In Silico Mathematical Modelling for Glioblastoma: A Critical Review and a Patient-Specific Case
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Continuum Models
3.2. Discrete and Hybrid Models
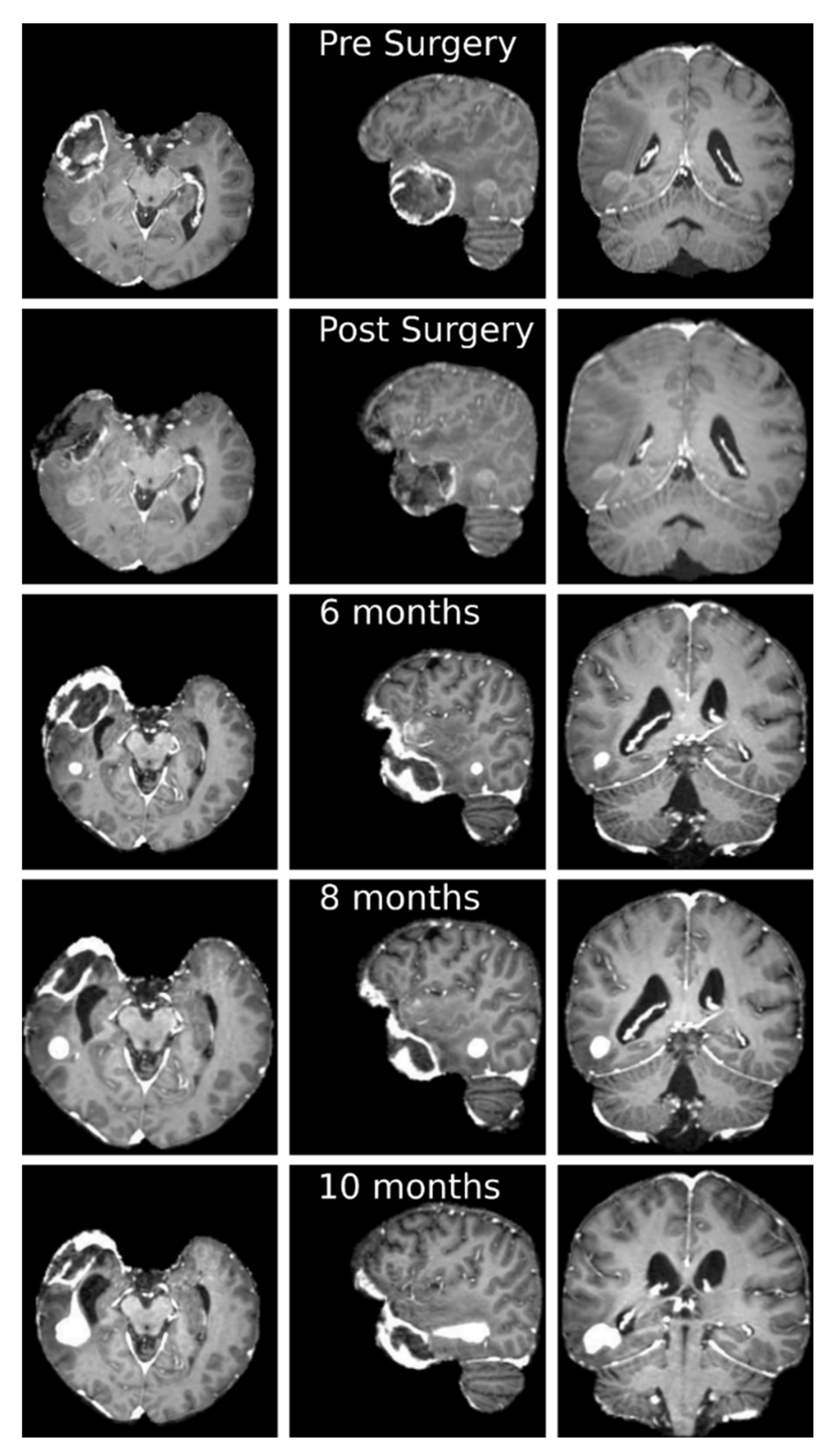
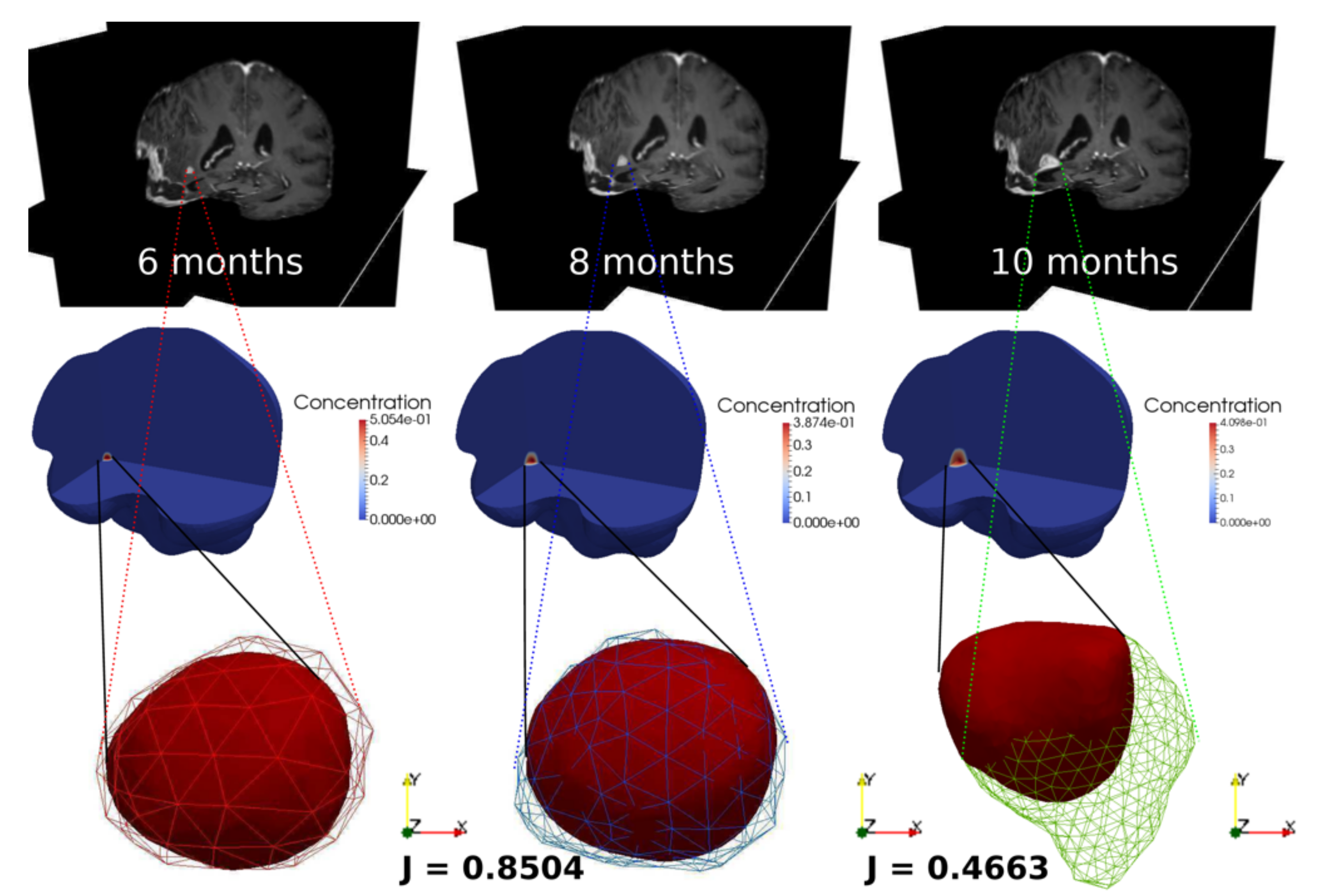
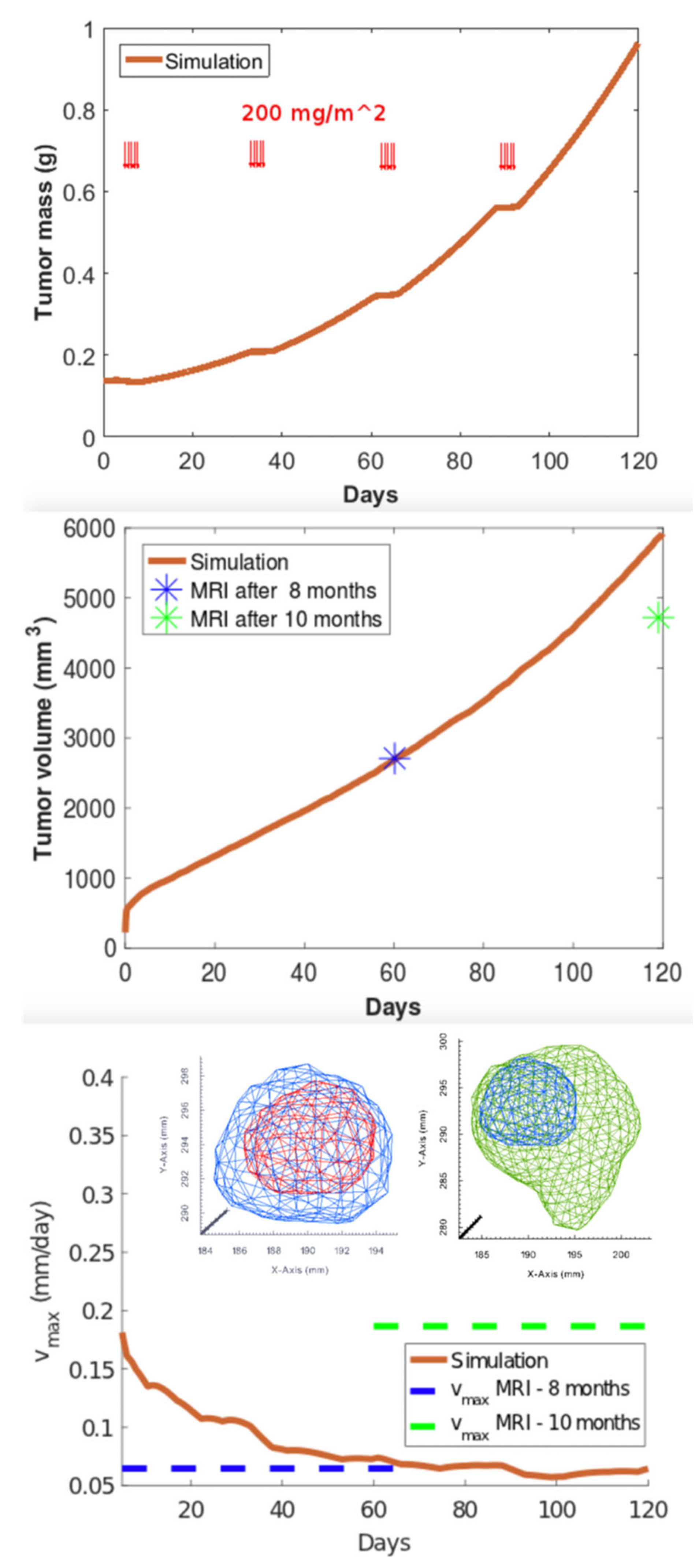
3.3. Clinical Case Study: Primary GBM Tumor
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. GLIOMATH: Clinical and Radiological Data Collection
Appendix A.1. Preoperative Screening, Radiological Protocol and Surgery
Appendix A.2. Postoperative Management
Appendix B. Mathematical Model
References
- Ostrom, Q.T.; Gittleman, H.; Truitt, G.; Boscia, A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011–2015. Neuro Oncol. 2018, 20, iv1–iv86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leece, R.; Xu, J.; Ostrom, Q.T.; Chen, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. Global incidence of malignant brain and other central nervous system tumors by histology, 2003–2007. Neuro Oncol. 2017, 19, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Van Zijl, P.C.M.; Laterra, J.; Salhotra, A.; Lal, B.; Mori, S.; Zhou, J. Unique patterns of diffusion directionality in rat brain tumors revealed by high-resolution diffusion tensor MRI. Magn. Reson. Med. 2007, 58, 454–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deisboeck, T.S.; Berens, M.E.; Kansal, A.R.; Torquato, S.; Stemmer-Rachamimov, A.O.; Chiocca, E.A. Pattern of self-organization in tumour systems: Complex growth dynamics in a novel brain tumour spheroid model. Cell Prolif. 2001, 34, 115–134. [Google Scholar] [CrossRef]
