Influence of Psychological Biomarkers on Therapeutic Adherence by Patients with Non-Alcoholic Fatty Liver Disease: A Moderated Mediation Model
Abstract
1. Introduction
2. Materials and Methods
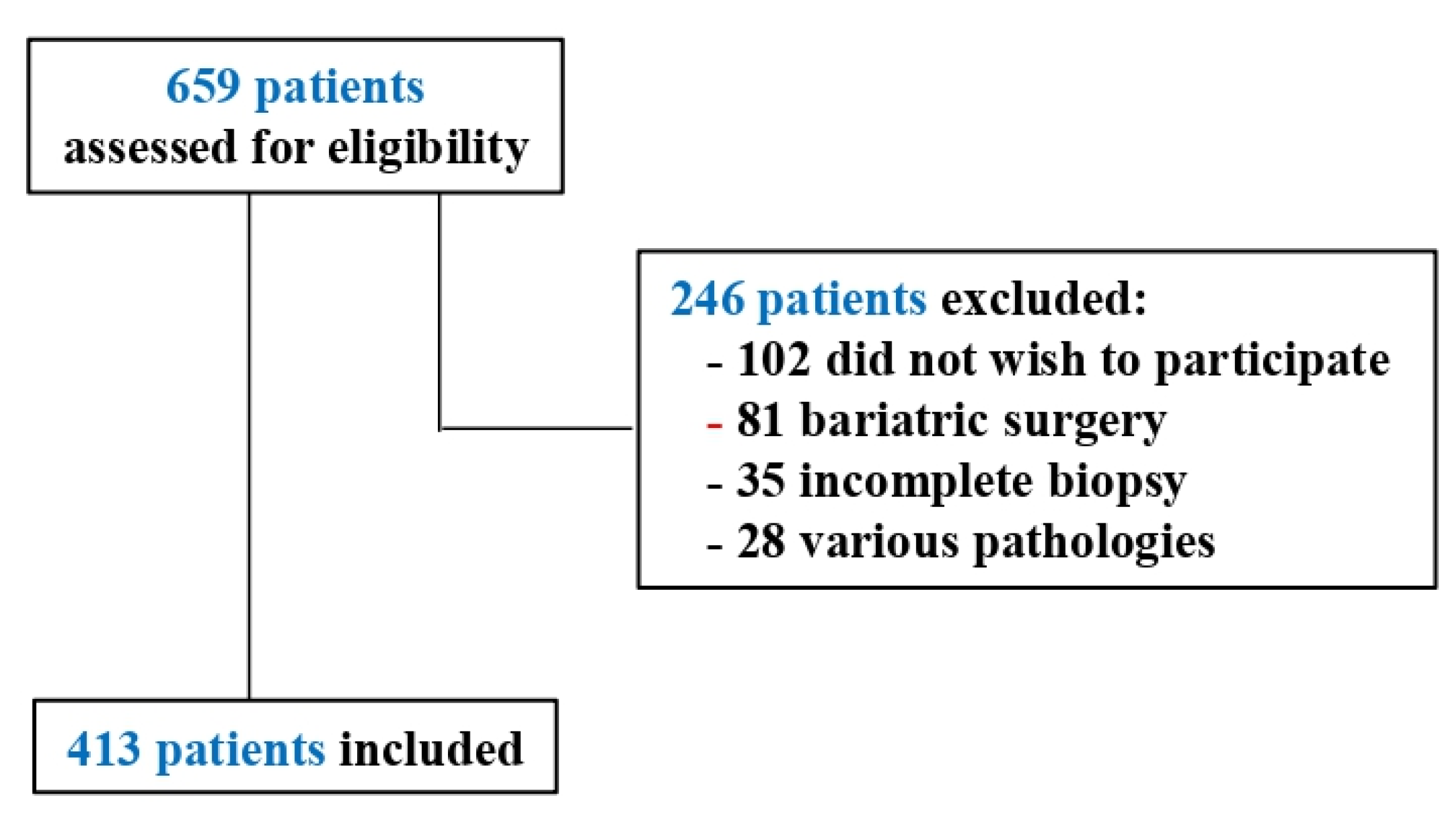
2.1. Participants
2.2. Instruments
2.3. Procedure
2.4. Statistical Analysis
3. Results
3.1. Sociodemographic and Clinic Variables
3.2. Correlation Analysis
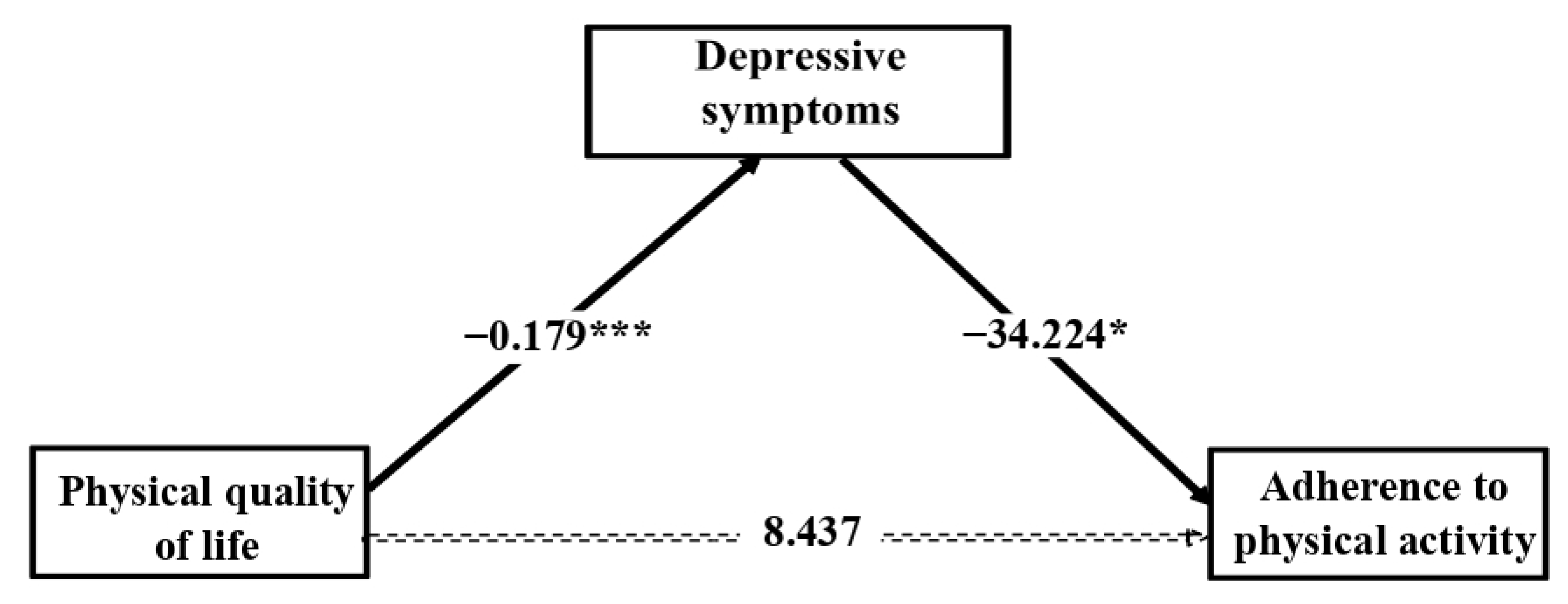
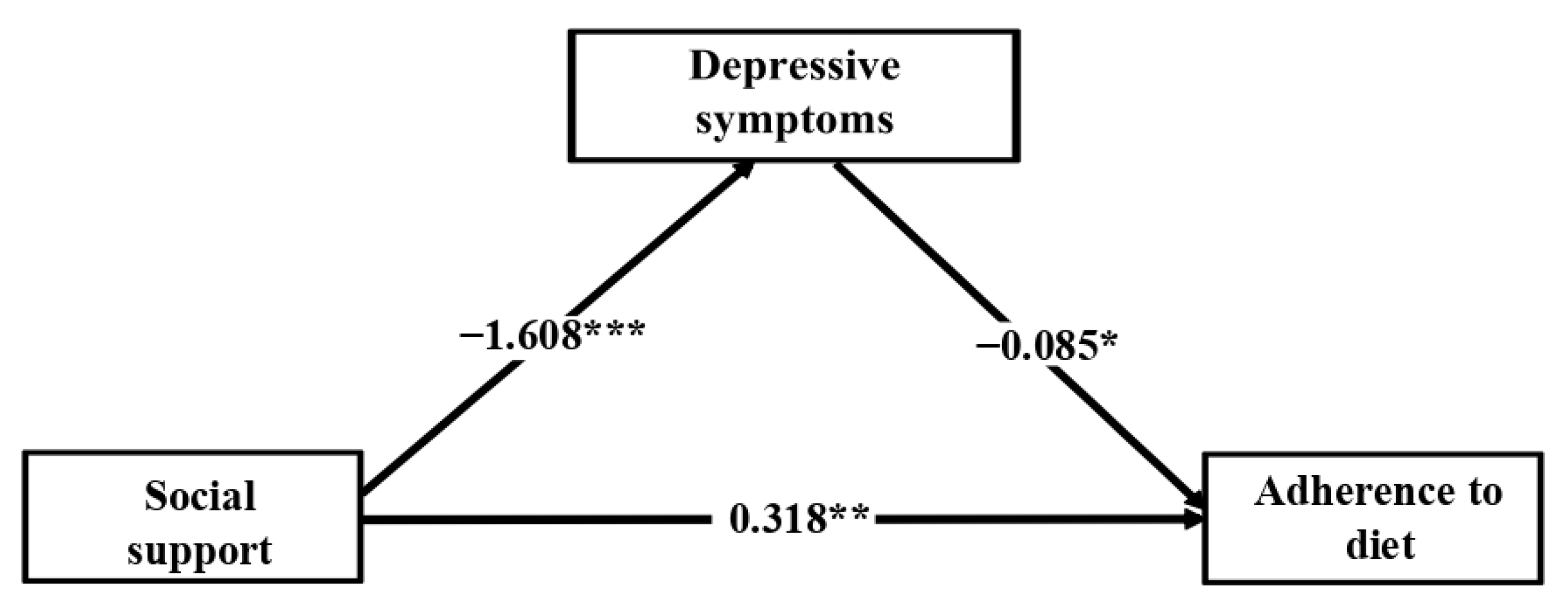
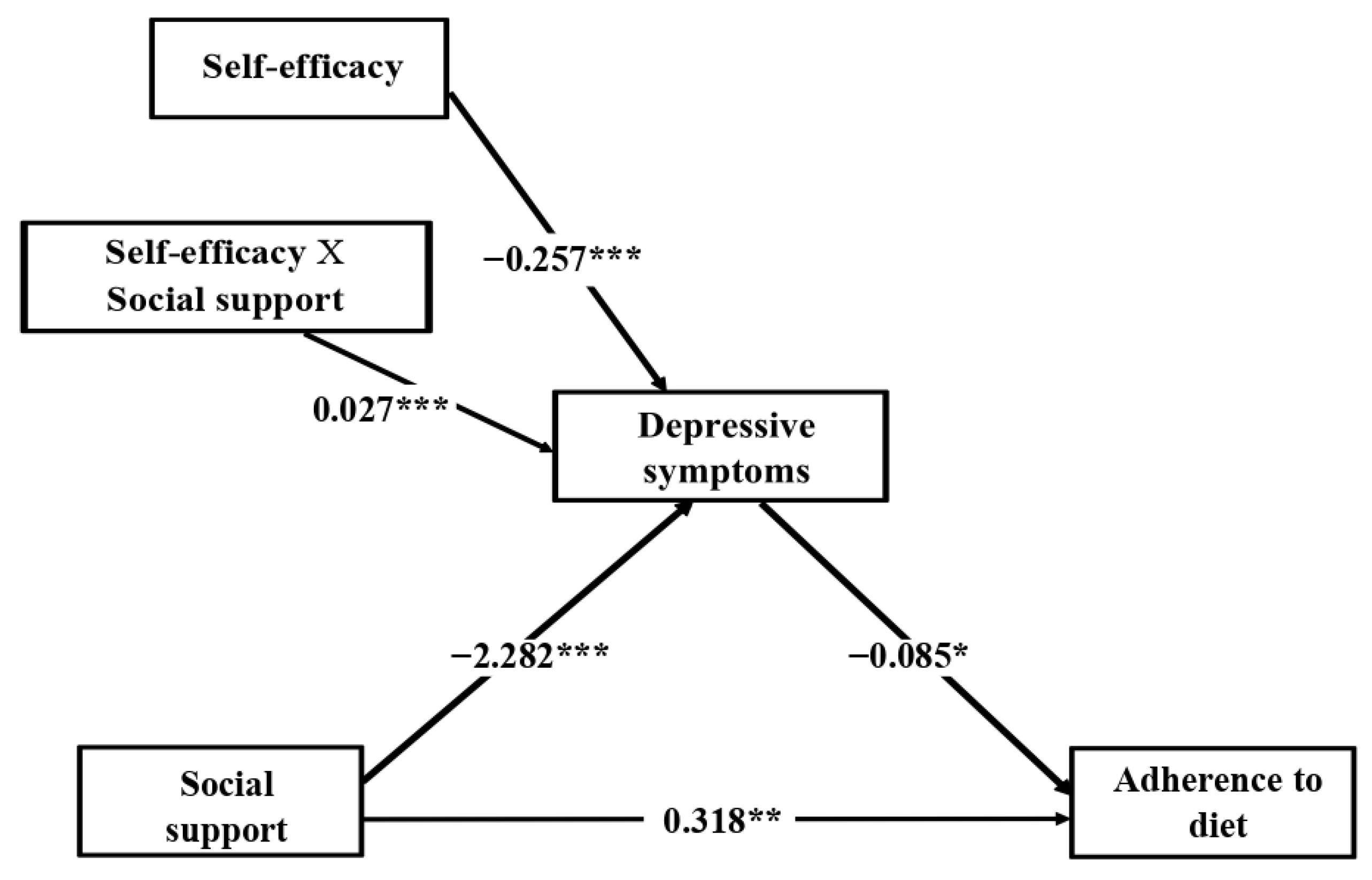
3.3. Mediation Analysis
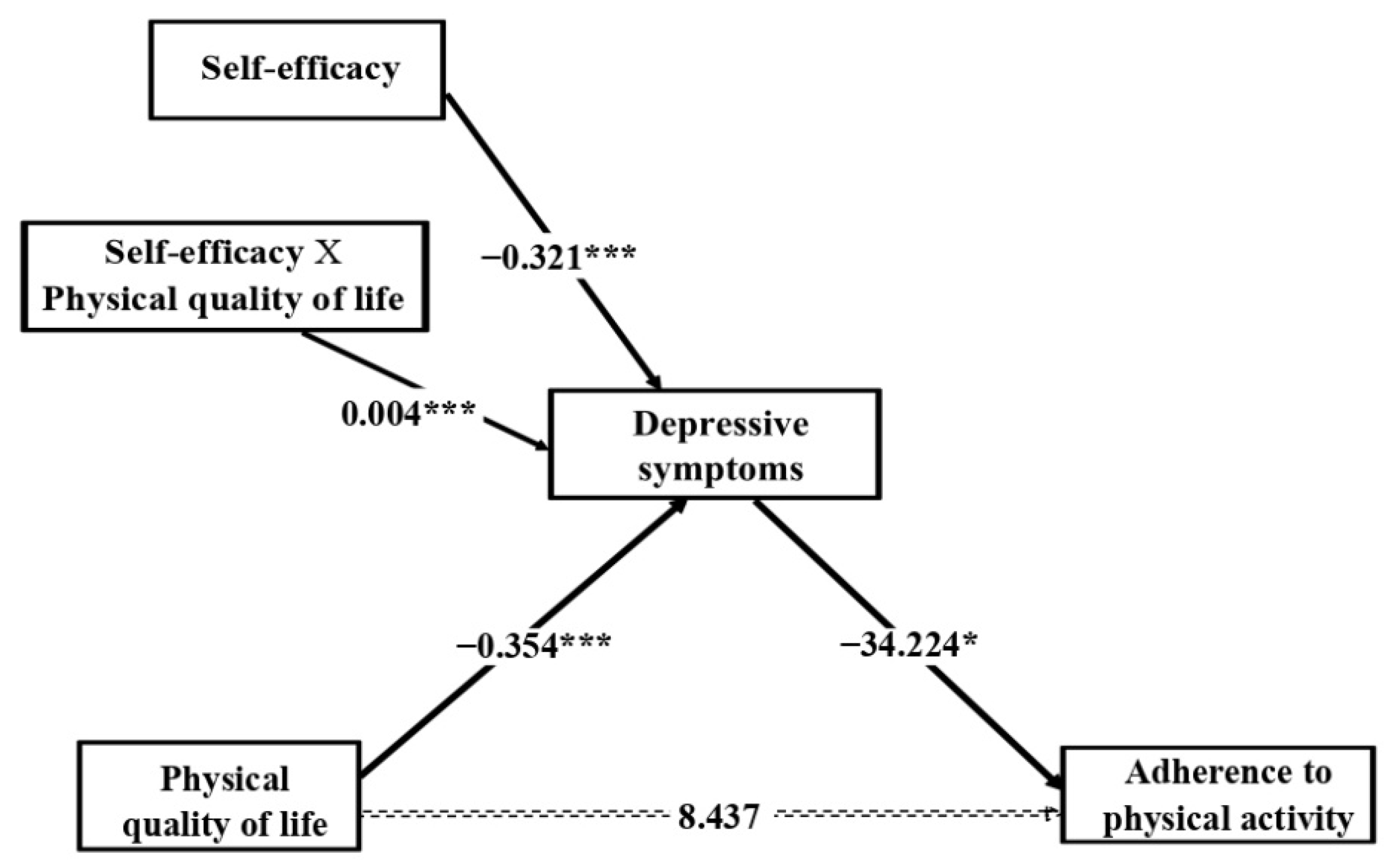
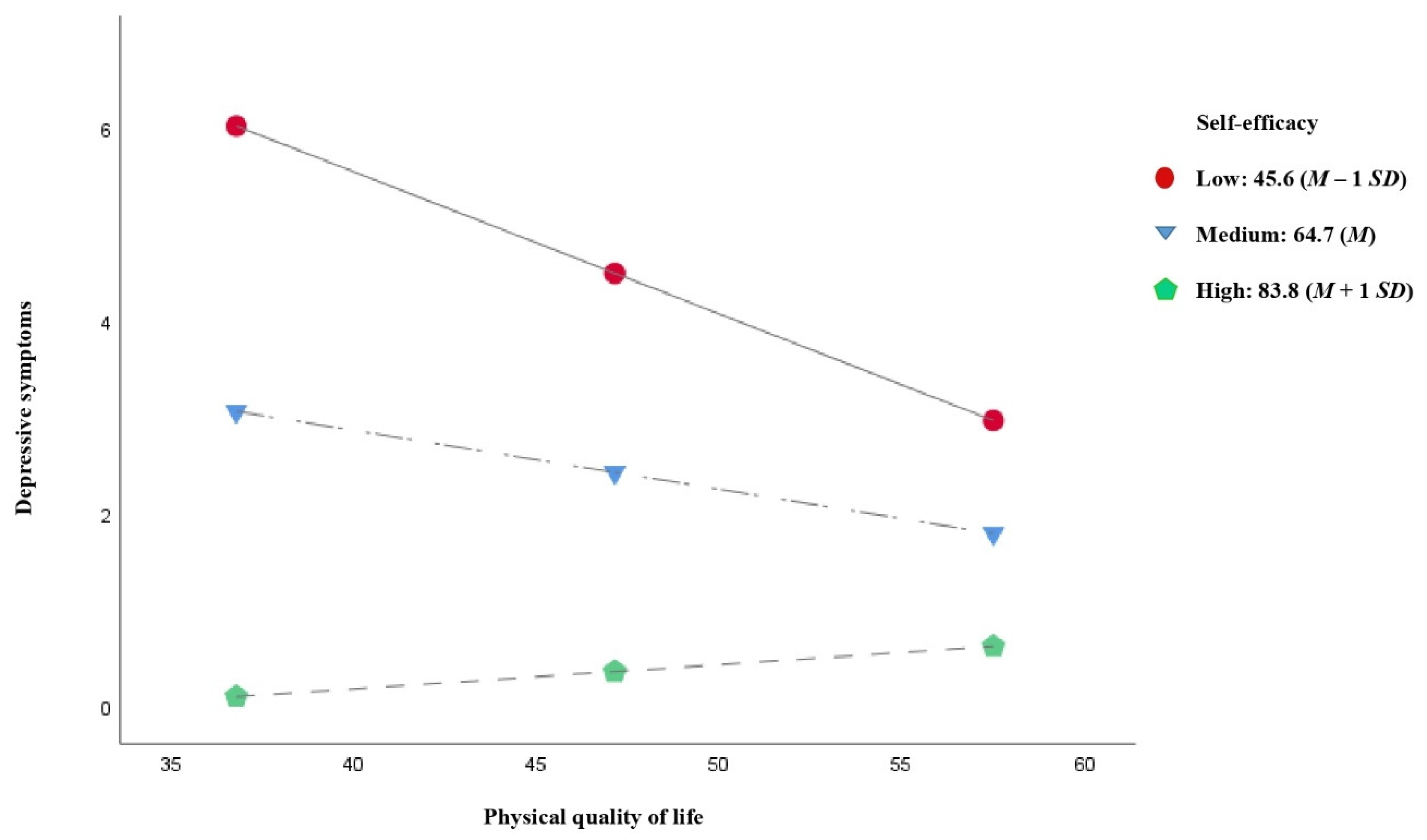
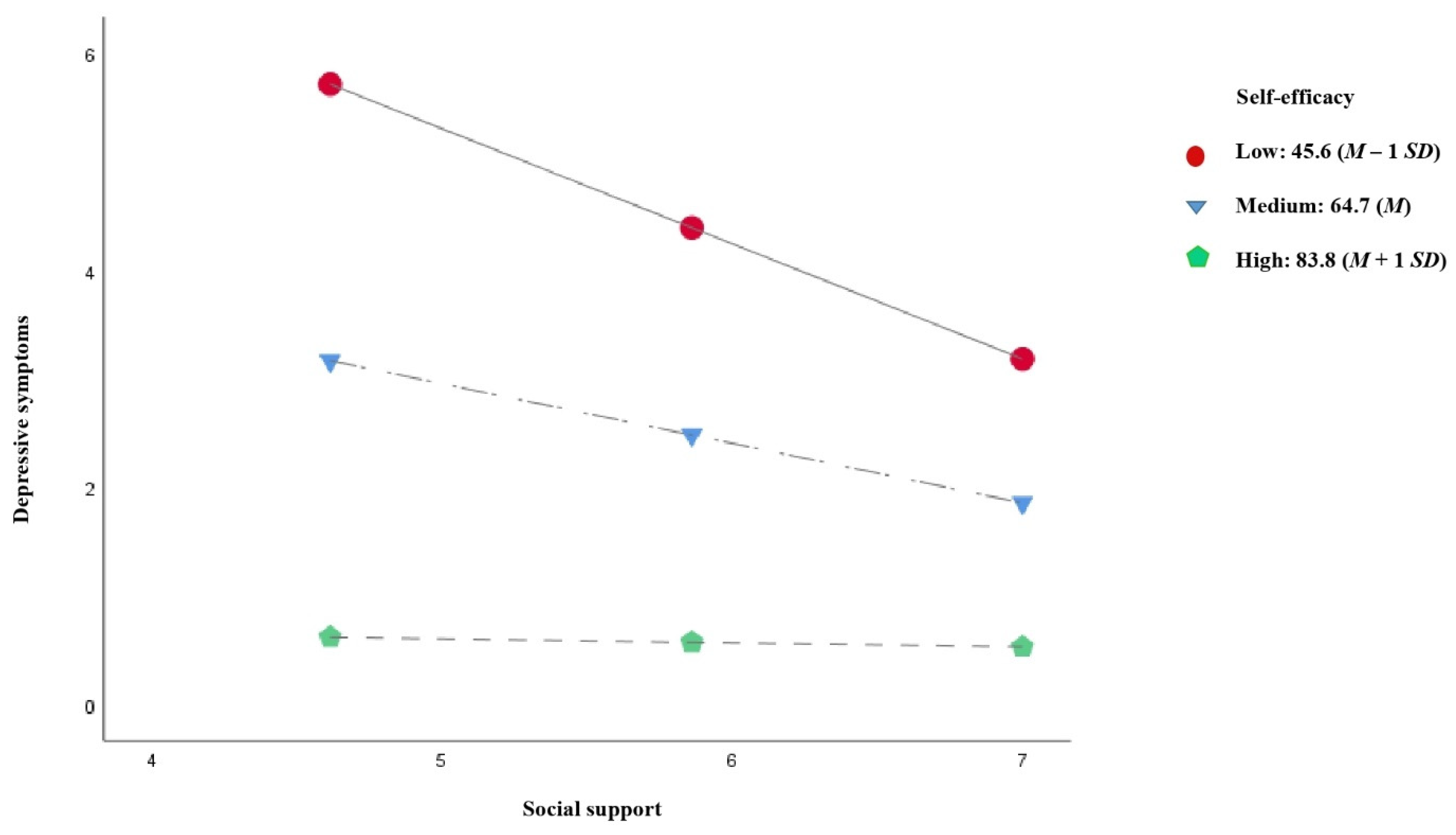
3.4. Moderated Mediation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marjot, T.; Moolla, A.; Cobbold, J.F.; Hodson, L.; Tomlinson, J.W. Nonalcoholic Fatty Liver Disease in Adults: Current Concepts in Etiology, Outcomes, and Management. Endocr. Rev. 2020, 41, 66–117. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Marchesini, G.; Pinto-Cortez, H.; Petta, S. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis: Implications for Liver Transplantation. Transplantation 2019, 103, 22–27. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Romero-Gómez, M.; Zelber-Sagi, S.; Trenell, M. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 2017, 67, 829–846. [Google Scholar] [CrossRef]
- Reginato, E.; Pippi, R.; Aiello, C.; Sbroma Tomaro, E.; Ranucci, C.; Buratta, L.; Bini, V.; Marchesini, G.; De Feo, P.; Fanelli, C. Effect of Short Term Intensive Lifestyle Intervention on Hepatic Steatosis Indexes in Adults with Obesity and/or Type 2 Diabetes. J. Clin. Med. 2019, 8, 851. [Google Scholar] [CrossRef] [PubMed]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015, 149, 367–378.e5. [Google Scholar] [CrossRef] [PubMed]
- Serfaty, L. Management of patients with non-alcoholic steatohepatitis (NASH) in real life. Liver Int. 2018, 38, 52–55. [Google Scholar] [CrossRef]
- Centis, E.; Moscatiello, S.; Bugianesi, E.; Bellentani, S.; Fracanzani, A.L.; Calugi, S.; Petta, S.; Dalle-Grave, R.; Marchesini, G. Stage of change and motivation to healthier lifestyle in non-alcoholic fatty liver disease. J. Hepatol. 2013, 58, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Assimakopoulos, K.; Karaivazoglou, K.; Tsermpini, E.-E.; Diamantopoulou, G.; Triantos, C. Quality of life in patients with nonalcoholic fatty liver disease: A systematic review. J. Psychosom. Res. 2018, 112, 73–80. [Google Scholar] [CrossRef]
- Golabi, P.; Otgonsuren, M.; Cable, R.; Felix, S.; Koenig, A.; Sayiner, M.; Younossi, Z.M. Non-alcoholic Fatty Liver Disease (NAFLD) is associated with impairment of Health Related Quality of Life (HRQOL). Health Qual. Life Outcomes 2016, 14, 18. [Google Scholar] [CrossRef]
- Huang, X.; Liu, X.; Yu, Y. Depression and Chronic Liver Diseases: Are There Shared Underlying Mechanisms? Front. Mol. Neurosci. 2017, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, A.A.; Price, J.K.; Stepanova, M.; Poms, L.W.; Fang, Y.; Moon, J.; Nader, F.; Younossi, Z.M. Depression in Patients with Nonalcoholic Fatty Liver Disease and Chronic Viral Hepatitis B and C. J. Psychosom. Res. 2011, 52, 127–132. [Google Scholar] [CrossRef]
