Rate of Post-Operative Pancreatic Fistula after Robotic-Assisted Pancreaticoduodenectomy with Pancreato-Jejunostomy versus Pancreato-Gastrostomy: A Retrospective Case Matched Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Endpoints
2.2. Definitions
2.3. Surgical Technique
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Testini, M.; Piccinni, G.; Lissidini, G.; Gurrado, A.; Tedeschi, M.; Franco, I.; Di Meo, G.; Pasculli, A.; De Luca, G.; Ribezzi, M.; et al. Surgical management of the pancreatic stump following pancreato-duodenectomy. J. Visc. Surg. 2016, 153, 193–202. [Google Scholar] [CrossRef]
- McMillan, M.T.; Malleo, G.; Bassi, C.; Allegrini, V.; Casetti, L.; Drebin, J.A.; Esposito, A.; Landoni, L.; Lee, M.K.; Pulvirenti, A.; et al. Multicenter, Prospective Trial of Selective Drain Management for Pancreatoduodenectomy Using Risk Stratification. Ann. Surg. 2017, 265, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.B.; Dua, M.M.; Worhunsky, D.J.; Poultsides, G.A.; Norton, J.A.; Visser, B.C. The First Decade of Laparoscopic Pancreaticoduodenectomy in the United States: Costs and Outcomes Using the Nationwide Inpatient Sample. Surg. Endosc. 2016, 30, 1778–1783. [Google Scholar] [CrossRef]
- Traverso, L.W.; Longmire, W.P., Jr. Preservation of the pylorus in pancreaticoduodenectomy. Surg. Gynecol. Obstet. 1978, 146, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Crippa, S.; Cirocchi, R.; Randolph, J.; Partelli, S.; Belfiori, G.; Piccioli, A.; Parisi, A.; Falconi, M. Pancreaticojejunostomy is comparable to pancreaticogastrostomy after pancreaticoduodenectomy: An updated meta-analysis of randomized controlled trials. Langenbeck’s Arch. Surg. 2016, 401, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Menahem, B.; Guittet, L.; Mulliri, A.; Alves, A.; Lubrano, J. Pancreaticogastrostomy is superior to pancreaticojejunostomy for prevention of pancreatic fistula after pancreaticoduodenectomy: An updated meta-analysis of randomized controlled trials. Ann. Surg. 2015, 261, 882–887. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, Q.; Gao, S.; Lou, J.; Li, G.; Zhang, Y.; Ma, T.; Zhang, Y.; Xu, Y.; Liang, T. Duct-to-Mucosa vs Invagination for Pancreaticojejunostomy after Pancreaticoduodenectomy: A Prospective, Randomized Controlled Trial from a Single Surgeon. J. Am. Coll. Surg. 2016, 222, 10–18. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, B.; Shi, S.; Qin, Y.; Ji, S.; Xu, W.; Liu, J.; Liu, L.; Liu, C.; Long, J.; et al. Papillary-like main pancreatic duct invaginated pancreaticojejunostomy versus duct-to-mucosa pancreaticojejunostomy after pancreaticoduodenectomy: A prospective randomized trial. Surgery 2015, 158, 1211–1218. [Google Scholar] [CrossRef]