- Swanson, K.R.; Alvord, E.C.; Murray, J.D. A quantitative model for differential motility of gliomas in grey and white matter. Cell Prolif. 2000, 33, 317–329. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; Van Den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Wen, P.Y.; Macdonald, D.R.; Reardon, D.A.; Cloughesy, T.F.; Sorensen, A.G.; Galanis, E.; DeGroot, J.; Wick, W.; Gilbert, M.R.; Lassman, A.B.; et al. Updated Response Assessment Criteria for High-Grade Gliomas: Response Assessment in Neuro-Oncology Working Group. J. Clin. Oncol. 2010, 28, 1963–1972. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Xu, J.; Kromer, C.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013. Neuro Oncol. 2016, 18, v1–v75. [Google Scholar] [CrossRef] [Green Version]
- Davis, M.E. Glioblastoma: Overview of disease and treatment. Clin. J. Oncol. Nurs. 2016, 20, S2–S8. [Google Scholar] [CrossRef] [Green Version]
- Rivera, M.; Norman, S.; Sehgal, R.; Juthani, R. Updates on Surgical Management and Advances for Brain Tumors. Curr. Oncol. Rep. 2021, 23, 1–9. [Google Scholar] [CrossRef]
- Petrecca, K.; Guiot, M.C.; Panet-Raymond, V.; Souhami, L. Failure pattern following complete resection plus radiotherapy and temozolomide is at the resection margin in patients with glioblastoma. J. Neurooncol. 2013, 111, 19–23. [Google Scholar] [CrossRef]
- Lemée, J.-M.; Clavreul, A.; Menei, P. Intratumoral heterogeneity in glioblastoma: Don’t forget the peritumoral brain zone. Neuro Oncol. 2015, 17, 1322–1332. [Google Scholar] [CrossRef]
- Weller, M.; Van Den Bent, M.; Tonn, J.C.; Stupp, R.; Preusser, M.; Cohen-Jonathan-Moyal, E.; Henriksson, R.; Rhun, E.L.; Balana, C.; Chinot, O.; et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017, 18, e315–e329. [Google Scholar] [CrossRef] [Green Version]
- Swanson, K.R.; Bridge, C.; Murray, J.D.; Alvord, E.C. Virtual and real brain tumors: Using mathematical modeling to quantify glioma growth and invasion. J. Neurol. Sci. 2003, 216, 1–10. [Google Scholar] [CrossRef]
