovascular Risk Factors in ANCA-Associated Vasculitis. Rheumatology 2019, 58, 2333–2335. [Google Scholar] [CrossRef] [PubMed]
- Harper, L.; Morgan, M.D.; Chanouzas, D.; Caulfield, H.K.; Coughlan, L.; Dean, C.; Fletcher, K.; Cramp, F.; Greenfield, S.; Hewitt, C.A.; et al. Treatment of Fatigue with Physical Activity and Behavioural Change Support in Vasculitis: Study Protocol for an Open-Label Randomised Controlled Feasibility Study. BMJ Open 2018, 8, e023769. [Google Scholar] [CrossRef] [PubMed]
- Avina-Zubieta, J.A.; Thomas, J.; Sadatsafavi, M.; Lehman, A.J.; Lacaille, D. Risk of Incident Cardiovascular Events in Patients with Rheumatoid Arthritis: A Meta-Analysis of Observational Studies. Ann. Rheum. Dis. 2012, 71, 1524–1529. [Google Scholar] [CrossRef] [PubMed]
- Meune, C.; Touzé, E.; Trinquart, L.; Allanore, Y. Trends in Cardiovascular Mortality in Patients with Rheumatoid Arthritis over 50 Years: A Systematic Review and Meta-Analysis of Cohort Studies. Rheumatology 2009, 48, 1309–1313. [Google Scholar] [CrossRef] [PubMed]
- Burggraaf, B.; van Breukelen-van der Stoep, D.F.; de Vries, M.A.; Klop, B.; van Zeben, J.; van de Geijn, G.-J.M.; van der Meulen, N.; Birnie, E.; Prinzen, L.; Castro Cabezas, M. Progression of Subclinical Atherosclerosis in Subjects with Rheumatoid Arthritis and the Metabolic Syndrome. Atherosclerosis 2018, 271, 84–91. [Google Scholar] [CrossRef]
| N | Total Study Population (n = 103) | |
|---|---|---|
| Demographics and clinical parameters | ||
| Age (years) (mean SD) | 103 | 52.88 ± 17.40 |
| Male, n (%) | 103 | 46 (44.66) |
| Cardiovascular risk factors | ||
| Older age (>50 years for men, >60 years for women), n (%) | 103 | 46 (44.66) |
| BMI (kg/m2) (mean SD) | 103 | 25.34 ± 4.87 |
| BMI > 30 kg/m2, n (%) | 103 | 17 (16.5) |
| Diabetes mellitus, n (%) | 103 | 7 (6.8) |
| Hypertension, n (%) | 103 | 54 (52.4) |
| Ever smokers, n (%) | 103 | 43 (41.8) |
| History of CVD, n (%) | 103 | 11 (10.7) |
| Dyslipidemia, n (%) | 103 | 19 (18.5) |