- Zhu, F.; Wang, M.; Wang, X.; Tian, R.; Shi, C.; Xu, M.; Shen, M.; Han, J.; Luo, N.; Qin, R. Modified Technique of Pancreaticogastrostomy for Soft Pancreas with Two Continuous Hemstitch Sutures: A Single-Center Prospective Study. J. Gastrointest. Surg. 2013, 17, 1306–1311. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Lai, M.; Wang, X.; Tu, B.; Cheng, N.; Gong, J. Pancreaticogastrostomy versus pancreaticojejunostomy reconstruction for the prevention of pancreatic fistula following pancreaticoduodenectomy. Cochrane Database Syst. Rev. 2016, 12, CD012257. [Google Scholar] [CrossRef]
- Marino, M.V.; Mirabella, A.; Ruiz, M.G.; Komorowski, A.L. Robotic-Assisted versus Laparoscopic Distal Pancreatectomy: The Results of a Case-Matched Analysis from a Tertiary Care Center. Dig. Surg. 2020, 37, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Podda, M.; Gerardi, C.; Di Saverio, S.; Marino, M.V.; Davies, R.J.; Pellino, G.; Pisanu, A. Robotic-assisted versus open pancreaticoduodenectomy for patients with benign and malignant periampullary disease: A systematic review and meta-analysis of short-term outcomes. Surg. Endosc. 2020, 34, 2390–2409. [Google Scholar] [CrossRef] [PubMed]
- Beane, J.D.; Zenati, M.; Hamad, A.; Hogg, M.E.; Zeh, H.J., III; Zureikat, A.H. Robotic pancreaticoduodenectomy with vascular resection: Outcomes and learning curve. Surgery 2019, 166, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Bao, P.Q.; Mazirka, P.O.; Watkins, K.T. Retrospective Comparison of Robot-Assisted Minimally Invasive Versus Open Pancreaticoduodenectomy for Periampullary Neoplasms. J. Gastrointest. Surg. 2014, 18, 682–689. [Google Scholar] [CrossRef]
- Topal, B.; Fieuws, S.; Aerts, R.; Weerts, J.; Feryn, T.; Roeyen, G.; Bertrand, C.; Hubert, C.; Janssens, M.; Closset, J. Belgian Section of Hepatobiliary and Pancreatic Surgery. Pancreaticojejunostomy versus pancreaticogastrostomy reconstruction after pancreaticoduodenectomy for pancreatic or periampullary tumours: A multicenter randomised trial. Lancet Oncol. 2013, 14, 655–662. [Google Scholar] [CrossRef]
- Callery, M.P.; Pratt, W.B.; Kent, T.S.; Chaikof, E.L.; Vollmer, C.M. A Prospectively Validated Clinical Risk Score Accurately Predicts Pancreatic Fistula after Pancreatoduodenectomy. J. Am. Coll. Surg. 2013, 216, 1–14. [Google Scholar] [CrossRef] [PubMed]
