Gene Expression as a Guide to the Development of Novel Therapies in Primary Glomerular Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Functional Enrichment Analysis and Drug Prediction
3. Results
3.1. Computational Systems Biology Approaches Reveal Glomerulonephritis-Specific Gene Signatures
3.2. Unique Biological Processes and Pathways Are Implicated in Distinct Primary Glomerular Diseases
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ponticelli, C.; Glassock, R.J. A definition, modern classification, and global epidemiology of primary glomerulonephritis. In Treatment of Primary Glomerulonephritis; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Floege, J.; Amann, K. Primary glomerulonephritides. Lancet 2016, 387, 2036–2048. [Google Scholar] [CrossRef]
- Floege, J.; Barbour, S.J.; Cattran, D.C.; Hogan, J.J.; Nachman, P.H.; Tang, S.C.W.; Wetzels, J.F.M.; Cheung, M.; Wheeler, D.C.; Winkelmayer, W.C.; et al. Management and treatment of glomerular diseases (part 1): Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019, 95, 268–280. [Google Scholar] [CrossRef]
- Rovin, B.H.; Caster, D.J.; Cattran, D.C.; Gibson, K.L.; Hogan, J.J.; Moeller, M.J.; Roccatello, D.; Cheung, M.; Wheeler, D.C.; Winkelmayer, W.C.; et al. Management and treatment of glomerular diseases (part 2): Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019, 95, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Frangou, E.A.; Bertsias, G.K.; Boumpas, D.T. Gene expression and regulation in systemic lupus erythematosus. Eur. J. Clin. Investig. 2013, 43, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Tryggvason, S.H.; Guo, J.; Nukui, M.; Norlin, J.; Haraldsson, B.; Jörnvall, H.; Tryggvason, K.; He, L. A meta-analysis of expression signatures in glomerular disease. Kidney Int. 2013, 84, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Kitano, H. Computational systems biology. Nature 2002, 420, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Lamb, J.; Crawford, E.D.; Peck, D.; Modell, J.W.; Blat, I.C.; Wrobel, M.J.; Lerner, J.; Brunet, J.-P.; Subramanian, A.; Ross, K.N.; et al. The Connectivity Map: Using Gene-Expression Signatures to Connect Small Molecules, Genes, and Disease. Science 2006, 313, 1929–1935. [Google Scholar] [CrossRef]
- Keenan, A.B.; Jenkins, S.L.; Jagodnik, K.M.; Koplev, S.; He, E.; Torre, D.; Wang, Z.; Dohlman, A.B.; Silverstein, M.C.; Lachmann, A.; et al. The Library of Integrated Network-Based Cellular Signatures NIH Program: System-Level Cataloging of Human Cells Response to Perturbations. Cell Syst. 2018, 6, 13–24. [Google Scholar] [CrossRef]
- Peyvandipour, A.; Saberian, N.; Shafi, A.; Donato, M.; Draghici, S. Systems biology: A novel computational approach for drug repurposing using systems biology. Bioinformatics 2018, 34, 2817–2825. [Google Scholar] [CrossRef]
- Duan, Q.; Reid, S.P.; Clark, N.R.; Wang, Z.; Fernandez, N.F.; Rouillard, A.D.; Readhead, B.; Tritsch, S.R.; Hodos, R.; Hafner, M.; et al. L1000CDS2: LINCS L1000 characteristic direction signatures search engine. NPJ Syst. Biol. Appl. 2016, 2, 16015. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Liu, R.; Li, Z.; Lin, J.; Wojciechowicz, M.L.; Huang, J.; Lee, K.; Ma’Ayan, A.; He, J.C. Connectivity Mapping Identifies BI-2536 as a Potential Drug to Treat Diabetic Kidney Disease. Diabetes 2021, 70, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Rowland, J.; Akbarov, A.; Eales, J.; Xu, X.; Dormer, J.; Guo, H.; Denniff, M.; Jiang, X.; Ranjzad, P.; Nazgiewicz, A.; et al. Uncovering genetic mechanisms of kidney aging through transcriptomics, genomics, and epigenomics. Kidney Int. 2019, 95, 624–635. [Google Scholar] [CrossRef]
- Oliveros, J.C. VENNY. An Interactive Tool for Comparing Lists with Venn Diagrams. Available online: http://bioinfogp.cnb.csic.es/tools/venny/index.html (accessed on 1 March 2021).
