Osteoglycin as a Potential Biomarker of Mild Kidney Function Impairment in Type 2 Diabetes Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical Evaluation
2.3. Biochemical Measurements
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
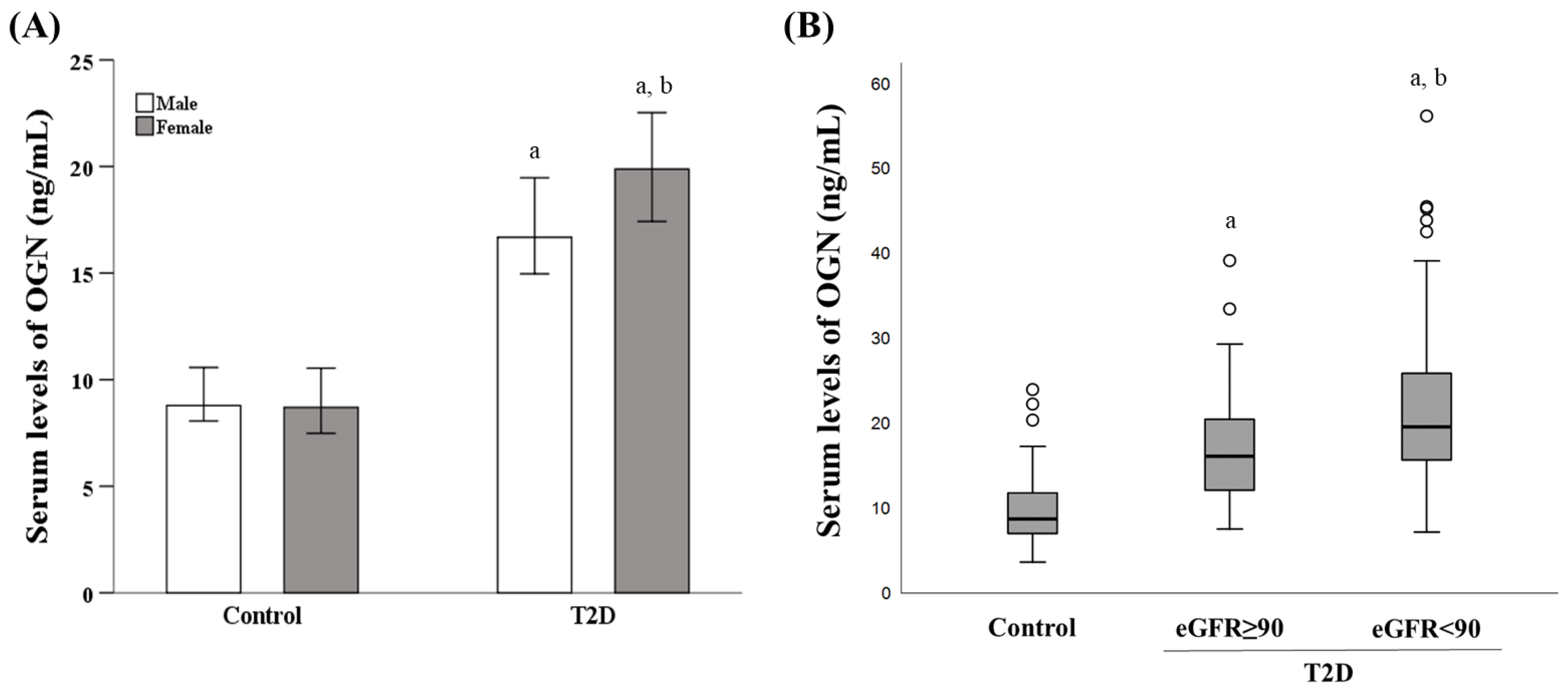
3.2. Influence of Diabetes Status, Sex, and eGFR on the Serum OGN Levels
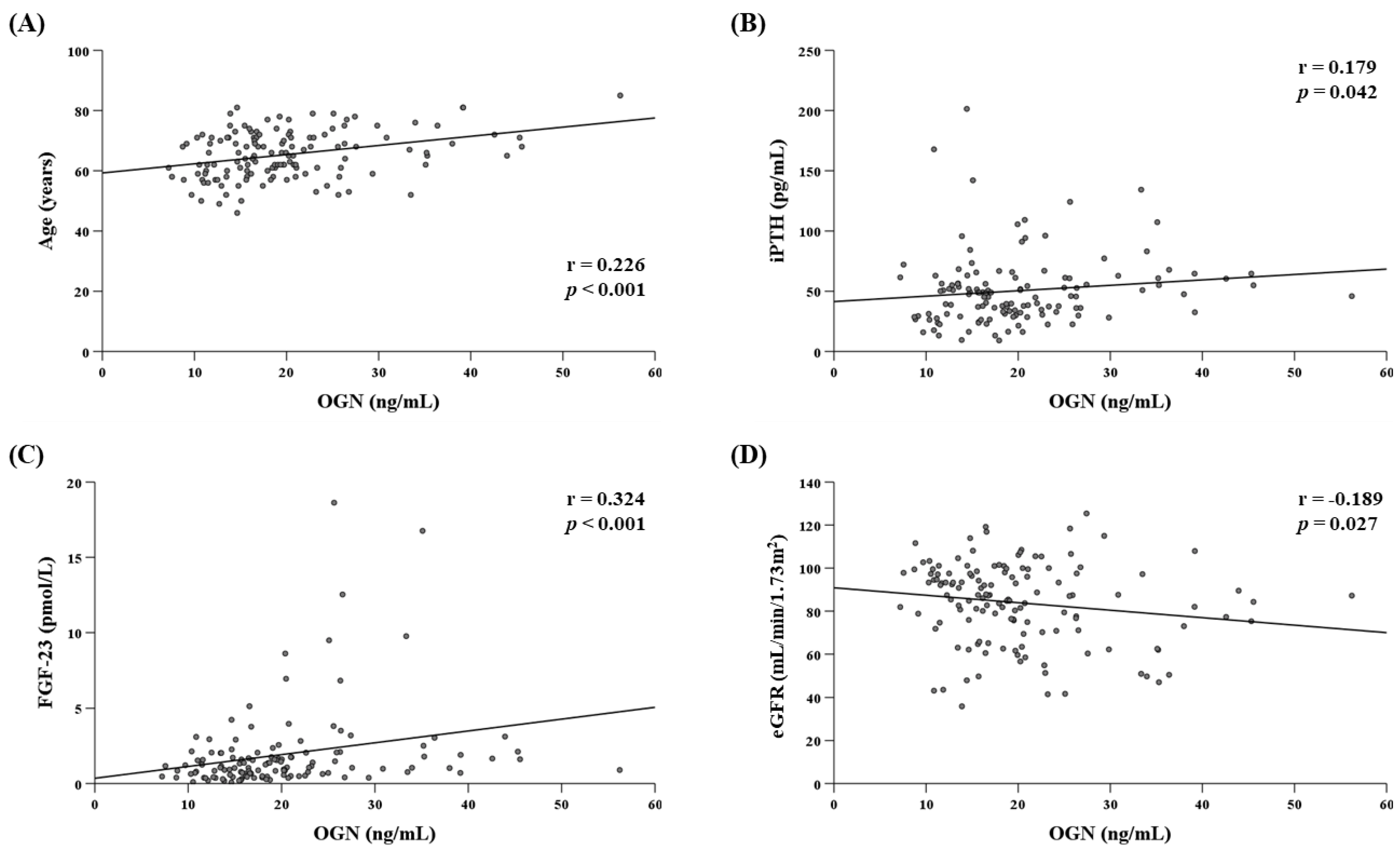
3.3. Determinants of Serum OGN Levels in the T2D Group
3.4. Association between FGF-23 Levels with Kidney Function and CVD
3.5. Usefulness of the OGN Serum Level to Estimate Impaired Kidney Function Risk in T2D Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, S.; Birk, D.E. The regulatory roles of small leucine-rich proteoglycans in extracellular matrix assembly. FEBS J. 2013, 280, 2120–2137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deckx, S.; Heymans, S.; Papageorgiou, A.-P. The diverse functions of osteoglycin: A deceitful dwarf, or a master regulator of disease? FASEB J. 2016, 30, 2651–2661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starup-Linde, J.; Viggers, R.; Handberg, A. Osteoglycin and bone–A systematic review. Curr. Osteoporos. Rep. 2019. [Google Scholar] [CrossRef] [PubMed]
- Van Aelst, L.N.L.; Voss, S.; Carai, P.; Van Leeuwen, R.; Vanhoutte, D.; Sanders-van Wijk, S.; Eurlings, L.; Swinnen, M.; Verheyen, F.K.; Verbeken, E.; et al. Osteoglycin prevents cardiac dilatation and dysfunction after myocardial infarction through infarct collagen strengthening. Circ. Res. 2015, 116, 425–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tasheva, E.S.; Corpuz, L.M.; Funderburgh, J.L.; Conrad, G.W. Differential splicing and alternative polyadenylation generate multiple mimecan MRNA transcripts. J. Biol. Chem. 1997, 272, 32551–32556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, K.; Matsumoto, E.; Higashimaki, Y.; Katagiri, T.; Sugimoto, T.; Seino, S.; Kaji, H. Role of osteoglycin in the linkage between muscle and bone. J. Biol. Chem. 2012, 287, 11616–11628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, N.J.; Ali, N.; Zhang, L.; Qi, Y.; Clarke, I.; Enriquez, R.F.; Brzozowska, M.; Lee, I.C.; Rogers, M.J.; Laybutt, D.R.; et al. Osteoglycin, a novel coordinator of bone and glucose homeostasis. Mol. Metab. 2018, 13, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Bentz, H.; Chang, R.J.; Thompson, A.Y.; Glaser, C.B.; Rosen, D.M. Amino acid sequence of bovine osteoinductive factor. J. Biol. Chem. 1990, 265, 5024–5029. [Google Scholar] [CrossRef]