- McMillan, M.T.; Ecker, B.L.; Behrman, S.W.; Callery, M.P.; Christein, J.D.; Drebin, J.A.; Fraker, D.L.; Kent, T.S.; Lee, M.K.; Roses, R.E.; et al. Externalized Stents for Pancreatoduodenectomy Provide Value Only in High-Risk Scenarios. J. Gastrointest. Surg. 2016, 20, 2052–2062. [Google Scholar] [CrossRef]
- Shubert, C.R.; Wagie, A.E.; Farnell, M.B.; Nagorney, D.M.; Que, F.G.; Lombardo, K.R.; Truty, M.J.; Smoot, R.L.; Kendrick, M.L. Clinical Risk Score to Predict Pancreatic Fistula after Pancreatoduodenectomy: Independent External Validation for Open and Laparoscopic Approaches. J. Am. Coll. Surg. 2015, 221, 689–698. [Google Scholar] [CrossRef]
- Schuh, F.; Mihaljevic, A.L.; Probst, P.; Trudeau, M.T.; Müller, P.C.; Marchegiani, G.; Besselink, M.G.; Uzunoglu, F.; Izbicki, J.R.; Falconi, M.; et al. A Simple Classification of Pancreatic Duct Size and Texture Predicts Postoperative Pancreatic Fistula. Ann. Surg. 2021. Epub ahead of print. [Google Scholar] [CrossRef]
- Bassi, C.; Marchegiani, G.; Dervenis, C.; Sarr, M.; Abu Hilal, M.; Adham, M.; Allen, P.; Andersson, R.; Asbun, H.J.; Besselink, M.G.; et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017, 161, 584–591. [Google Scholar] [CrossRef] [Green Version]
- Clavien, P.A.; Barkun, J.; De Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; De Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, S.Y.; Wang, J.W.; Lau, W.Y.; Cai, X.J.; Mou, Y.P.; Liu, Y.B.; Li, J.T. Conventional versus binding pancreaticojejunostomy after pancreaticoduodenectomy: A prospective randomized trial. Ann. Surg. 2007, 245, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Wente, M.N.; Bassi, C.; Dervenis, C.; Fingerhut, A.; Gouma, D.J.; Izbicki, J.R.; Neoptolemos, J.P.; Padbury, R.T.; Sarr, M.G.; Traverso, L.W.; et al. Delayed gastric emptying (DGE) after pancreatic surgery: A suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007, 142, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Wente, M.N.; Veit, J.A.; Bassi, C.; Dervenis, C.; Fingerhut, A.; Gouma, D.J.; Izbicki, J.R.; Neoptolemos, J.P.; Padbury, R.T.; Sarr, M.G.; et al. Postpancreatectomy hemorrhage (PPH)–An International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 2007, 142, 20–25. [Google Scholar] [CrossRef]
- Kawai, M.; Tani, M.; Terasawa, H.; Ina, S.; Hirono, S.; Nishioka, R.; Miyazawa, M.; Uchiyama, K.; Yamaue, H. Early removal of prophylactic drains reduces the risk of intra-abdominal infections in patients with pancreatic head resection: Prospective study for 104 consecutive patients. Ann. Surg. 2006, 244, 1–7. [Google Scholar] [CrossRef]