- Daniele, T.M.D.C.; De Bruin, V.M.S.; De Oliveira, D.S.N.; Pompeu, C.M.R.; E Forti, A.C. Associations among physical activity, comorbidities, depressive symptoms and health-related quality of life in type 2 diabetes. Arq. Bras. Endocrinol. Metabol. 2013, 57, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Mazzeschi, C.; Pazzagli, C.; Buratta, L.; Reboldi, G.P.; Battistini, D.; Piana, N.; Pippi, R.; Fatone, C.; De Feo, P. Mutual Interactions between Depression/Quality of Life and Adherence to a Multidisciplinary Lifestyle Intervention in Obesity. J. Clin. Endocrinol. Metab. 2012, 97, E2261–E2265. [Google Scholar] [CrossRef]
- Karfopoulou, E.; Anastasiou, C.A.; Avgeraki, E.; Kosmidis, M.H.; Yannakoulia, M. The role of social support in weight loss maintenance: Results from the MedWeight study. J. Behav. Med. 2016, 39, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Blasiole, J.A.; Shinkunas, L.; Labrecque, D.R.; Arnold, R.M.; Zickmund, S.L. Mental and physical symptoms associated with lower social support for patients with hepatitis C. World J. Gastroenterol. 2006, 12, 4665–4672. [Google Scholar] [CrossRef]
- Steese, S.; Dollette, M.; Phillips, W.; Hossfeld, E.; Matthews, G.; Taormina, G. Understanding Girls’ Circle as an intervention on perceived social support, body image, self-efficacy, locus of control, and self-esteem. Adolescence 2006, 41, 55–74. [Google Scholar]
- Sepúlveda, V.A.C.; Romero, G.A.L.; Jaramillo, V.L. Coping strategies and their relation with depression and anxiety in pedi-atric residents in a third level pediatric hospital. Bol. Med. Hosp. Infant. Mex. 2012, 69, 347–354. [Google Scholar]
- Chang, M.-W.; Nitzke, S.; Guilford, E.; Adair, C.H.; Hazard, D.L. Motivators and Barriers to Healthful Eating and Physical Activity among Low-Income Overweight and Obese Mothers. J. Am. Diet. Assoc. 2008, 108, 1023–1028. [Google Scholar] [CrossRef]
- Naicker, K.; Øverland, S.; Johnson, J.A.; Manuel, D.; Skogen, J.C.; Sivertsen, B.; Colman, I. Symptoms of anxiety and depression in type 2 diabetes: Associations with clinical diabetes measures and self-management outcomes in the Norwegian HUNT study. Psychoneuroendocrinology 2017, 84, 116–123. [Google Scholar] [CrossRef]
- Simon, G.E.; Ludman, E.J.; Linde, J.A.; Operskalski, B.H.; Ichikawa, L.; Rohde, P.; Finch, E.A.; Jeffery, R.W. Association between obesity and depression in middle-aged women. Gen. Hosp. Psychiatry 2008, 30, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.S.; Brown, M.B.; Funnell, M.M.; Anderson, R.M. Social Support, Quality of Life, and Self-Care Behaviors Among African Americans With Type 2 Diabetes. Diabetes Educ. 2008, 34, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Funuyet-Salas, J.; Pérez-San-Gregorio, M.Á.; Martín-Rodríguez, A.; Romero-Gómez, M. Psychological Biomarkers and Fibrosis: An Innovative Approach to Non-alcoholic Fatty Liver Disease. Front. Med. 2020, 7, 585425. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, D.; Strauser, D.R. Operationalizing Self-Efficacy, Related Social Cognitive Variables, and Moderating Effects: Implications for rehabilitation research and practice. Rehabil. Couns. Bull. 2009, 52, 251–258. [Google Scholar] [CrossRef]