- Alfonso, J.C.L.; Talkenberger, K.; Seifert, M.; Klink, B.; Hawkins-Daarud, A.; Swanson, K.R.; Hatzikirou, H.; Deutsch, A. The biology and mathematical modelling of glioma invasion: A review. J. R. Soc. Interface 2017, 14, 20170490. [Google Scholar] [CrossRef]
- Cristini, V.; Lowengrub, J. Multiscale Modeling of Cancer: An Integrated Experimental and Mathematical Modeling Approach; Cambridge University Press: Cambridge, UK, 2010; ISBN 9780511781452. [Google Scholar]
- Lowengrub, J.S.; Frieboes, H.B.; Jin, F.; Chuang, Y.L.; Li, X.; MacKlin, P.; Wise, S.M.; Cristini, V. Nonlinear modelling of cancer: Bridging the gap between cells and tumours. Nonlinearity 2010, 23, R1–R9. [Google Scholar] [CrossRef] [Green Version]
- Ohgaki, H.; Dessen, P.; Jourde, B.; Horstmann, S.; Nishikawa, T.; Di Patre, P.L.; Burkhard, C.; Schüler, D.; Probst-Hensch, N.M.; Maiorka, P.C.; et al. Genetic pathways to glioblastoma: A population-based study. Cancer Res. 2004, 64, 6892–6899. [Google Scholar] [CrossRef] [Green Version]
- Hatzikirou, H.; Deutsch, A.; Schaller, C.; Simon, M.; Swanson, K. Mathematical Modelling of Glioblastoma Tumour Development: A Review. Math. Model. Methods Appl. Sci. 2005, 15, 1779–1794. [Google Scholar] [CrossRef] [Green Version]
- Swanson, K.R.; Rostomily, R.C.; Alvord, E.C. A mathematical modelling tool for predicting survival of individual patients following resection of glioblastoma: A proof of principle. Br. J. Cancer 2008, 98, 113–119. [Google Scholar] [CrossRef]
- Harpold, H.L.P.; Alvord, E.C.; Swanson, K.R. The evolution of mathematical modeling of glioma proliferation and invasion. J. Neuropathol. Exp. Neurol. 2007, 66, 1–9. [Google Scholar] [CrossRef]