- Heberle, H.; Meirelles, G.V.; Da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 1–7. [Google Scholar] [CrossRef]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. g:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef] [PubMed]
- Motulsky, H. GraphPad Prism Version 5.0 Regression Guide; GraphPad Software Inc.: San Diego, CA, USA, 2007. [Google Scholar]
- Wang, Z.; Lachmann, A.; Keenan, A.B.; Ma’Ayan, A. L1000FWD: Fireworks visualization of drug-induced transcriptomic signatures. Bioinformatics 2018, 34, 2150–2152. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Shrivastava, S.; Hassanali, M.; Stothard, P.; Chang, Z.; Woolsey, J. DrugBank: A comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006, 34, D668–D672. [Google Scholar] [CrossRef]
- Hodgin, J.B.; Borczuk, A.C.; Nasr, S.H.; Markowitz, G.S.; Nair, V.; Martini, S.; Eichinger, F.; Vining, C.; Berthier, C.C.; Kretzler, M.; et al. A Molecular Profile of Focal Segmental Glomerulosclerosis from Formalin-Fixed, Paraffin-Embedded Tissue. Am. J. Pathol. 2010, 177, 1674–1686. [Google Scholar] [CrossRef]
- Ju, W.; Greene, C.S.; Eichinger, F.; Nair, V.; Hodgin, J.B.; Bitzer, M.; Lee, Y.-S.; Zhu, Q.; Kehata, M.; Li, M.; et al. Defining cell-type specificity at the transcriptional level in human disease. Genome Res. 2013, 23, 1862–1873. [Google Scholar] [CrossRef]
- Reich, H.N.; Tritchler, D.; Cattran, D.C.; Herzenberg, A.M.; Eichinger, F.; Boucherot, A.; Henger, A.; Berthier, C.C.; Nair, V.; Cohen, C.D.; et al. A Molecular Signature of Proteinuria in Glomerulonephritis. PLoS ONE 2010, 5, e13451. [Google Scholar] [CrossRef]
- Bian, Y.; Han, J.; Kannabiran, V.; Mohan, S.; Cheng, H.; Friedman, J.; Zhang, L.; VanWaes, C.; Chen, Z. MEK Inhibitor PD-0325901 Overcomes Resistance to CK2 Inhibitor CX-4945 and Exhibits Anti-Tumor Activity in Head and Neck Cancer. Int. J. Biol. Sci. 2015, 11, 411–422. [Google Scholar] [CrossRef]
- Delaney, A.M.; Printen, J.A.; Chen, H.; Fauman, E.B.; Dudley, D.T. Identification of a Novel Mitogen-Activated Protein Kinase Kinase Activation Domain Recognized by the Inhibitor PD 184352. Mol. Cell. Biol. 2002, 22, 7593–7602. [Google Scholar] [CrossRef]
- Radhakrishnan, J.; Cattran, D.C. The KDIGO practice guideline on glomerulonephritis: Reading between the (guide)lines—application to the individual patient. Kidney Int. 2012, 82, 840–856. [Google Scholar] [CrossRef]
- Rosenberg, A.Z.; Kopp, J.B. Focal Segmental Glomerulosclerosis. Clin. J. Am. Soc. Nephrol. 2017, 12, 502–517. [Google Scholar] [CrossRef] [PubMed]
- Maas, R.J.; Deegens, J.K.; Wetzels, J.F. Permeability factors in idiopathic nephrotic syndrome: Historical perspectives and lessons for the future. Nephrol. Dial. Transplant. 2014, 29, 2207–2216. [Google Scholar] [CrossRef] [PubMed]
- De Vriese, A.S.; Sethi, S.; Nath, K.A.; Glassock, R.J.; Fervenza, F.C. Differentiating Primary, Genetic, and Secondary FSGS in Adults: A Clinicopathologic Approach. J. Am. Soc. Nephrol. 2018, 29, 759–774. [Google Scholar] [CrossRef] [PubMed]