| Sedentary lifestyle (yes), n (%) | 103 | 20 (19.4) |
| Comorbidities and health-related scores | ||
| Osteoporosis, n (%) | 103 | 28 (27.2) |
| SF-36 score (mean SD) | 53 | |
| Physical score | 41.69 ± 10.21 | |
| Mental score | 42.10 ± 9.86 | |
| Vasculitis characteristics | ||
| Disease duration (months) (median, IQR) | 103 | 54.05 (11.11; 99.14) |
| MPO-ANCA, n (%) | 103 | 25 (24.3) |
| PR3-ANCA, n (%) | 103 | 44 (42.7) |
| BVAS score (mean SD) | 103 | 4.50 ± 8.31 |
| VDI score (mean SD) | 103 | 2.30 ± 2.05 |
| HAQ score (mean SD) | 60 | 0.31 ± 0.45 |
| Treatments | ||
| Use of GC, n (%) | 103 | 86 (83.50) |
| Daily current dose of GC, (mg) (median IQR) | 86 | 12 (5; 30) |
| Cumulative dose of GC, (g) (median IQR) | 100 | 11.26 (6.00; 21.42) |
| Current immunosuppressive agents, n (%) | 103 | 77 (74.76%) |
| Aspirin, n (%) | 103 | 17 (16.5) |
| Statins, n (%) | 103 | 16 (15.53) |
| Anti-hypertensive agents, n (%) | 103 | 39 (37.86) |
| Anti-diabetics, n (%) | 103 | 7 (6.80) |
| Biological characteristics | ||
| CRP (mg/L) (mean SD) | 99 | 9.15 ± 18.59 |
| Hb1Ac (mean SD) | 92 | 5.66 ± 0.75 |
| LDL cholesterol (mean SD) | 94 | 1.19 ± 0.45 |
| HDL cholesterol (mean SD) | 96 | 0.74 ± 0.29 |
| Ratio proteinuria/creatininuria (mean SD) | 96 | 29.98 ± 65.99 |
| Number of CVRFs | Number of Patients without MACEs | Number of Patients in Whom ≥1 MACE Occurred, n (%) |
|---|---|---|
| 0 | 20 | 1 (4.8%) |
| 1 | 21 | 1 (4.5%) |
| 2 | 28 | 2 (6.7%) |
| ≥ 3 | 18 | 12 (40%) |
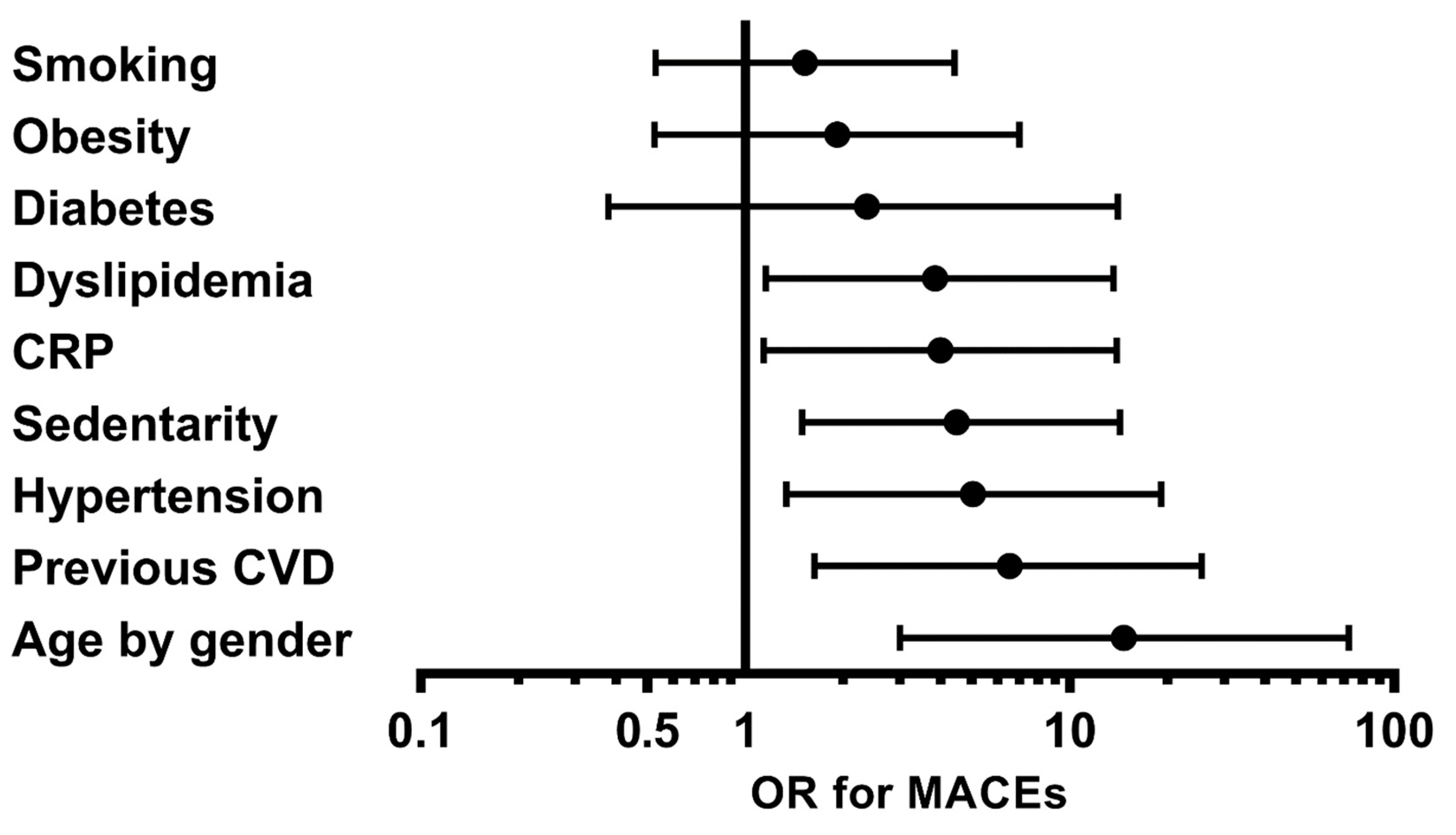
| Cardiovascular Risk Factor (CVRF) | Patients Who Had MACEs among Those with This CVRF, n (%) | Patients Who Had MACEs among Those without This CVRF, n (%) | p-Value | OR (95% CI) |
|---|---|---|---|---|
| Older age (>50 for men; >60 for women) | 14 (30.4%) | 2 (3.5%) | 0.001 | 14.71 (2.98–72.68) |
| Personal medical history of CVD | 5 (45.5%) | 11 (12%) | 0.007 | 6.54 (1.66–25.71) |
| Sedentary lifestyle | 7 (35%) | 9 (10.8%) | 0.011 | 4.50 (1.42–14.29) |
| Hypertension | 13 (24.1%) | 3 (6.1%) | 0.017 | 5.04 (1.33–19.12) |