- Bandura, A. Self-efficacy: Toward a unifying theory of behaviour change. Psychol. Rev. 1977, 84, 191–215. [Google Scholar] [CrossRef] [PubMed]
- Bandura, A. Social Foundations of thought and Action: A Social Cognitive Theory; Prentice-Hall: Englewood Cliffs, NY, USA, 1986. [Google Scholar]
- Frith, J.; Day, C.P.; Robinson, L.; Elliott, C.; Jones, D.E.; Newton, J.L. Potential strategies to improve uptake of exercise interventions in non-alcoholic fatty liver disease. J. Hepatol. 2010, 52, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Adam, J.; Folds, L. Depression, Self-efficacy, and Adherence in Patients with Type 2 Diabetes. J. Nurse Pract. 2014, 10, 646–652. [Google Scholar] [CrossRef]
- Dutton, G.R.; Tan, F.; Provost, B.C.; Sorenson, J.L.; Allen, B.; Smith, D. Relationship between self-efficacy and physical activity among patients with type 2 diabetes. J. Behav. Med. 2009, 32, 270–277. [Google Scholar] [CrossRef]
- Leung, A.W.Y.; Chan, R.S.M.; Sea, M.M.M.; Woo, J. Identifying psychological predictors of adherence to a community-based lifestyle modification program for weight loss among Chinese overweight and obese adults. Nutr. Res. Pract. 2019, 13, 415–424. [Google Scholar] [CrossRef]
- Leung, A.W.Y.; Chan, R.S.M.; Sea, M.M.M.; Woo, J. Psychological Factors of Long-Term Dietary and Physical Activity Adherence among Chinese Adults with Overweight and Obesity in a Community-Based Lifestyle Modification Program: A Mixed-Method Study. Nutrients 2020, 12, 1379. [Google Scholar] [CrossRef] [PubMed]
- Zelber-Sagi, S.; Bord, S.; Dror-Lavi, G.; Smith, M.L.; Towne, S.D.T., Jr.; Buch, A.; Webb, M.; Yeshua, H.; Nimer, A.; Shibolet, O. Role of illness perception and self-efficacy in lifestyle modification among non-alcoholic fatty liver disease patients. World J. Gastroenterol. 2017, 23, 1881–1890. [Google Scholar] [CrossRef]
- Maruish, M.E. User’s Manual for the SF-12v2 Health Survey; QualityMetric Incorporated: Lincoln, RI, USA, 2012. [Google Scholar]
- Ware, J.E.; Kosinski, M.; Turner-Bowker, D.M.; Gandek, B. How to Score Version 2 of the Sf-12 Health Survey (with a Supplement Documenting Version 1); QualityMetric Incorporated: Lincoln, RI, USA, 2002. [Google Scholar]
- Zimet, G.D.; Dahlem, N.W.; Zimet, S.G.; Farley, G.K. The Multidimensional Scale of Perceived Social Support. J. Pers. Assess. 1988, 52, 30–41. [Google Scholar] [CrossRef]
- Landeta, O.; Calvete, E. Adaptación y validación de la escala multidimensional de apoyo social percibido. Ansiedad Estrés 2002, 8, 173–182. [Google Scholar]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Caro, I.; Ibáñez, E. La escala hospitalaria de ansiedad y depression: Su utilidad práctica en Psicología de la Salud. Bol. Psicol. 1992, 36, 43–69. [Google Scholar]