- Maas, R.J.; Deegens, J.K.; Smeets, B.; Moeller, M.J.; Wetzels, J.F. Minimal change disease and idiopathic FSGS: Manifestations of the same disease. Nat. Rev. Nephrol. 2016, 12, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Fogo, A.B. Causes and pathogenesis of focal segmental glomerulosclerosis. Nat. Rev. Nephrol. 2015, 11, 76–87. [Google Scholar] [CrossRef]
- McCarthy, E.T.; Sharma, M.; Savin, V.J. Circulating Permeability Factors in Idiopathic Nephrotic Syndrome and Focal Segmental Glomerulosclerosis. Clin. J. Am. Soc. Nephrol. 2010, 5, 2115–2121. [Google Scholar] [CrossRef]
- Wei, C.; El Hindi, S.; Li, J.; Fornoni, A.; Goes, N.; Sageshima, J.; Maiguel, D.; Karumanchi, S.A.; Yap, H.-K.; Saleem, M.; et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat. Med. 2011, 17, 952–960. [Google Scholar] [CrossRef]
- Audard, V.; Zhang, S.-Y.; Copie-Bergman, C.; Rucker-Martin, C.; Ory, V.; Candelier, M.; Baia, M.; Lang, P.; Pawlak, A.; Sahali, D. Occurrence of minimal change nephrotic syndrome in classical Hodgkin lymphoma is closely related to the induction of c-mip in Hodgkin-Reed Sternberg cells and podocytes. Blood 2010, 115, 3756–3762. [Google Scholar] [CrossRef]
- Shalhoub, R. Pathogenesis of Lipoid Nephrosis: A Disorder of T-Cell Function. Lancet 1974, 304, 556–560. [Google Scholar] [CrossRef]
- Vivarelli, M.; Massella, L.; Ruggiero, B.; Emma, F. Minimal Change Disease. Clin. J. Am. Soc. Nephrol. 2017, 12, 332–345. [Google Scholar] [CrossRef]
- Audard, V.; Pawlak, A.; Candelier, M.; Lang, P.; Sahali, D. Upregulation of Nuclear Factor-Related Kappa B Suggests a Disorder of Transcriptional Regulation in Minimal Change Nephrotic Syndrome. PLoS ONE 2012, 7, e30523. [Google Scholar] [CrossRef] [PubMed]
- Aviles, D.H.; Vehaskari, V.M.; Manning, J.; Ochoa, A.C.; Zea, A.H. Decreased expression of T-cell NF-κB p65 subunit in steroid-resistant nephrotic syndrome. Kidney Int. 2004, 66, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.N.; Tang, S.C.W.; Schena, F.P.; Novak, J.; Tomino, Y.; Fogo, A.B.; Glassock, R.J. IgA nephropathy. Nat. Rev. Dis. Prim. 2016, 2, 16001. [Google Scholar] [CrossRef]
- Gutiérrez, E.; Carvaca-Fontán, F.; Luzardo, L.; Morales, E.; Alonso, M.; Praga, M. A Personalized Update on IgA Nephropathy: A New Vision and New Future Challenges. Nephron 2020, 144, 555–571. [Google Scholar] [CrossRef]
- Ronco, P.; Debiec, H. Molecular Pathogenesis of Membranous Nephropathy. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 287–313. [Google Scholar] [CrossRef]
- Behnert, A.; Fritzler, M.J.; Teng, B.; Zhang, M.; Bollig, F.; Haller, H.; Škoberne, A.; Mahler, M.; Schiffer, M. An Anti-Phospholipase A2 Receptor Quantitative Immunoassay and Epitope Analysis in Membranous Nephropathy Reveals Different Antigenic Domains of the Receptor. PLoS ONE 2013, 8, e61669. [Google Scholar] [CrossRef]
- Tomas, N.M.; Beck, L.H.; Meyer-Schwesinger, C.; Seitz-Polski, B.; Ma, H.; Zahner, G.; Dolla, G.; Hoxha, E.; Helmchen, U.; Dabert-Gay, A.-S.; et al. Thrombospondin Type-1 Domain-Containing 7A in Idiopathic Membranous Nephropathy. N. Engl. J. Med. 2014, 371, 2277–2287. [Google Scholar] [CrossRef]