- Marino, M.V.; Podda, M.; Ruiz, M.G.; Fernandez, C.C.; Guarrasi, D.; Fleitas, M.G. Robotic-assisted versus open pancreaticoduodenectomy: The results of a case-matched comparison. J. Robot. Surg. 2019, 14, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Giulianotti, P.C.; Gonzalez-Heredia, R.; Esposito, S.; Masrur, M.; Gangemi, A.; Bianco, F.M. Trans-gastric pancreaticogastrostomy reconstruction after pylorus-preserving robotic Whipple: A proposal for a standardized technique. Surg. Endosc. 2018, 32, 2169–2174. [Google Scholar] [CrossRef]
- Hu, B.-Y.; Wan, T.; Zhang, W.-Z.; Dong, J.-H. Risk factors for postoperative pancreatic fistula: Analysis of 539 successive cases of pancreaticoduodenectomy. World J. Gastroenterol. 2016, 22, 7797–7805. [Google Scholar] [CrossRef]
- Pedrazzoli, S. Pancreatoduodenectomy (PD) and postoperative pancreatic fistula (POPF): A systematic review and analysis of the POPF-related mortality rate in 60,739 patients retrieved from the English literature published between 1990 and 2015. Medicine 2017, 96, e6858. [Google Scholar] [CrossRef]
- Vollmer, C.M., Jr.; Sanchez, N.; Gondek, S.; McAuliffe, J.; Kent, T.S.; Christein, J.D.; Callery, M.P.; Pancreatic Surgery Mortality Study Group. Pancreatic Surgery Mortality Study Group. A root-cause analysis of mortality following major pancreatectomy. J. Gastrointest. Surg. 2012, 16, 89–102. [Google Scholar] [CrossRef]
- Boggi, U.; Napoli, N.; Costa, F.; Kauffmann, E.F.; Menonna, F.; Iacopi, S.; Vistoli, F.; Amorese, G. Robotic-assisted pancreatic resections. World J. Surg. 2016, 40, 2497–2506. [Google Scholar] [CrossRef]
- Figueras, J.; Sabater, L.; Planellas, P.; Muñoz-Forner, E.; Lopez-Ben, S.; Falgueras, L.; Sala-Palau, C.; Albiol, M.; Ortega-Serrano, J.; Castro-Gutierrez, E. Randomized clinical trial of pancreaticogastrostomy versus pancreaticojejunostomy on the rate and severity of pancreatic fistula after pancreaticoduodenectomy. BJS 2013, 100, 1597–1605. [Google Scholar] [CrossRef]
- Keck, T.; Wellner, U.F.; Bahra, M.; Klein, F.; Sick, O.; Niedergethmann, M.; Wilhelm, T.J.; Farkas, S.A.; Börner, T.; Bruns, C.; et al. Pancreatogastrostomy Versus Pancreatojejunostomy for RECOnstruction After PANCreatoduodenectomy (RECOPANC, DRKS 00000767). Ann. Surg. 2016, 263, 440–449. [Google Scholar] [CrossRef]