- Kukita, A.; Bonewald, L.; Rosen, D.; Seyedin, S.; Mundy, G.R.; Roodman, G.D. Osteoinductive factor inhibits formation of human osteoclast-like cells. Proc. Natl. Acad. Sci. USA 1990, 87, 3023–3026. [Google Scholar] [CrossRef] [Green Version]
- Shanahan, C.M.; Cary, N.R.; Osbourn, J.K.; Weissberg, P.L. Identification of osteoglycin as a component of the vascular matrix. differential expression by vascular smooth muscle cells during neointima formation and in atherosclerotic plaques. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2437–2447. [Google Scholar] [CrossRef]
- Cheng, J.M.; Akkerhuis, K.M.; Meilhac, O.; Oemrawsingh, R.M.; Garcia-Garcia, H.M.; van Geuns, R.-J.; Piquer, D.; Merle, D.; du Paty, E.; Galéa, P.; et al. Circulating osteoglycin and NGAL/MMP9 complex concentrations predict 1-year major adverse cardiovascular events after coronary angiography. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1078–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adler, A.I.; Stevens, R.J.; Manley, S.E.; Bilous, R.W.; Cull, C.A.; Holman, R.R.; UKPDS Group. Development and progression of nephropathy in type 2 diabetes: The United Kingdom Prospective Diabetes study (UKPDS 64). Kidney Int. 2003, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloomgarden, Z.T. Diabetic nephropathy. Diabetes Care 2008, 31, 823–827. [Google Scholar] [CrossRef] [Green Version]
- Buyadaa, O.; Magliano, D.J.; Salim, A.; Koye, D.N.; Shaw, J.E. Risk of rapid kidney function decline, all-cause mortality, and major cardiovascular events in nonalbuminuric chronic kidney disease in type 2 diabetes. Diabetes Care 2020, 43, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Penno, G.; Solini, A.; Orsi, E.; Bonora, E.; Fondelli, C.; Trevisan, R.; Vedovato, M.; Cavalot, F.; Lamacchia, O.; Scardapane, M.; et al. Non-albuminuric renal impairment is a strong predictor of mortality in individuals with type 2 diabetes: The Renal Insufficiency And Cardiovascular Events (RIACE) Italian Multicentre study. Diabetologia 2018, 61, 2277–2289. [Google Scholar] [CrossRef] [Green Version]