- Protopapa, M.; Zygogianni, A.; Stamatakos, G.S.; Antypas, C.; Armpilia, C.; Uzunoglu, N.K.; Kouloulias, V. Clinical implications of in silico mathematical modeling for glioblastoma: A critical review. J. Neurooncol. 2018, 136, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.C.; Giverso, C.; Faggiano, E.; Boffano, C.; Acerbi, F.; Ciarletta, P. Towards the personalized treatment of glioblastoma: Integrating patient-specific clinical data in a continuous mechanical model. PLoS ONE 2015, 10, e0132887. [Google Scholar]
- Acerbi, F.; Agosti, A.; Falco, J.; Marchesi, S.; Vetrano, I.G.; DiMeco, F.; Bizzi, A.; Ferroli, P.; Scita, G.; Ciarletta, P. Mechano-biological features in a patient-specific computational model of glioblastoma. In Neuromethods; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Ciarletta, P.; Ambrosi, D.; Maugin, G.A.; Preziosi, L. Mechano-transduction in tumour growth modelling. Eur. Phys. J. E 2013, 36. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D. Preferred Reporting Items for Systematic Reviews and MetaAnalyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Marcu, L.G.; Harriss-Phillips, W.M. In silico modelling of treatment-induced tumour cell kill: Developments and advances. Comput. Math. Methods Med. 2012, 2012, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Papadogiorgaki, M.; Koliou, P.; Kotsiakis, X.; Zervakis, M.E. Mathematical modelling of spatio-temporal glioma evolution. Theor. Biol. Med. Model. 2013, 10, 47. [Google Scholar] [CrossRef] [Green Version]
- Owen, L.N.; Steel, G.G. The growth and cell population kinetics of spontaneous tumours in domestic animals. Br. J. Cancer 1969, 23, 493–509. [Google Scholar] [CrossRef] [Green Version]
- Tracqui, P.; Cruywagen, G.C.; Woodward, D.E.; Bartoo, G.T.; Murray, J.D.; Alvord, E.C. A mathematical model of glioma growth: The effect of chemotherapy on spatio???temporal growth. Cell Prolif. 1995, 28, 17–31. [Google Scholar] [CrossRef]