- Baessler, J.; Schwarcer, R. Evaluación de la autoeficacia: Adaptación española de la escala de autoeficacia general. Ansiedad Estrés 1996, 2, 1–8. [Google Scholar]
- Sanjuán-Suárez, P.; Pérez-García, A.M.; Bermúdez-Moreno, J. Escala de autoeficacia general: Datos psicométricos de la adaptación para población española. Psicothema 2000, 12, 509–513. [Google Scholar]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Fornell, C.; Larcker, D.F. Evaluating structural equations models with unobservable variables and measurement error. J. Mark. Res. 1981, 18, 39–50. [Google Scholar] [CrossRef]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A Short Screener Is Valid for Assessing Mediterranean Diet Adherence among Older Spanish Men and Women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.F.; Rockwood, N.J. Regression-based statistical mediation and moderation analysis in clinical research: Observations, recommendations, and implementation. Behav. Res. Ther. 2017, 98, 39–57. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis Second Edition: A Regression-Based Approach, 2nd ed.; The Guilford Press: New York, NY, USA, 2018. [Google Scholar]
- Hayes, A.F.; Matthes, J. Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behav. Res. Methods 2009, 41, 924–936. [Google Scholar] [CrossRef] [PubMed]
- Franco, I.; Bianco, A.; Mirizzi, A.; Campanella, A.; Bonfiglio, C.; Sorino, P.; Notarnicola, M.; Tutino, V.; Cozzolongo, R.; Giannuzzi, V.; et al. Physical Activity and Low Glycemic Index Mediterranean Diet: Main and Modification Effects on NAFLD Score. Results from a Randomized Clinical Trial. Nutrients 2020, 13, 66. [Google Scholar] [CrossRef]
- Kim, A.; Krishnan, A.; Hamilton, J.P.; Woreta, T.A. The Impact of Dietary Patterns and Nutrition in Nonalcoholic Fatty Liver Disease. Gastroenterol. Clin. N. Am. 2021, 50, 217–241. [Google Scholar] [CrossRef]
- Salehi-Sahlabadi, A.; Sadat, S.; Beigrezaei, S.; Pourmasomi, M.; Feizi, A.; Ghiasvand, R.; Hadi, A.; Clark, C.C.T.; Miraghajani, M. Dietary patterns and risk of non-alcoholic fatty liver disease. BMC Gastroenterol. 2021, 21, 41. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Corey, K.E.; Lim, J.K. AGA Clinical Practice Update on Lifestyle Modification Using Diet and Exercise to Achieve Weight Loss in the Management of Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology 2021, 160, 912–918. [Google Scholar] [CrossRef]
- Funuyet-Salas, J.; Pérez-San-Gregorio, M.Á.; Martín-Rodríguez, A.; Romero-Gómez, M. Quality of Life and Coping in Nonalcoholic Fatty Liver Disease: Influence of Diabetes and Obesity. Int. J. Environ. Res. Public Health 2021, 18, 3503. [Google Scholar] [CrossRef] [PubMed]
- Aller, R.; Burgueño-Gómez, B.; Sigüenza, R.; Fernández-Rodríguez, C.; Fernández, N.; Antolín, B.; Durà, M.; Pina, M.; Lo-renzo, S.; García, C.; et al. Comparative study of overweight and obese patients with nonalcoholic fatty liver disease. Rev. Española Enferm. Dig. 2019, 111, 256–263. [Google Scholar] [CrossRef]