- Debiec, H.; Guigonis, V.; Mougenot, B.; Decobert, F.; Haymann, J.-P.; Bensman, A.; Deschênes, G.; Ronco, P.M. Antenatal Membranous Glomerulonephritis Due to Anti–Neutral Endopeptidase Antibodies. N. Engl. J. Med. 2002, 346, 2053–2060. [Google Scholar] [CrossRef]
- Cybulsky, A.V.; Rennke, H.G.; Feintzeig, I.D.; Salant, D. Complement-induced glomerular epithelial cell injury. Role of the membrane attack complex in rat membranous nephropathy. J. Clin. Investig. 1986, 77, 1096–1107. [Google Scholar] [CrossRef]
- Reinhard, L.; Stahl, R.A.; Hoxha, E. Is primary membranous nephropathy a complement mediated disease? Mol. Immunol. 2020, 128, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Motavalli, R.; Etemadi, J.; Soltani-Zangbar, M.S.; Ardalan, M.-R.; Kahroba, H.; Roshangar, L.; Nouri, M.; Aghebati-Maleki, L.; Khiavi, F.M.; Abediazar, S.; et al. Altered Th17/Treg ratio as a possible mechanism in pathogenesis of idiopathic membranous nephropathy. Cytokine 2021, 141, 155452. [Google Scholar] [CrossRef] [PubMed]
- Rana, K.; Yan, Y.W.; Buzza, M.; Tonna, S.; Ke, W.Z.; Lin, T.; Sin, L.; Padavarat, S.; Savige, J. The Genetics of Thin Basement Membrane Nephropathy. Semin. Nephrol. 2005, 25, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Massaro, F.; Molica, M.; Breccia, M. Ponatinib: A Review of Efficacy and Safety. Curr. Cancer Drug Targets 2017, 18, 847–856. [Google Scholar] [CrossRef]
- Nishiya, N. JAK3 inhibitor VI is a mutant specific inhibitor for epidermal growth factor receptor with the gatekeeper mutation T790M. World J. Biol. Chem. 2015, 6, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, N.; Liu, B.; Ling, J.; Yang, W.; Pang, X.; Li, T. Pracinostat (SB939), a histone deacetylase inhibitor, suppresses breast cancer metastasis and growth by inactivating the IL-6/STAT3 signalling pathways. Life Sci. 2020, 248, 117469. [Google Scholar] [CrossRef]
- Carboni, J.M.; Wittman, M.; Yang, Z.; Lee, F.; Greer, A.; Hurlburt, W.; Hillerman, S.; Cao, C.; Cantor, G.H.; Dell-John, J.; et al. BMS-754807, a small molecule inhibitor of insulin-like growth factor-1R/IR. Mol. Cancer Ther. 2009, 8, 3341–3349. [Google Scholar] [CrossRef]
- Nan, J.; Du, Y.; Chen, X.; Bai, Q.; Wang, Y.; Zhang, X.; Zhu, N.; Zhang, J.; Hou, J.; Wang, Q.; et al. TPCA-1 Is a Direct Dual Inhibitor of STAT3 and NF-κB and Regresses Mutant EGFR-Associated Human Non–Small Cell Lung Cancers. Mol. Cancer Ther. 2014, 13, 617–629. [Google Scholar] [CrossRef]
- Frangou, E.; Chrysanthopoulou, A.; Mitsios, A.; Kambas, K.; Arelaki, S.; Angelidou, I.; Arampatzioglou, A.; Gakiopoulou, H.; Bertsias, G.K.; Verginis, P.; et al. REDD1/autophagy pathway promotes thromboinflammation and fibrosis in human systemic lupus erythematosus (SLE) through NETs decorated with tissue factor (TF) and interleukin-17A (IL-17A). Ann. Rheum. Dis. 2019, 78, 238–248. [Google Scholar] [CrossRef]
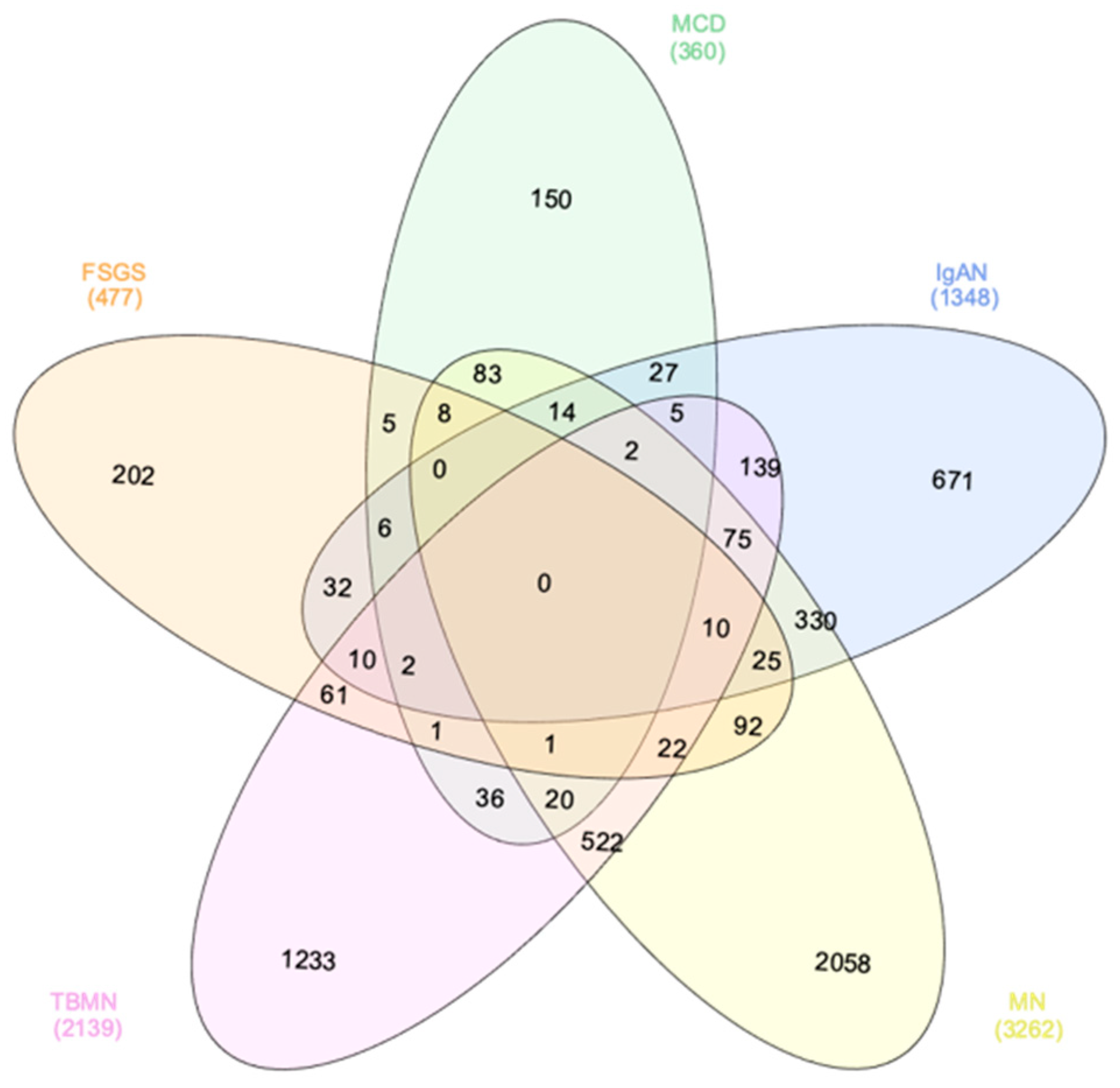
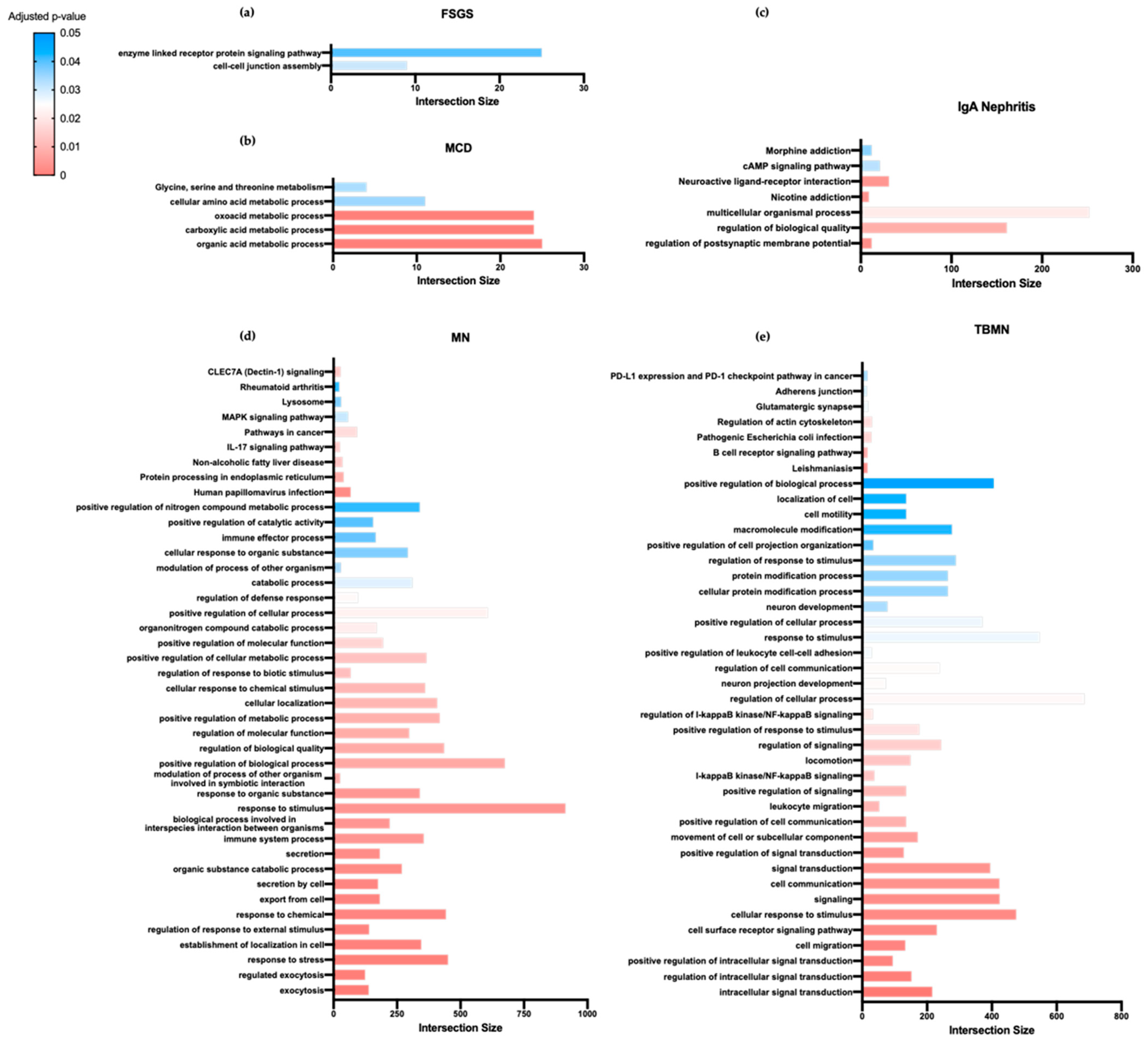
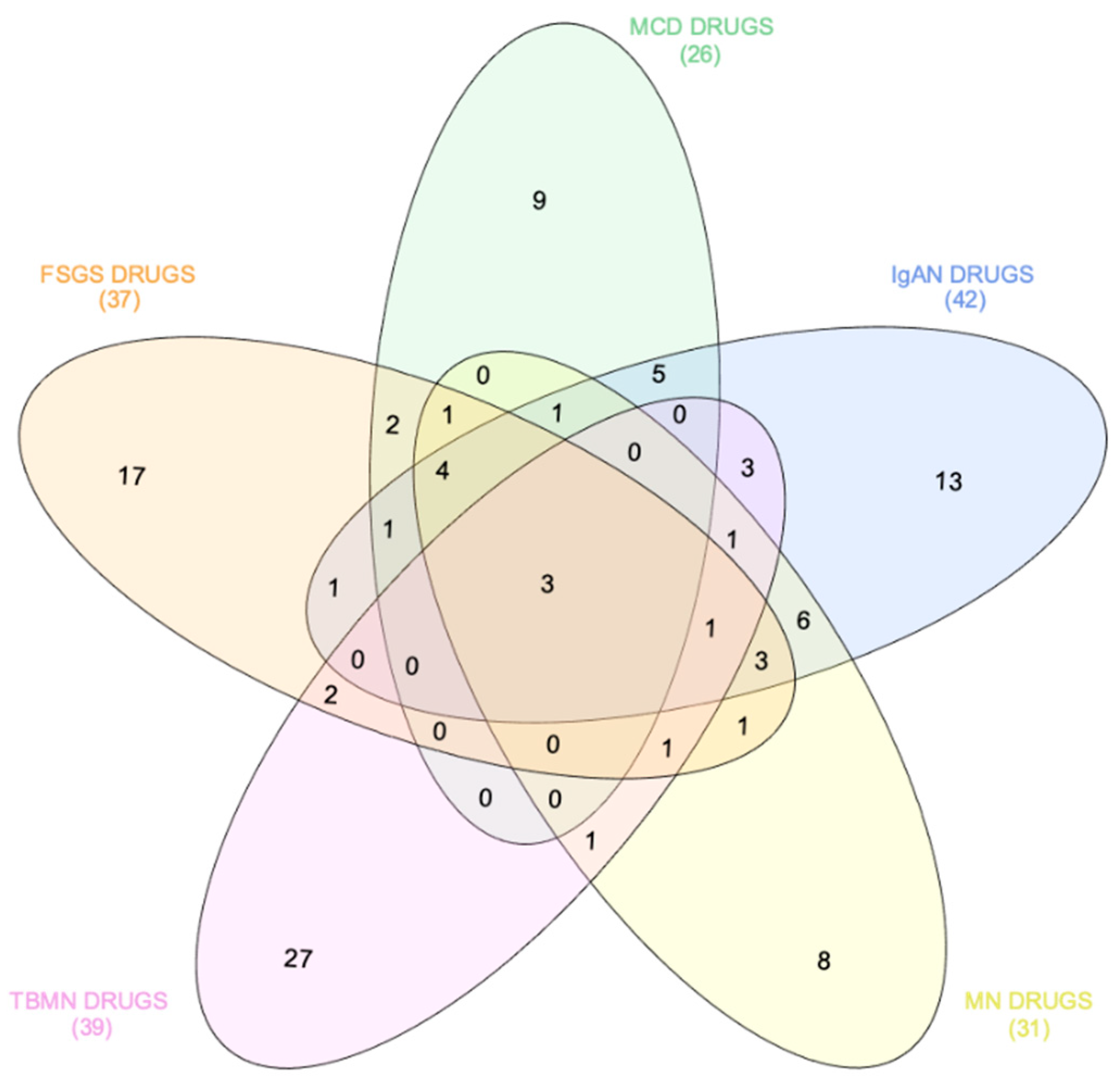
| Specific Drugs | Rank | Score | Perturbation (Drug) | Cell Line | Dose | Time |
|---|---|---|---|---|---|---|