- MacIsaac, R.J.; Tsalamandris, C.; Panagiotopoulos, S.; Smith, T.J.; McNeil, K.J.; Jerums, G. Nonalbuminuric renal insufficiency in type 2 diabetes. Diabetes Care 2004, 27, 195–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dwyer, J.P.; Parving, H.-H.; Hunsicker, L.G.; Ravid, M.; Remuzzi, G.; Lewis, J.B. Renal dysfunction in the presence of normoalbuminuria in type 2 diabetes: Results from the DEMAND study. Cardiorenal. Med. 2012, 2, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, M.C.; Macisaac, R.J.; Jerums, G.; Weekes, A.; Moran, J.; Shaw, J.E.; Atkins, R.C. Nonalbuminuric renal impairment in type 2 diabetic patients and in the general population (National Evaluation of the Frequency of Renal Impairment CO-Existing with NIDDM [NEFRON] 11). Diabetes Care 2009, 32, 1497–1502. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wang, Y.; Zheng, R.; Zhao, Z.; Ma, Y. Osteoinductive factor is a novel biomarker for the diagnosis of early diabetic nephropathy. Int. J. Clin. Exp. Pathol. 2015, 8, 3110–3115. [Google Scholar] [PubMed]
- Wei, W.; Tu, M.; Huang, R.; Chen, T. Serum osteoinductive factor is associated with microalbuminuria and diabetic nephropathy in type 2 diabetes. Medicine 2018, 97, e11759. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of medical care in diabetes-2017: Summary of revisions. Diabetes Care 2017, 40, S4–S5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Topolski, T.D.; LoGerfo, J.; Patrick, D.L.; Williams, B.; Walwick, J.; Patrick, M.B. The Rapid Assessment of Physical Activity (RAPA) among older adults. Prev. Chronic Dis. 2006, 3, A118. [Google Scholar] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, B.-Y.; Li, X.-L.; Wang, Y.-J.; Zhang, Z.; Pei, F.; Wang, Q.-Z.; Zhang, J.; Cai, Y.-W.; Cheng, M.; et al. Restoration of mimecan expression by grape seed procyanidin B2 through regulation of nuclear factor-KappaB in mice with diabetic nephropathy. Iran J. Kidney Dis. 2016, 10, 325–331. [Google Scholar] [PubMed]
- Dunkler, D.; Gao, P.; Lee, S.F.; Heinze, G.; Clase, C.M.; Tobe, S.; Teo, K.K.; Gerstein, H.; Mann, J.F.E.; Oberbauer, R.; et al. Risk prediction for early CKD in type 2 diabetes. Clin. J. Am. Soc. Nephrol. 