- Lotfi, A.; Saneei, P.; Hekmatdost, A.; Salehisahlabadi, A.; Shiranian, A.; Ghiasvand, R. The relationship between dietary antioxidant intake and physical activity rate with nonalcoholic fatty liver disease (NAFLD): A case—Control study. Clin. Nutr. ESPEN 2019, 34, 45–49. [Google Scholar] [CrossRef]
- Vela, M.V.B.; Abete, I.; Zulet, M.D.L.Á.; Tur, J.A.; Pintó, X.; Corbella, E.; González, M.Á.M.; Corella, D.; Macías, M.; Tinahones, F.; et al. Risk factors differentially associated with non-alcoholic fatty liver disease in males and females with metabolic syndrome. Rev. Esp. Enferm. Dig. 2019, 112, 94–100. [Google Scholar] [CrossRef]
- Khalatbari-Soltani, S.; Marques-Vidal, P.; Imamura, F.; Forouhi, N.G. Prospective association between adherence to the Mediterranean diet and hepatic steatosis: The Swiss CoLaus cohort study. BMJ Open 2020, 10, e040959. [Google Scholar] [CrossRef] [PubMed]
- Cuschieri, S.; Libra, M. Adherence to the Mediterranean Diet in Maltese Adults. Int. J. Environ. Res. Public Health 2020, 18, 10. [Google Scholar] [CrossRef]
- Giraldi, L.; Miele, L.; Aleksovska, K.; Manca, F.; Leoncini, E.; Biolato, M.; Arzani, D.; Pirro, M.A.; Marrone, G.; Cefalo, C.; et al. Mediterranean diet and the prevention of non-alcoholic fatty liver disease: Results from a case-control study. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7391–7398. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.H.; Kim, H.J.; Park, E.-C.; Jang, S.-I. Association between sitting time and non-alcoholic fatty liver disease in South Korean population: A cross-sectional study. Lipids Health Dis. 2020, 19, 212. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Stepanova, M.; Henry, L.; Racila, A.; Lam, B.; Pham, H.T.; Hunt, S. A disease-specific quality of life instrument for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis: CLDQ-NAFLD. Liver Int. 2017, 37, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Ohmori, Y.; Ito, H.; Morita, A.; Deura, K.; Miyachi, M.; Saku Cohort Study Group. Associations between depression and unhealthy behaviours related to metabolic syndrome: A cross sectional study. Asia Pac. J. Clin. Nutr. 2017, 26, 130–140. [Google Scholar] [CrossRef]
- Burgess, E.; Hassmén, P.; Pumpa, K.L. Determinants of adherence to lifestyle intervention in adults with obesity: A systematic review. Clin. Obes. 2017, 7, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Gerber, L.H.; Weinstein, A.; Pawloski, L. Role of Exercise in Optimizing the Functional Status of Patients with Nonalcoholic Fatty Liver Disease. Clin. Liver Dis. 2014, 18, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.; Liao, M.; Allegrante, J.P.; Mosca, L. Low Social Support Level is Associated with Non-Adherence to Diet at 1 Year in the Family Intervention Trial for Heart Health (FIT Heart). J. Nutr. Educ. Behav. 2010, 42, 380–388. [Google Scholar] [CrossRef]