| FSGS-specific | 3 | 0.0344 | Tanespimycin | HEPG2 | 10.0 um | 24 h |
| 4 | 0.0323 | JAK3 Inhibitor VI | PC3 | 10.0 um | 6 h | |
| 48 | 0.0237 | BRD-A17065207 | SKB | 10.0 um | 24 h | |
| 10 | 0.0301 | Ponatinib | HEPG2 | 3.33 um | 24 h | |
| 11 | 0.0301 | NVP-AUY922 | HEPG2 | 0.37 um | 24 h | |
| 23 | 0.0258 | BRD-K53414658 | A375 | 10.0 um | 24 h | |
| 28 | 0.0258 | KIN001-265 | HEPG2 | 0.37 um | 24 h | |
| 31 | 0.0258 | CGP-60474 | MCF10A | 1.11 um | 24 h | |
| 33 | 0.0258 | GSK-1059615 | BT20 | 10.0 um | 24 h | |
| 34 | 0.0258 | AZD-5438 | BT20 | 10.0 um | 24 h | |
| 36 | 0.0258 | GSK-2126458 | LNCAP | 1.11 um | 24 h | |
| 38 | 0.0258 | AZ-628 | A375 | 3.33 um | 24 h | |
| 39 | 0.0237 | Z-Leu3-VS | HCC515 | 10.0 um | 24 h | |
| 41 | 0.0237 | CGP 71683 hydrochloride | HCC515 | 10.0 um | 24 h | |
| 42 | 0.0237 | KETOROLAC TROMETHAMINE | HCC515 | 10.0 um | 24 h | |
| 45 | 0.0237 | 16-HYDROXYTRIPTOLIDE | HA1E | 0.08 um | 24 h | |
| 46 | 0.0237 | 89671 | HA1E | 0.09 um | 24 h | |
| MCD-specific | 11 | 0.0453 | BRD-K13810148 | MCF7 | 10.0 um | 24 h |
| 16 | 0.0453 | Pracinostat | A549 | 10.0 um | 24 h | |
| 18 | 0.0425 | NORETHINDRONE | VCAP | 10.0 um | 24 h | |
| 25 | 0.0425 | 5-Fluorocytosine | VCAP | 10.0 um | 24 h | |
| 30 | 0.0397 | TWS119 | HCC515 | 10.0 um | 24 h | |
| 37 | 0.0397 | DL-PDMP | A375 | 64.0 um | 24 h | |
| 42 | 0.0397 | Taxifolin-(+/−) | VCAP | 10.0 um | 24 h | |
| 44 | 0.0397 | EI-293 | PC3 | 10.0 um | 24 h | |
| 46 | 0.0397 | Neratinib | HCC515 | 3.33 um | 24 h | |
| IgAN-specific | 3 | 0.0296 | BMS-754807 | A375 | 10.0 um | 24 h |
| 4 | 0.0296 | BRD-K19295594 | A375 | 11.1 um | 24 h | |
| 11 | 0.0281 | BRD-K65814004 | MCF7 | 10.0 um | 24 h | |
| 18 | 0.0266 | DG-041 | A375 | 40.0 um | 24 h | |
| 25 | 0.0258 | 15-Deoxy-12,14-prostaglandin J2 | A375 | 10.0 um | 24 h | |
| 27 | 0.0258 | Dovitinib | A375 | 1.11 um | 24 h | |
| 28 | 0.0258 | Mitoxantrone | A375 | 0.12 um | 24 h | |
| 32 | 0.0251 | 7b-cis | A375 | 10.0 um | 24 h | |
| 39 | 0.0243 | MK-0591 | A375 | 80.0 um | 24 h | |
| 41 | 0.0243 | Piperlongumine (HPLC) | PC3 | 10.0 um | 24 h | |
| 43 | 0.0243 | S1367 | MCF7 | 10.0 um | 24 h | |
| 46 | 0.0243 | NVP-BEZ235 | A549 | 0.37 um | 24 h | |
| 47 | 0.0243 | SB590885 | HT29 | 3.33 um | 24 h | |
| MN-specific | 9 | 0.0183 | Foretinib | HT29 | 10.0 um | 24 h |
| 34 | 0.0151 | WH-4-025 | HT29 | 10.0 um | 24 h | |
| 13 | 0.0170 | GDC-0980 | HT29 | 10.0 um | 24 h | |
| 23 | 0.0157 | TPCA-1 | HT29 | 10.0 um | 24 h | |
| 25 | 0.0157 | S1175 | MCF7 | 10.0 um | 24 h | |
| 31 | 0.0154 | BRD-K41859756 | HT29 | 10.0 um | 24 h | |
| 41 | 0.0148 | Palbociclib | HME1 | 10.0 um | 24 h | |
| 49 | 0.0145 | Erlotinib | MCF10A | 3.33 um | 24 h | |
| TBMN-specific | 14 | 0.0243 | Narciclasine | PC3 | 10.0 um | 6 h |
| 10 | 0.0252 | BRD-A93236127 | MCF7 | 10.0 um | 24 h | |
| 12 | 0.0247 | BRD-A62809825 | HT29 | 10.0 um | 24 h | |
| 13 | 0.0247 | Homoharringtonine | HT29 | 10.0 um | 6 h | |
| 16 | 0.0238 | ARP 101 | MCF7 | 10.0 um | 24 h | |
| 17 | 0.0228 | CAY10594 | HT29 | 20.0 um | 24 h | |
| 18 | 0.0228 | BRD-K91370081 | SKB | 10.0 um | 24 h | |