- Jin, Y.; Feng, Y.-Y.; Qi, X.-G.; Hao, G.; Yu, Y.-Q.; Li, J.-T.; Peng, S.-Y. Pancreatogastrostomy vs pancreatojejunostomy after pancreaticoduodenectomy: An updated meta-analysis of RCTs and our experience. World J. Gastrointest. Surg. 2019, 11, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.V.; Podda, M.; Pisanu, A.; di Saverio, S.; Fleitas, M.G. Robotic-assisted Pancreaticoduodenectomy: Technique Description and Performance Evaluation after 60 Cases. Surg. Laparosc. Endosc. Percutaneous Tech. 2020, 30, 156–163. [Google Scholar] [CrossRef] [PubMed]
- McMillan, M.T.; Zureikat, A.H.; Hogg, M.E.; Kowalsky, S.J.; Zeh, H.J.; Sprys, M.H.; Vollmer, C.M. A Propensity Score–Matched Analysis of Robotic vs Open Pancreatoduodenectomy on Incidence of Pancreatic Fistula. JAMA Surg. 2017, 152, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Kantor, O.; Talamonti, M.S.; Pitt, H.A.; Vollmer, C.M.; Riall, T.S.; Hall, B.L.; Wang, C.-H.; Baker, M.S. Using the NSQIP Pancreatic Demonstration Project to Derive a Modified Fistula Risk Score for Preoperative Risk Stratification in Patients Undergoing Pancreaticoduodenectomy. J. Am. Coll. Surg. 2017, 224, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Ramanathan, R.; Zenati, M.S.; Al Abbas, A.; Hogg, M.E.; Zeh, H.J.; Zureikat, A.H. Robotic Pancreaticoduodenectomy Is Associated with Decreased Clinically Relevant Pancreatic Fistulas: A Propensity-Matched Analysis. J. Gastrointest. Surg. 2019, 24, 1111–1118. [Google Scholar] [CrossRef]
- Mungroop, T.H.; Van Rijssen, L.B.; Van Klaveren, D.; Smits, F.J.; Van Woerden, V.; Linnemann, R.J.; De Pastena, M.; Klompmaker, S.; Marchegiani, G.; Ecker, B.L.; et al. Alternative fistula risk score for pancreatoduodenectomy (a-FRS): Design and international external validation. Ann. Surg. 2019, 269, 937–943. [Google Scholar] [CrossRef]
- Polanco, P.M.; Zenati, M.S.; Hogg, M.E.; Shakir, M.; Boone, B.A.; Bartlett, D.L.; Zeh, H.J.; Zureikat, A.H. An analysis of risk factors for pancreatic fistula after robotic pancreaticoduodenectomy: Outcomes from a consecutive series of standardized pancreatic reconstructions. Surg. Endosc. 2016, 30, 1523–1529. [Google Scholar] [CrossRef]
- Lee, S.E.; Jang, J.Y.; Lim, C.S.; Kang, M.J.; Kim, S.H.; Kim, M.A.; Kim, S.W. Measurement of pancreatic fat by magnetic resonance imaging: Predicting the occurrence of pancreatic fistula after pancreatoduodenectomy. Ann. Surg. 2010, 251, 932–936. [Google Scholar] [CrossRef] [PubMed]
| Unsuitability for pneumoperitoneum |
| ASA score > III |