2015, 10, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Solini, A.; Penno, G.; Bonora, E.; Fondelli, C.; Orsi, E.; Arosio, M.; Trevisan, R.; Vedovato, M.; Cignarelli, M.; Andreozzi, F.; et al. Diverging association of reduced glomerular filtration rate and albuminuria with coronary and noncoronary events in patients with type 2 diabetes: The Renal Insufficiency and Cardiovascular Events (RIACE) Italian Multicenter study. Diabetes Care 2012, 35, 143–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fliser, D.; Kollerits, B.; Neyer, U.; Ankerst, D.P.; Lhotta, K.; Lingenhel, A.; Ritz, E.; Kronenberg, F.; MMKD Study Group; Kuen, E.; et al. Fibroblast Growth Factor 23 (FGF23) predicts progression of chronic kidney disease: The Mild to Moderate Kidney Disease (MMKD) study. J. Am. Soc. Nephrol. 2007, 18, 2600–2608. [Google Scholar] [CrossRef] [PubMed]
- Coen, G.; Manni, M.; Mantella, D.; Pierantozzi, A.; Balducci, A.; Condò, S.; DiGiulio, S.; Yancovic, L.; Lippi, B.; Manca, S.; et al. Are PTH serum levels predictive of coronary calcifications in haemodialysis patients? Nephrol. Dial. Transplant. 2007, 22, 3262–3267. [Google Scholar] [CrossRef]
- Yeung, S.M.H.; Bakker, S.J.L.; Laverman, G.D.; De Borst, M.H. Fibroblast Growth Factor 23 and adverse clinical outcomes in type 2 diabetes: A bitter-sweet symphony. Curr. Diab. Rep. 2020, 20, 50. [Google Scholar] [CrossRef] [PubMed]
- Frimodt-Møller, M.; von Scholten, B.J.; Reinhard, H.; Jacobsen, P.K.; Hansen, T.W.; Persson, F.I.; Parving, H.-H.; Rossing, P. Growth Differentiation Factor-15 and Fibroblast Growth Factor-23 are associated with mortality in type 2 diabetes–An observational follow-up study. PLoS ONE 2018, 13, e0196634. [Google Scholar] [CrossRef] [Green Version]
- Chan, G.C.; Divers, J.; Russell, G.B.; Langefeld, C.D.; Wagenknecht, L.E.; Hsu, F.-C.; Xu, J.; Smith, S.C.; Palmer, N.D.; Hicks, P.J.; et al. FGF23 concentration and APOL1 genotype are novel predictors of mortality in African Americans with type 2 diabetes. Diabetes Care 2018, 41, 178–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isakova, T.; Xie, H.; Yang, W.; Xie, D.; Anderson, A.H.; Scialla, J.; Wahl, P.; Gutiérrez, O.M.; Steigerwalt, S.; He, J.; et al. Fibroblast Growth Factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 2011, 305, 2432–2439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kendrick, J.; Cheung, A.K.; Kaufman, J.S.; Greene, T.; Roberts, W.L.; Smits, G.; Chonchol, M.; HOST Investigators. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J. Am. Soc. Nephrol. 2011, 22, 1913–1922. [Google Scholar] [CrossRef] [PubMed]