| 19 | 0.0223 | BRD-K32896438 | MCF7 | 10.0 um | 24 h | |
| 20 | 0.0223 | BRD-K23478508 | MCF7 | 10.0 um | 24 h | |
| 21 | 0.0223 | BRD-K53308430 | VCAP | 10.0 um | 24 h | |
| 22 | 0.0218 | Wortmannin | PC3 | 10.0 um | 24 h | |
| 23 | 0.0218 | BJM-ctd2-9 | SW620 | 10.0 um | 6 h | |
| 24 | 0.0218 | HY-10518 | VCAP | 10.0 um | 24 h | |
| 26 | 0.0218 | BRD-K06009608 | VCAP | 10.0 um | 24 h | |
| 28 | 0.0218 | LDN-193189 | SKBR3 | 10.0 um | 3 h | |
| 50 | 0.0204 | BRD-K92317137 | PC3 | 10.0 um | 6 h | |
| 30 | 0.0213 | PK-11195 | HT29 | 160.0 um | 24 h | |
| 31 | 0.0213 | MST- 312 | PC3 | 11.1 um | 24 h | |
| 32 | 0.0213 | BRD-K80786583 | MCF7 | 10.0 um | 24 h | |
| 33 | 0.0213 | BRD-K78122587 | MCF7 | 10.0 um | 24 h | |
| 37 | 0.0213 | GDC-0941 | LNCAP | 1.11 um | 24 h | |
| 38 | 0.0209 | PERHEXILINE MALEATE | HT29 | 10.0 um | 24 h | |
| 40 | 0.0209 | Dorsomorphin dihydrochloride | MCF7 | 10.0 um | 24 h | |
| 41 | 0.0209 | BRD-U33728988 | SKB | 10.0 um | 24 h | |
| 44 | 0.0209 | BRD-A80502530 | MCF7 | 10.0 um | 24 h | |
| 45 | 0.0209 | PHA-665752 | HT29 | 0.04 um | 24 h | |
| 47 | 0.0209 | JW-7-24-1 | A549 | 10.0 um | 24 h |
| Disease | Rank | Score | Perturbation (Drug) | Cell Line | Dose | Time |
|---|---|---|---|---|---|---|
| FSGS | 7 | 0.0323 | PD-0325901 | HEPG2 | 10.0 um | 24 h |
| MCD | 1 | 0.0567 | HT29 | 0.12 um | 24 h | |
| IgAN | 45 | 0.0243 | A375 | 1.11 um | 24 h | |
| TBNM | 36 | 0.0213 | HT29 | 0.12 um | 24 h | |
| MN | 5 | 0.0192 | HT29 | 0.12 um | 24 h | |
| FSGS | 9 | 0.0301 | PD-184352 | A375 | 3.33 um | 24 h |
| MCD | 7 | 0.0482 | HT29 | 10.0 um | 24 h | |
| IgAN | 16 | 0.0273 | HT29 | 10.0 um | 24 h | |
| TBNM | 27 | 0.0218 | HT29 | 10.0 um | 24 h | |
| MN | 12 | 0.0170 | HT29 | 10.0 um | 24 h | |
| FSGS | 44 | 0.0237 | KU 0060648 trihydrochloride | A375 | 10.0 um | 24 h |
| MCD | 20 | 0.0425 | A375 | 10.0 um | 24 h | |
| IgAN | 24 | 0.0258 | A375 | 10.0 um | 24 h | |
| TBNM | 8 | 0.0257 | MCF7 | 10.0 um | 24 h | |
| MN | 20 | 0.0157 | A375 | 10.0 um | 24 h |
| Perturbation (Drug) | Mechanism of Action (MOA) | FDA Approval | Disease Target | Side Effects |
|---|---|---|---|---|
| Tanespimycin | HSP inhibitor | Yes | Multiple myeloma | N/A |
| JAK3 Inhibitor VI | Unknown | No | Unknown | N/A |
| BRD-A17065207 | Protein synthesis inhibitor | No | Unknown | N/A |
| Ponatinib | Bcr-Abl kinase inhibitor | Yes | Chronic myeloid leukemia | Hypertension |
| FLT3 inhibitor | Acute lymphoblastic leukemia | Rash | ||
| PDGFR tyrosine kinase receptor inhibitor | Abdominal pain | |||
| Fatigue | ||||
| Headache | ||||
| Dry skin | ||||
| Constipation | ||||
| Arthralgia | ||||
| Nausea | ||||
| Pyrexia | ||||
| Thrombocytopenia | ||||
| Anemia | ||||
| Neutropenia | ||||
| Lymphopenia | ||||
| Leukopenia | ||||
| NVP-AUY922 | HSP inhibitor | No | Unknown | N/A |
| BRD-K53414658 | VEGFR inhibitor | No | Unknown | N/A |
| KIN001-265 | Unknown | No | Unknown | N/A |
| CGP-60474 | Unknown | No | Unknown | N/A |
| GSK-1059615 | Unknown | No | Unknown | N/A |
| AZD-5438 | CDK inhibitor | No | Unknown | N/A |
| GSK-2126458 | Unknown | No | Unknown | N/A |