- Swanson, K.R.; Alvord, E.C.; Murray, J.D. Virtual brain tumours (gliomas) enhance the reality of medical imaging and highlight inadequacies of current therapy. Br. J. Cancer 2002, 86, 14–18. [Google Scholar] [CrossRef] [Green Version]
- Swanson, K.R.; Harpold, H.L.P.; Peacock, D.L.; Rockne, R.; Pennington, C.; Kilbride, L.; Grant, R.; Wardlaw, J.M.; Alvord, E.C. Velocity of Radial Expansion of Contrast-enhancing Gliomas and the Effectiveness of Radiotherapy in Individual Patients: A Proof of Principle. Clin. Oncol. 2008, 20, 301–308. [Google Scholar] [CrossRef]
- Jbabdi, S.; Mandonnet, E.; Duffau, H.; Capelle, L.; Swanson, K.R.; Pélégrini-Issac, M.; Guillevin, R.; Benali, H. Simulation of anisotropic growth of low-grade gliomas using diffusion tensor imaging. Magn. Reson. Med. 2005, 54, 616–624. [Google Scholar] [CrossRef]
- Cristini, V.; Li, X.; Lowengrub, J.S.; Wise, S.M. Nonlinear simulations of solid tumor growth using a mixture model: Invasion and branching. J. Math. Biol. 2009, 58, 723–763. [Google Scholar] [CrossRef] [Green Version]
- Macklin, P.; Lowengrub, J. Nonlinear simulation of the effect of microenvironment on tumor growth. J. Theor. Biol. 2007, 245, 677–704. [Google Scholar] [CrossRef] [Green Version]
- Lowengrub, J.; Nie, Q.; Cristini, V.; Li, X. Nonlinear three-dimensional simulation of solid tumor growth. Discret. Contin. Dyn. Syst. Ser. B 2007, 7, 581–604. [Google Scholar]
- Wang, C.H.; Rockhill, J.K.; Mrugala, M.; Peacock, D.L.; Lai, A.; Jusenius, K.; Wardlaw, J.M.; Cloughesy, T.; Spence, A.M.; Rockne, R.; et al. Prognostic significance of growth kinetics in newly diagnosed glioblastomas revealed by combining serial imaging with a novel biomathematical model. Cancer Res. 2009, 69, 9133–9140. [Google Scholar] [CrossRef] [Green Version]