- Barberia, A.M.; Attree, M.; Todd, C.; Todd, C. Understanding eating behaviours in Spanish women enrolled in a weight-loss treatment. J. Clin. Nurs. 2008, 17, 957–966. [Google Scholar] [CrossRef]
- Ball, K.; Jeffery, R.W.; Abbott, G.; McNaughton, S.A.; Crawford, D. Is healthy behavior contagious: Associations of social norms with physical activity and healthy eating. Int. J. Behav. Nutr. Phys. Act. 2010, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Somerset, S.M.; Graham, L.; Markwell, K. Depression scores predict adherence in a dietary weight loss intervention trial. Clin. Nutr. 2011, 30, 593–598. [Google Scholar] [CrossRef]
- Harvey, J.N.; Lawson, V.L. The importance of health belief models in determining self-care behaviour in diabetes. Diabet. Med. 2009, 26, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Navidian, A.; Kermansaravi, F.; Imani, M. The relationship between weight-efficacy of life style and overweight and obesity. Iran J. Endocrinol. Metab. 2013, 14, 557–564. [Google Scholar]
- Gutteling, J.J.; Duivenvoorden, H.J.; Busschbach, J.J.V.; de Man, R.A.; Darlington, A.-S.E. Psychological Determinants of Health-Related Quality of Life in Patients With Chronic Liver Disease. Psychosomatics 2010, 51, 157–165. [Google Scholar] [CrossRef]
- Linde, J.A.; Jeffery, R.W.; Levy, R.L.; Sherwood, N.E.; Utter, J.; Pronk, N.P.; Boyle, R.G. Binge eating disorder, weight control self-efficacy, and depression in overweight men and women. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.J.; VanWormer, J.J.; Crain, A.L.; Boucher, J.L.; Histon, T.; Caplan, W.; Bowman, J.D.; Pronk, N.P. Weight-Loss Outcomes: A Systematic Review and Meta-Analysis of Weight-Loss Clinical Trials with a Minimum 1-Year Follow-Up. J. Am. Diet. Assoc. 2007, 107, 1755–1767. [Google Scholar] [CrossRef] [PubMed]
- Funuyet-Salas, J.; Martín-Rodríguez, A.; Conrad, R.; Pérez-San-Gregorio, M.Á. Psychological Biomarker Profile in NAFLD/NASH with Advanced Fibrosis. In NAFLD and NASH. Biomarkers in Detection, Diagnosis and Monitoring, 1st ed.; Romero-Gómez, M., Ed.; Springer Nature: Cham, Switzerland, 2020; pp. 205–223. [Google Scholar] [CrossRef]
- Hunt, C.W.; Grant, J.S.; Pritchard, D.A. An empirical study of self-efficacy and social support in diabetes self-management: Implications for home healthcare nurses. Home Healthc. Nurse 2012, 30, 255–262. [Google Scholar] [CrossRef]
- Bellentani, S.; Dalle-Grave, R.; Suppini, A.; Marchesini, G. Fatty Liver Italian Network. Behavior therapy for nonalcoholic fatty liver disease: The need for a multidisciplinary approach. Hepatology 2008, 47, 746–754. [Google Scholar] [CrossRef]
- Fabricatore, A.N. Behavior Therapy and Cognitive-Behavioral Therapy of Obesity: Is There a Difference? J. Am. Diet. Assoc. 2007, 107, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Hussien, J.; Brunet, J.; Romain, A.J.; Lemelin, L.; Baillot, A. Living with severe obesity: Adults’ physical activity preferences, self-efficacy to overcome barriers and motives. Disabil. Rehabil. 2020, 1, 1–10. [Google Scholar] [CrossRef]