| Body mass index (BMI) < 35 kg/m2 |
| Borderline or Locally advanced tumours |
| Intraperitoneal or extraperitoneal metastases |
| Tumor size > 5 cm |
| Patients who underwent total pancreatectomy |
| Patients requiring concomitant organ or vascular resection |
| Conversion to open |
| Variables | PJ (n = 40) | PG (n = 20) | Overall (n = 60) | p Value |
|---|---|---|---|---|
| Age, years, median (IQR) | 63.2 (55.6–71.4) | 61.9 (53.8–68.5) | 62.9 (54.1–71.1) | 0.688 |
| Sex, n (%) | ||||
| 27 (67.5%) | 13 (65%) | 40 (66.7%) | 0.627 |
| 13 (32.5%) | 7 (35%) | 20 (33.3%) | 0.799 |
| BMI, Kg/m2, mean (±SD) | 25.1 ± 3.4 | 24.8 ± 2.8 | 25 ± 3.2 | 0.824 |
| ASA score, mean (±SD) | 2.5 ± 0.06 | 2.2 ± 0.04 | 2.4 ± 0.7 | 0.856 |
| Pathology | ||||
| 30 (75%) | 15 (75%) | 45 (75%) | 1 |
| 21 | 8 | 29 | |
| 3 | 2 | 5 | |
| 2 | 2 | 4 | |
| 2 | 2 | 4 | |
| 1 | 1 | 2 | |
| 1 | / | 1 | |
| 10 (25%) | 5 (25%) | 15 (25%) | 1 |
| 4 | 2 | 6 | |
| 3 | 1 | 4 | |
| 2 | 1 | 3 | |
| 1 | 1 | 2 | |
| Tumor size, cm, mean (±SD) | 2.86 ± 1.7 | 2.55 ± 1.4 | 2.8 ± 1.6 | 0.822 |
| Neoadjuvant CHT, n (%) | 6 (15%) | 2 (10%) | 8 (13.3%) | 0.479 |
| Pancreatic texture, n (%) | 1 | |||
| 16 (40%) | 8 (40%) | 24 (40%) | |
| 24 (60%) | 12 (60%) | 36 (60%) | |
| Wirsung duct diameter, median ± SD | 3.4 ± 2.4 | 2.9 ± 2.5 | 3.2 ± 2.4 | 0.627 |
| 31 (77.5%) | 14 (70%) | 45 (75%) | 0.669 |
| 9 (22.5%) | 6 (30%) | 15 (25%) | 0.611 |
| ISGPS classification | ||||
| 19 (47.5%) | 9 (45%) | 28 (46.7%) | 0.821 |
| 5 (12.5%) | 3 (15%) | 8 (13.3%) | 0.793 |
| 12 (30%) | 5 (25%) | 17 (28.3%) | 0.645 |
| 4 (10%) | 3 (15%) | 7 (11.7%) | 0.612 |
| Mean CRS-POPF ± SD | 4.6 ± 2.2 | 5.1 ± 1.8 | 4.7 ± 2.1 | 0.433 |
| Histopathology, n (%) | ||||
| 8 (20%) | 5 (25%) | 13 (21.7%) | 0.523 |
| 32 (80%) | 15 (75%) | 47 (78.3%) | |
| Estimated blood loss | ||||
| 8 (20%) | 4 (20%) | 12 (20%) | 1 |
| 32 (80%) | 16 (80%) | 48 (80%) | |
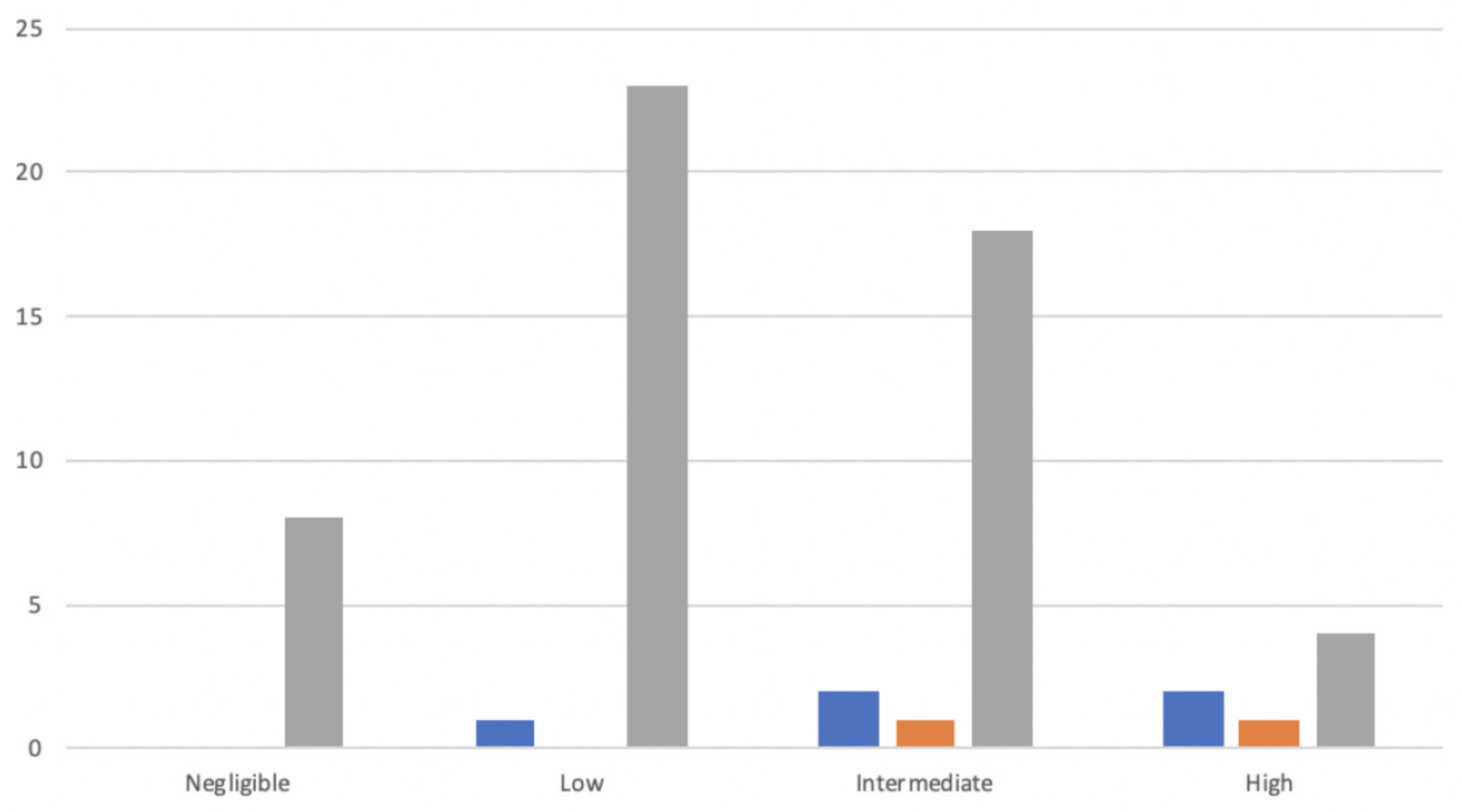
| Categories of POPF risk, n (%) | 0.788 | |||
| 6 (15%) | 2 (10%) | 8 (13.3%) | |
| 16 (40%) | 6 (30%) | 22 (%) | |
| 14 (35%) | 9 (45%) | 23 (35%) | |
| 4 (10%) | 3 (15%) | 7 (11.7%) |
| Variables | PJ (n = 40) | PG (n = 20) | Overall (n = 60) | p Value |
|---|---|---|---|---|
| Operative time, min, median ± SD | 375 ± 102 | 315 ± 110 | 355 ± 103 | 0.345 |
| Time of the anastomoses, min, median ± SD | 32 ± 11 | 25 ± 14 | 30.2 ± 12 | 0.002 |
| Estimated blood loss, ml, median (IQR) | 270 (180–600) | 295 (200–700) | 275 (180–600) | 0.442 |