- Larsson, T.; Nisbeth, U.; Ljunggren, O.; Jüppner, H.; Jonsson, K.B. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003, 64, 2272–2279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niewczas, M.A.; Gohda, T.; Skupien, J.; Smiles, A.M.; Walker, W.H.; Rosetti, F.; Cullere, X.; Eckfeldt, J.H.; Doria, A.; Mayadas, T.N.; et al. Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J. Am. Soc. Nephrol. 2012, 23, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Looker, H.C.; Colombo, M.; Hess, S.; Brosnan, M.J.; Farran, B.; Dalton, R.N.; Wong, M.C.; Turner, C.; Palmer, C.N.A.; Nogoceke, E.; et al. Biomarkers of rapid chronic kidney disease progression in type 2 diabetes. Kidney Int. 2015, 88, 888–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinzel, A.; Kammer, M.; Mayer, G.; Reindl-Schwaighofer, R.; Hu, K.; Perco, P.; Eder, S.; Rosivall, L.; Mark, P.B.; Ju, W.; et al. Validation of plasma biomarker candidates for the prediction of EGFR decline in patients with type 2 diabetes. Diabetes Care 2018, 41, 1947–1954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tancredi, M.; Rosengren, A.; Svensson, A.-M.; Kosiborod, M.; Pivodic, A.; Gudbjörnsdottir, S.; Wedel, H.; Clements, M.; Dahlqvist, S.; Lind, M. Excess mortality among persons with type 2 diabetes. N. Engl. J. Med. 2015, 373, 1720–1732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.-H.; Yang, W.-C.; Tsai, S.-T.; Tung, T.-H.; Chou, P. A community-based study of chronic kidney disease among type 2 diabetics in Kinmen, Taiwan. Diabetes Res. Clin. Pract. 2007, 75, 306–312. [Google Scholar] [CrossRef]
- Shen, Y.; Ding, F.H.; Zhang, R.Y.; Zhang, Q.; Lu, L.; Shen, W.F. Association of serum mimecan with angiographic coronary collateralization in patients with stable coronary artery disease and chronic total occlusion. Atherosclerosis 2016, 252, 75–81. [Google Scholar] [CrossRef]
- Seki, T.; Saita, E.; Kishimoto, Y.; Ibe, S.; Miyazaki, Y.; Miura, K.; Ohmori, R.; Ikegami, Y.; Kondo, K.; Momiyama, Y. Low levels of plasma osteoglycin in patients with complex coronary lesions. J. Atheroscler. Thromb. 2018, 25, 1149–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, X.; Zhao, L.; Zhu, J.; Gu, H.; Li, H.; Wang, L.; Xu, W.; Chen, J. Serum mimecan is associated with arterial stiffness in hypertensive patients. J. Am. Heart Assoc. 