| Dyslipidemia | 6 (31.6%) | 10 (11.9%) | 0.03 | 3.86 (1.14–13.09) |
| Obesity | 4 (23.5%) | 12 (14%) | 0.32 | 1.93 (0.53–7.00) |
| Diabetes mellitus | 2 (28.6%) | 14 (14.6%) | 0.34 | 2.38 (0.40–14.06) |
| Ever smoker | 8 (18.6%) | 8 (13.3%) | 0.44 | 1.53 (0.52–4.47) |
| OR (95% CI) | p | |
|---|---|---|
| Number of cardiovascular risk factors | 1.74 (1.28–2.37) | <0.001 |
| Use of glucocorticoids | 1.16 (0.50–2.72) | 0.733 |
| Use of cyclophosphamide | 0.56 (0.17–1.85) | 0.339 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roubille, C.; Henriquez, S.; Mercuzot, C.; Duflos, C.; Dunogue, B.; Briot, K.; Guillevin, L.; Terrier, B.; Fesler, P. Impact of Cardiovascular Risk Factors on the Occurrence of Cardiovascular Events in Antineutrophil Cytoplasmic Antibody (ANCA)-Associated Vasculitides. J. Clin. Med. 2021, 10, 2299. https://doi.org/10.3390/jcm10112299
Roubille C, Henriquez S, Mercuzot C, Duflos C, Dunogue B, Briot K, Guillevin L, Terrier B, Fesler P. Impact of Cardiovascular Risk Factors on the Occurrence of Cardiovascular Events in Antineutrophil Cytoplasmic Antibody (ANCA)-Associated Vasculitides. Journal of Clinical Medicine. 2021; 10(11):2299. https://doi.org/10.3390/jcm10112299
Chicago/Turabian StyleRoubille, Camille, Soledad Henriquez, Cédric Mercuzot, Claire Duflos, Bertrand Dunogue, Karine Briot, Loic Guillevin, Benjamin Terrier, and Pierre Fesler. 2021. "Impact of Cardiovascular Risk Factors on the Occurrence of Cardiovascular Events in Antineutrophil Cytoplasmic Antibody (ANCA)-Associated Vasculitides" Journal of Clinical Medicine 10, no. 11: 2299. https://doi.org/10.3390/jcm10112299
APA StyleRoubille, C., Henriquez, S., Mercuzot, C., Duflos, C., Dunogue, B., Briot, K., Guillevin, L., Terrier, B., & Fesler, P. (2021). Impact of Cardiovascular Risk Factors on the Occurrence of Cardiovascular Events in Antineutrophil Cytoplasmic Antibody (ANCA)-Associated Vasculitides. Journal of Clinical Medicine, 10(11), 2299. https://doi.org/10.3390/jcm10112299