| AZ-628 | RAF inhibitor | No | Unknown | N/A |
| Z-Leu3-VS | Unknown | No | Unknown | N/A |
| CGP 71683 hydrochloride | Neuropeptide receptor antagonist | No | Unknown | N/A |
| Ketorolac TROMETHAMINE | Cyclooxygenase inhibitor | Yes | Unknown | Abdominal pain |
| Dyspepsia | ||||
| Nausea | ||||
| Headaches | ||||
| Vomiting | ||||
| Epigastric pain | ||||
| Gastrointestinal bleeding | ||||
| Drowsiness | ||||
| Acute renal failure | ||||
| Hypertension | ||||
| Respiratory depression | ||||
| Coma | ||||
| 16-HYDROXYTRIPTOLIDE | RNA polymerase inhibitor | No | Unknown | N/A |
| 89671 | Unknown | No | Unknown | N/A |
| BRD-K13810148 | HDAC inhibitor | No | Unknown | N/A |
| Pracinostat | HDAC inhibitor | No | Unknown | N/A |
| Norethindrone | Progesterone receptor agonist | Yes | Menopause | Adverse effects of contraceptives |
| Osteoporosis | nausea | |||
| Vaginal atrophy | vomiting | |||
| 5-Fluorocytosine | Inhibition of RNA and DNA biosynthesis | Yes | Bacterial septicemia | Nausea |
| Endocarditis | Diarrhea | |||
| Urinary tract infections | Vomiting | |||
| Meningitis | Abdominal pain | |||
| TWS119 | Glycogen synthase kinase-3β inhibitor | No | Unknown | N/A |
| DL-PDMP | Unknown | No | Unknown | N/A |
| Taxifolin-(+/−) | Opioid receptor antagonist | No | Unknown | N/A |
| EI-293 | Unknown | No | Unknown | N/A |
| Neratinib | EGFR inhibitor | Yes | Breast cancer | Hepatotoxicity |
| Diarrhea | ||||
| Vomiting | ||||
| Dehydration | ||||
| Cellulitis | ||||
| Renal failure | ||||
| Erysipelas | ||||
| Alanine aminotransferase increase | ||||
| Aspartate aminotransferase increase | ||||
| Nausea | ||||
| Fatigue | ||||
| Abdominal pain | ||||
| BMS-754807 | IGF-1 inhibitor | No | Unknown | N/A |
| BRD-K19295594 | BCL inhibitor | No | Unknown | N/A |
| MCL1 inhibitor | ||||
| BRD-K65814004 | Nitric oxide synthase inhibitor | No | Unknown | N/A |
| DG-041 | Unknown | No | Unknown | N/A |
| 15-Deoxy-12,14-prostaglandin J2 | Natural peroxisome | No | Unknown | N/A |
| Proliferator-activated | ||||
| receptor-γ (PPAR-γ) agonist | ||||
| Dovitinib | EGFR inhibitor | No | Unknown | N/A |
| FGFR inhibitor | ||||
| FLT3 inhibitor | ||||
| PDGFR tyrosine kinase receptor inhibitor | ||||
| VEGFR inhibitor | ||||
| Mitoxantrone | Topoisomerase inhibitor | Yes | Multiple sclerosis | Leukopenia with infection |
| Prostate cancer | ||||
| Acute myeloid leukemia | ||||
| 7b-cis | Unknown | No | Unknown | N/A |
| MK-0591 | Leukotriene synthesis inhibitor | No | Unknown | N/A |
| Piperlongumine (HPLC) | Unknown | No | Unknown | N/A |
| S1367 | Topoisomerase inhibitor | No | Unknown | N/A |
| NVP-BEZ235 | mTOR inhibitor, PI3K inhibitor | No | Unknown | N/A |
| SB590885 | Unknown | No | Unknown | N/A |
| Foretinib | VEGFR inhibitor | No | Unknown | N/A |
| WH-4-025 | Unknown | No | Unknown | N/A |
| GDC-0980 | mTOR inhibitor, PI3K inhibitor | No | Unknown | N/A |
| TPCA-1 | IKK inhibitor | No | Unknown | N/A |
| S1175 | HSP inhibitor | No | Unknown | N/A |
| BRD-K41859756 | HSP inhibitor | No | Unknown | N/A |
| Palbociclib | CDK inhibitor | Yes | Breast cancer | Neutropenia |