| Intraoperative blood transfusion, n (%) | 3 (7.5%) | 1 (5%) | 4 (6.7%) | 0.766 |
| Post-operative complications, n (%) | 19 (47.5%) | 9 (45%) | 28 (46.6%) | 0.635 |
| −11 (27.5%) | −6 (30%) | −17 (28.3%) | 0.826 |
| −8 (20%) | −3 (15%) | −11 (18.3%) | 0.542 |
| 5 (12.5%) | 3 (15%) | 8 (13.3%) | 0.524 |
| CR-POPF | 5 (12.5%) | 2 (10%) | 7 (11.7%) | 0.827 |
| −3 (7.5%) | −1 (5%) | −4 (6.7%) | 0.789 |
| −2 (5%) | −1 (5%) | −3 (5%) | 0.977 |
| Delayed gastric emptying, n (%) | 2 (5%) | 1 (5%) | 3 (5%) | 0.928 |
| Grade C Postoperative hemorrhage, n (%) | 1 (2.5%) | 2 (10%) | 3 (5%) | 0.338 |
| Pancreatitis, n (%) | 1 (2.5%) | / | 1 (1.4%) | 0.782 |
| Bile leakage, n (%) | 1 (2.5%) | 1 (5%) | 2 (3.3%) | 0.654 |
| Ascites, n (%) | 1 (2.5%) | / | 1 (1.4%) | 0.782 |
| Intra-abdominal collection, n (%) | 3 (7.5%) | / | 3 (4.3%) | 0.002 |
| Length of hospital stays, days, median ± SD | 14 ± 4 | 11 ± 6 | 15.8 ± 5 | 0.223 |
| Readmission, n (%) | 4 (10%) | 1 (5%) | 5 (8.3%) | 0.524 |
| Reoperation, n (%) | 2 (5%) | 1 (5%) | 3 (5%) | 0.928 |
| Mortality 90-days, n (%) | 2 (5%) | 1 (5%) | 3 (5%) | 0.928 |
| Variables | PJ (n = 20) | PG (n = 20) | p Value |
|---|---|---|---|
| Histopathology, n (%) | |||
| - PDAC/IPMN | 14 (70%) | 15 (75%) | 0.855 |
| - Ampullary, Duodenal, Cystic | 6 (30%) | 5 (25%) | 0.793 |
| Pancreatic texture, n (%) | |||
| - Soft | 13 (65%) | 13 (65%) | 1 |
| - Hard | 7 (35%) | 7 (35%) | 1 |
| Pancreatic duct diameter, mm, n (%) | |||
| - ≥3 | 13 (65%) | 14 (70%) | 0.643 |
| - <3 | 7 (35%) | 6 (30%) | 0.635 |
| ISGPS Classification | |||
| - A | 9 (45%) | 9 (45%) | 1 |
| - B | 3 (15%) | 3 (15%) | 1 |
| - C | 5 (25%) | 5 (25%) | 1 |
| - D | 3 (15%) | 3 (15%) | 1 |
| Intraoperative blood loss, mL, n (%) | |||
| - ≥500 | 5 (25%) | 4 (20%) | 0.617 |
| - <500 | 15 (75%) | 16 (80%) | 0.539 |
| Median Operative time, min (IQR) | 330 (270.2–395.8) | 315 (265–382) | 0.75 |
| Anastomotic time, min (IQR) | 46 (28–52) | 25 (18–40) | 0.002 |
| Morbidity rate, n (%) | 11 (55%) | 9 (45%) | 0.721 |
| - Minor | 5 (25%) | 6 (30%) | 0.586 |
| - Major | 6 (30%) | 3 (15%) | 0.324 |
| Biochemical Leak, n (%) | 4 (20%) | 3 (15%) | 0.721 |
| CR–POPF, n (%) | 3 (15%) | 2 (10%) | 0.478 |
| Delayed gastric emptying, n (%) | 1 (5%) | 1 (5%) | 1 |
| Post-pancreatectomy hemorrhage, n (%) | / | 2 (10%) | 0.003 |
| Intra-abdominal collection, n (%) | 3 (15%) | / | 0.002 |
| Reoperation, n (%) | 2 (10%) | 1 (5%) | 0.474 |
| Median length of hospital stays, days (IQR) | 14.2 (12.4–22) | 11.5 (9.5–19) | 0.165 |