2015, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moncayo-Arlandi, J.; López-García, A.; Fernández, M.C.; Durán, A.C.; Fernández, B. Osteoglycin deficiency does not affect atherosclerosis in mice. Atherosclerosis 2014, 237, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Tengryd, C.; Nielsen, S.H.; Cavalera, M.; Bengtsson, E.; Genovese, F.; Karsdal, M.; Dunér, P.; Orho-Melander, M.; Nilsson, J.; Edsfeldt, A.; et al. The proteoglycan mimecan is associated with carotid plaque vulnerability and increased risk of future cardiovascular death. Atherosclerosis 2020, 313, 88–95. [Google Scholar] [CrossRef] [PubMed]

| eGFR (mL/min/1.73 m2) | |||
|---|---|---|---|
| eGFR ≥ 90 | eGFR < 90 | p | |
| Patients (n) | 62 | 85 | |
| Men/women (%) | 56/44 | 60/40 | 0.666 |
| Age (years) | 62 ± 7 | 68 ± 8 | <0.001 * |
| eGFR (mL/min/1.73 m2) | 100 ± 7 | 69 ± 15 | <0.001 * |
| CLINICAL EVALUATION | |||
| Body weight (kg) | 86 ± 15 | 87 ± 13 | 0.838 |
| Height (cm) | 165 ± 0.09 | 165 ± 0.08 | 0.717 |
| BMI (kg/m2) | 32 ± 5 | 32 ± 4 | 0.944 |
| Waist circumference (cm) | 106 ± 11 | 106 ± 10 | 0.900 |
| Diabetes duration (years) | 14 ± 10 | 15 ± 9 | 0.468 |
| Systolic blood pressure (mmHg) | 133 ± 17 | 137 ± 18 | 0.133 |
| Diastolic blood pressure (mmHg) | 79 ± 9 | 79 ± 12 | 0.833 |
| UACR ≥ 30 mg/g (%) | 21 | 24 | 0.657 |
| Hypertension (%) | 76 | 92 | 0.007 * |
| Dyslipidemia (%) | 82 | 93 | 0.045 * |
| CVD (%) | 25.8 | 43.5 | 0.027 * |
| Osteoporosis (%) | 9.7 | 5.9 | 0.399 |
| Smoker or ex-smoker (%) | 48 | 46 | 0.843 |
| Alcohol consumption excessive (%) | 20 | 13 | 0.271 |
| Sedentarism (%) | 15 | 17 | 0.735 |
| CURRENT MEDICATION USE | |||
| Insulin (%) | 10 | 14 | 0.756 |
| Oral antidiabetic drugs (%) | 31 | 28 | 0.551 |
| Insulin + Oral antidiabetic drugs (%) | 59 | 58 | 0.423 |
| BIOCHEMICAL MEASUREMENTS | |||
| FPG (mg/dL) | 150 ± 52 | 150 ± 55 | 0.989 |
| HbA1c (mmol/mol) | 62 ± 16 | 63 ± 15 | 0.792 |
| HbA1c (%) | 7.8 ± 1.4 | 7.9 ± 1.3 | 0.792 |
| TG (mg/dL) | 158 ± 71 | 166 ± 85 | 0.568 |
| HDL-c (mg/dL) | 47 ± 13 | 44 ± 10 | 0.128 |
| LDL-c (mg/dL) | 98 ± 44 | 88 ± 37 | 0.127 |
| Calcium (mg/dL) | 9.8 ± 0.4 | 9.7 ± 0.4 | 0.368 |
| Phosphorous (mg/dL) | 3.4 ± 0.5 | 3.3 ± 0.4 | 0.098 |
| 25(OH)D (ng/mL) | 20 ± 8 | 22 ± 9 | 0.167 |
| iPTH (pg/mL) | 44 ± 25 | 56 ± 34 | 0.029 * |