- Cohen, S.M. Concept Analysis of Adherence in the Context of Cardiovascular Risk Reduction. Nurs. Forum 2009, 44, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi-Roshan, M.; Salari, A.; Soltanipour, S. Reliability and Validity of the 14-point mediterranean diet adherence screener among the Iranian high risk population. Mediterr. J. Nutr. Metab. 2018, 11, 323–329. [Google Scholar] [CrossRef]
- Vieira, L.M.; Gottschall, C.B.; Vinholes, D.B.; Martinez-Gonzalez, M.A.; Marcadenti, A. Translation and cross-cultural adaptation of 14-item Mediterranean Diet Adherence Screener and low-fat diet adherence questionnaire. Clin. Nutr. ESPEN 2020, 39, 180–189. [Google Scholar] [CrossRef]
| M (SD) | Adherence to Physical Activity M (SD) | r (p) | Adherence to Diet M (SD) | r (p) | |
|---|---|---|---|---|---|
| Age | 55.1 (11.6) | 925.9 (1130.2) | −0.08 (0.112) | 8.1 (2.3) | 0.18 (<0.001) |
| BMI | 30.8 (5.2) | 925.9 (1130.2) | −0.17 (<0.001) | 8.1 (2.3) | −0.11 (0.025) |
| Total N (%) | Adherence to physical activity M (SD) | t/F (p) | Adherence to diet M (SD) | t/F (p) | |
| Gender Male Female | 252 (61.0) 161 (39.0) | 1027.1 (1228.5) 767.5 (938.2) | t(1, 398.052) = 2.43 (0.016) | 7.9 (2.3) 8.5 (2.3) | t(1, 411) = −2.92 (0.004) |
| Marital status With partner Without partner | 321 (77.7) 92 (22.3) | 905.4 (1126.3) 997.6 (1147.3) | t(1, 411) = 0.69 (0.491) | 8.2 (2.2) 7.9 (2.6) | t(1, 133.560) = −1.09 (0.279) |
| Education Low Medium High | 182 (44.1) 118 (28.6) 113 (27.3) | 883.7 (997.2) 941.8 (1241.7) 977.3 (1214.7) | F(2, 410) = 0.25 (0.775) | 8.4 (2.2) 7.9 (2.2) 8.0 (2.6) | F(2, 410) = 2.50 (0.083) |
| Employment Working Not working | 198 (47.9) 215 (52.1) | 947.8 (1269.5) 905.7 (987.4) | t(1, 411) = 0.38 (0.706) | 7.9 (2.3) 8.4 (2.3) | t(1, 411) = −2.25 (0.025) |
| NASH Absence Presence | 178 (43.1) 235 (56.9) | 955.4 (1210.8) 903.6 (1067.3) | t(1, 411) = 0.46 (0.646) | 8.3 (2.4) 8.0 (2.2) | t(1, 411) = 1.37 (0.170) |
| Significant fibrosis Absence Presence | 257 (62.2) 156 (37.8) | 954.7 (1233.4) 878.5 (937.7) | t(1, 411) = 0.66 (0.508) | 8.1 (2.3) 8.1 (2.3) | t(1, 411) = −0.09 (0.927) |
| Type 2 Diabetes Absence Presence | 279 (67.5) 134 (32.4) | 914.0 (1135.2) 950.8 (1123.6) | t(1, 411) = 0.31 (0.757) | 8.1 (2.3) 8.2 (2.4) | t(1, 411) = −0.23 (0.815) |
| Obesity Absence Presence | 198 (47.9) 215 (52.1) | 1128.7 (1265.3) 739.2 (955.3) | t(1, 365.427) = 3.51 (0.001) | 8.4 (2.3) 7.9 (2.3) | t(1, 411) = 2.06 (0.040) |
| Variables | M (SD) | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|
| 1. Adherence to physical activity | 925.9 (1130.2) | 1 | 0.21 *** | 0.19 *** | 0.16 ** | 0.18 *** | −0.19 *** |
| 2. Adherence to diet | 8.1 (2.3) | 0.21 *** | 1 | 0.09 | 0.22 *** | 0.18 *** | −0.20 *** |
| 3. Physical quality of life | 46.9 (10.5) | 0.19 *** | 0.09 | 1 | 0.34 *** | 0.46 *** | −0.52 *** |
| 4. Social support | 5.9 (1.2) | 0.16 ** | 0.22 *** | 0.34 *** | 1 | 0.56 *** | −0.56 *** |
| 5. Self-efficacy | 64.7 (18.8) | 0.18 *** | 0.18 *** | 0.46 *** | 0.56 *** | 1 | −0.70 *** |
| 6. Depressive symptoms | 2.8 (3.8) | −0.19 *** | −0.20 *** | −0.52 *** | −0.56 *** | −0.70 *** | 1 |