| Variables | CR-POPF (n = 7) | No-POPF (n = 53) | Univariate p Value | Odds Ratio | 95% CI |
|---|---|---|---|---|---|
| Age | |||||
| 4 | 27 | 0.76 | ||
| 3 | 26 | |||
| Sex | |||||
| 4 | 36 | 0.57 | ||
| 3 | 17 | |||
| BMI | |||||
| 5 | 14 | <0.05 | 6.96 | (1.2–40.1) |
| 2 | 39 | |||
| Diabetes | |||||
| 1 | 10 | 0.77 | ||
| 6 | 43 | |||
| ASA score | |||||
| 3 | 29 | 0.55 | ||
| 4 | 24 | |||
| Pancreatic duct diameter | |||||
| 2 | 43 | |||
| 5 | 10 | <0.05 | 10.7 | (1.8–63.6) |
| Underlying pathology | |||||
| PDAC/IPMN/etc. | 4 | 43 | 0.16 | ||
| Ampullary/Cystic/Duodenal | 3 | 10 | |||
| Tumor size | |||||
| 2 | 21 | 0.59 | ||
| 5 | 33 | |||
| Texture of the pancreas | |||||
| 6 | 18 | <0.05 | 11.66 | (1.3–104.4) |
| 1 | 35 | - | ||
| Operative time | |||||
| 4 | 34 | 0.71 | ||
| 3 | 19 | |||
| Blood loss | |||||
| 5 | 7 | <0.05 | 10.95 | (2.1–56.3) |
| 2 | 46 | - | ||
| Reconstruction type | |||||
| 5 | 35 | 0.77 | ||
| 2 | 18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marino, M.V.; Heng Chiow, A.K.; Mirabella, A.; Vaccarella, G.; Komorowski, A.L. Rate of Post-Operative Pancreatic Fistula after Robotic-Assisted Pancreaticoduodenectomy with Pancreato-Jejunostomy versus Pancreato-Gastrostomy: A Retrospective Case Matched Comparative Study. J. Clin. Med. 2021, 10, 2181. https://doi.org/10.3390/jcm10102181
Marino MV, Heng Chiow AK, Mirabella A, Vaccarella G, Komorowski AL. Rate of Post-Operative Pancreatic Fistula after Robotic-Assisted Pancreaticoduodenectomy with Pancreato-Jejunostomy versus Pancreato-Gastrostomy: A Retrospective Case Matched Comparative Study. Journal of Clinical Medicine. 2021; 10(10):2181. https://doi.org/10.3390/jcm10102181
Chicago/Turabian StyleMarino, Marco V., Adrian Kah Heng Chiow, Antonello Mirabella, Gianpaolo Vaccarella, and Andrzej L. Komorowski. 2021. "Rate of Post-Operative Pancreatic Fistula after Robotic-Assisted Pancreaticoduodenectomy with Pancreato-Jejunostomy versus Pancreato-Gastrostomy: A Retrospective Case Matched Comparative Study" Journal of Clinical Medicine 10, no. 10: 2181. https://doi.org/10.3390/jcm10102181
APA StyleMarino, M. V., Heng Chiow, A. K., Mirabella, A., Vaccarella, G., & Komorowski, A. L. (2021). Rate of Post-Operative Pancreatic Fistula after Robotic-Assisted Pancreaticoduodenectomy with Pancreato-Jejunostomy versus Pancreato-Gastrostomy: A Retrospective Case Matched Comparative Study. Journal of Clinical Medicine, 10(10), 2181. https://doi.org/10.3390/jcm10102181