| FGF-23 (pmol/L) | 0.86 (0.47–1.70) | 1.25 (0.77–2.44) | 0.028 * |
| OGN (ng/mL) | 16.14 (12.13–20.48) | 19.59 (15.70–26.90) | 0.002 * |
| Variables | B | 95% CI (Lower Limit/Upper Limit) | p |
|---|---|---|---|
| Age | 0.319 | 0.091/0.547 | 0.007 * |
| Sex | −3.090 | −6.550/0.371 | 0.080 |
| iPTH | 0.018 | −0.043/0.080 | 0.554 |
| FGF-23 | 0.838 | 0.275/1.400 | 0.004 * |
| eGFR | −0.036 | −0.127/0.056 | 0.440 |
| Insulin treatment | −0.089 | −0.274/0.097 | 0.347 |
| Current medication | −3.131 | −6.796/0.535 | 0.093 |
| UACR ≥ 30 mg/g | 0.024 | −0.003/0.051 | 0.076 |
| Presence of osteoporosis | −0.559 | −6.128/5.010 | 0.842 |
| Presence of CVD | −3.243 | −6.641/0.156 | 0.061 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Salvatierra, S.; García-Fontana, C.; Andújar-Vera, F.; Grau-Perales, A.B.; Martínez-Heredia, L.; Avilés-Pérez, M.D.; Hayón-Ponce, M.; Iglesias-Baena, I.; Riquelme-Gallego, B.; Muñoz-Torres, M.; et al. Osteoglycin as a Potential Biomarker of Mild Kidney Function Impairment in Type 2 Diabetes Patients. J. Clin. Med. 2021, 10, 2209. https://doi.org/10.3390/jcm10102209
González-Salvatierra S, García-Fontana C, Andújar-Vera F, Grau-Perales AB, Martínez-Heredia L, Avilés-Pérez MD, Hayón-Ponce M, Iglesias-Baena I, Riquelme-Gallego B, Muñoz-Torres M, et al. Osteoglycin as a Potential Biomarker of Mild Kidney Function Impairment in Type 2 Diabetes Patients. Journal of Clinical Medicine. 2021; 10(10):2209. https://doi.org/10.3390/jcm10102209
Chicago/Turabian StyleGonzález-Salvatierra, Sheila, Cristina García-Fontana, Francisco Andújar-Vera, Alejandro Borja Grau-Perales, Luis Martínez-Heredia, María Dolores Avilés-Pérez, María Hayón-Ponce, Iván Iglesias-Baena, Blanca Riquelme-Gallego, Manuel Muñoz-Torres, and et al. 2021. "Osteoglycin as a Potential Biomarker of Mild Kidney Function Impairment in Type 2 Diabetes Patients" Journal of Clinical Medicine 10, no. 10: 2209. https://doi.org/10.3390/jcm10102209
APA StyleGonzález-Salvatierra, S., García-Fontana, C., Andújar-Vera, F., Grau-Perales, A. B., Martínez-Heredia, L., Avilés-Pérez, M. D., Hayón-Ponce, M., Iglesias-Baena, I., Riquelme-Gallego, B., Muñoz-Torres, M., & García-Fontana, B. (2021). Osteoglycin as a Potential Biomarker of Mild Kidney Function Impairment in Type 2 Diabetes Patients. Journal of Clinical Medicine, 10(10), 2209. https://doi.org/10.3390/jcm10102209