| Self-Efficacy | Effect (SE) | t (p) | Bootstrapped 95% CI | |
|---|---|---|---|---|
| Lower | Upper | |||
| Low: 45.6 (M − 1 SD) | −0.147 (0.016) | −9.06 (<0.001) | −0.179 | −0.115 |
| Medium: 64.7 (M) | −0.061 (0.014) | −4.34 (<0.001) | −0.089 | −0.033 |
| High: 83.8 (M + 1 SD) | 0.025 (0.020) | 1.25 (0.210) | −0.014 | 0.064 |
| Self-Efficacy | Effect (BootSE) | Bootstrapped 95% CI | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Effect 1 | Low: 45.6 (M − 1 SD) | 5.043 (1.957) | 1.391 | 8.915 |
| Effect 2 | Medium: 64.7 (M) | 2.091 (0.904) | 0.509 | 3.963 |
| Effect 3 | High: 83.8 (M + 1 SD) | −0.861 (0.912) | −3.030 | 0.534 |
| Effect 2 − Effect 1 | −2.952 (1.230) | −5.513 | −0.760 | |
| Effect 3 − Effect 1 | −5.903 (2.460) | −10.969 | −1.520 | |
| Effect 3 − Effect 2 | −2.952 (1.230) | −5.513 | −0.760 | |
| Self-Efficacy | Effect (SE) | t (p) | Bootstrapped 95% CI | |
|---|---|---|---|---|
| Lower | Upper | |||
| Low: 45.6 (M − 1 SD) | −1.060 (0.134) | −7.92 (<0.001) | −1.323 | −0.797 |
| Medium: 64.7 (M) | −0.549 (0.128) | −4.30 (<0.001) | −0.799 | −0.298 |
| High: 83.8 (M + 1 SD) | −0.037 (0.177) | −0.21 (0.832) | −0.385 | 0.310 |
| Self-Efficacy | Effect (BootSE) | Bootstrapped 95% CI | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Effect 1 | Low: 45.6 (M − 1 SD) | 0.090 (0.042) | 0.013 | 0.177 |
| Effect 2 | Medium: 64.7 (M) | 0.047 (0.023) | 0.006 | 0.097 |
| Effect 3 | High: 83.8 (M + 1 SD) | 0.003 (0.015) | −0.027 | 0.036 |
| Effect 2 − Effect 1 | −0.043 (0.021) | −0.088 | −0.006 | |
| Effect 3 − Effect 1 | −0.087 (0.042) | −0.176 | −0.013 | |
| Effect 3 − Effect 2 | −0.043 (0.021) | −0.088 | −0.006 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Funuyet-Salas, J.; Martín-Rodríguez, A.; Pérez-San-Gregorio, M.Á.; Romero-Gómez, M. Influence of Psychological Biomarkers on Therapeutic Adherence by Patients with Non-Alcoholic Fatty Liver Disease: A Moderated Mediation Model. J. Clin. Med. 2021, 10, 2208. https://doi.org/10.3390/jcm10102208
Funuyet-Salas J, Martín-Rodríguez A, Pérez-San-Gregorio MÁ, Romero-Gómez M. Influence of Psychological Biomarkers on Therapeutic Adherence by Patients with Non-Alcoholic Fatty Liver Disease: A Moderated Mediation Model. Journal of Clinical Medicine. 2021; 10(10):2208. https://doi.org/10.3390/jcm10102208
Chicago/Turabian StyleFunuyet-Salas, Jesús, Agustín Martín-Rodríguez, María Ángeles Pérez-San-Gregorio, and Manuel Romero-Gómez. 2021. "Influence of Psychological Biomarkers on Therapeutic Adherence by Patients with Non-Alcoholic Fatty Liver Disease: A Moderated Mediation Model" Journal of Clinical Medicine 10, no. 10: 2208. https://doi.org/10.3390/jcm10102208
APA StyleFunuyet-Salas, J., Martín-Rodríguez, A., Pérez-San-Gregorio, M. Á., & Romero-Gómez, M. (2021). Influence of Psychological Biomarkers on Therapeutic Adherence by Patients with Non-Alcoholic Fatty Liver Disease: A Moderated Mediation Model. Journal of Clinical Medicine, 10(10), 2208. https://doi.org/10.3390/jcm10102208