| Leukopenia | ||||
| Anemia | ||||
| Fatigue | ||||
| Nausea | ||||
| Diarrhea | ||||
| Respiratory infection | ||||
| Headache | ||||
| Thrombocytopenia | ||||
| Vomiting | ||||
| Decreased appetite | ||||
| Erlotinib | EGFR inhibitor | Yes | Non-small-cell lung cancer | Diarrhea |
| pancreatic cancer | Rash | |||
| Liver transaminase elevation | ||||
| Narciclasine | Unknown | No | Unknown | N/A |
| BRD-A93236127 | ATPase inhibitor | No | Unknown | N/A |
| BRD-A62809825 | Unknown | No | Unknown | N/A |
| Homoharringtonine | Protein synthesis inhibitor | Yes | Chronic myeloid leukemia | Myelosuppression |
| Bleeding | ||||
| Hyperglycemia | ||||
| Fetal harm | ||||
| ARP 101 | Unknown | No | Unknown | N/A |
| CAY10594 | Unknown | No | Unknown | N/A |
| BRD-K91370081 | DNA synthesis inhibitor | No | Unknown | N/A |
| BRD-K32896438 | Unknown | No | Unknown | N/A |
| BRD-K23478508 | ATPase inhibitor | Yes | Congestive heart failure | Nausea |
| Atrial fibrillation | Vomiting | |||
| Visual changes | ||||
| Arrhythmia | ||||
| BRD-K53308430 | Unknown | No | Unknown | N/A |
| Wortmannin | PI3K inhibitor | No | Unknown | N/A |
| BJM-ctd2-9 | Androgen receptor antagonist | No | Unknown | N/A |
| HY-10518 | Unknown | No | Unknown | N/A |
| BRD-K06009608 | Unknown | No | Unknown | N/A |
| LDN-193189 | Bone morphogenic protein inhibitor | No | Unknown | N/A |
| BRD-K92317137 | Unknown | No | Unknown | N/A |
| PK-11195 | Benozodiazepine receptor antagonist | No | Unknown | N/A |
| MST-312 | Unknown | No | Unknown | N/A |
| BRD-K80786583 | Unknown | No | Unknown | N/A |
| BRD-K78122587 | T-type calcium channel blocker | No | Unknown | N/A |
| GDC-0941 | PI3K inhibitor | No | Unknown | N/A |
| PERHEXILINE MALEATE | Unknown | No | Unknown | N/A |
| Dorsomorphin dihydrochloride | Unknown | No | Unknown | N/A |
| BRD-U33728988 | Unknown | No | Unknown | N/A |
| BRD-A80502530 | Unknown | No | Unknown | N/A |
| PHA-665752 | c-MET inhibitor | No | Unknown | N/A |
| JW-7-24-1 | Unknown | No | Unknown | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garantziotis, P.; Doumas, S.A.P.; Boletis, I.; Frangou, E. Gene Expression as a Guide to the Development of Novel Therapies in Primary Glomerular Diseases. J. Clin. Med. 2021, 10, 2262. https://doi.org/10.3390/jcm10112262
Garantziotis P, Doumas SAP, Boletis I, Frangou E. Gene Expression as a Guide to the Development of Novel Therapies in Primary Glomerular Diseases. Journal of Clinical Medicine. 2021; 10(11):2262. https://doi.org/10.3390/jcm10112262
Chicago/Turabian StyleGarantziotis, Panagiotis, Stavros A. P. Doumas, Ioannis Boletis, and Eleni Frangou. 2021. "Gene Expression as a Guide to the Development of Novel Therapies in Primary Glomerular Diseases" Journal of Clinical Medicine 10, no. 11: 2262. https://doi.org/10.3390/jcm10112262
APA StyleGarantziotis, P., Doumas, S. A. P., Boletis, I., & Frangou, E. (2021). Gene Expression as a Guide to the Development of Novel Therapies in Primary Glomerular Diseases. Journal of Clinical Medicine, 10(11), 2262. https://doi.org/10.3390/jcm10112262