- Rockne, R.; Alvord, E.C.; Rockhill, J.K.; Swanson, K.R. A mathematical model for brain tumor response to radiation therapy. J. Math. Biol. 2009, 58, 561–578. [Google Scholar] [CrossRef] [Green Version]
- Rockne, R.; Rockhill, J.K.; Mrugala, M.; Spence, A.M.; Kalet, I.; Hendrickson, K.; Lai, A.; Cloughesy, T.; Alvord, E.C.; Swanson, K.R. Predicting the efficacy of radiotherapy in individual glioblastoma patients in vivo: A mathematical modeling approach. Phys. Med. Biol. 2010, 55, 3271. [Google Scholar] [CrossRef] [Green Version]
- Roniotis, A.; Marias, K.; Sakkalis, V.; Manikis, G.C.; Zervakis, M. Simulating radiotherapy effect in high-grade glioma by using diffusive modeling and brain atlases. J. Biomed. Biotechnol. 2012. [Google Scholar] [CrossRef] [Green Version]
- Holdsworth, C.H.; Corwin, D.; Stewart, R.D.; Rockne, R.; Trister, A.D.; Swanson, K.R.; Phillips, M. Adaptive IMRT using a multiobjective evolutionary algorithm integrated with a diffusion-invasion model of glioblastoma. Phys. Med. Biol. 2012, 57, 8274. [Google Scholar] [CrossRef]
- Unkelbach, J.; Menze, B.H.; Konukoglu, E.; Dittmann, F.; Ayache, N.; Shih, H.A. Radiotherapy planning for glioblastoma based on a tumor growth model: Implications for spatial dose redistribution. Phys. Med. Biol. 2014, 59, 771–789. [Google Scholar] [CrossRef] [Green Version]
- Neal, M.L.; Trister, A.D.; Cloke, T.; Sodt, R.; Ahn, S.; Baldock, A.L.; Bridge, C.A.; Lai, A.; Cloughesy, T.F.; Mrugala, M.M.; et al. Discriminating Survival Outcomes in Patients with Glioblastoma Using a Simulation-Based, Patient-Specific Response Metric. PLoS ONE 2013, 8, e51951. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Adjei, A.A. Targeting Angiogenesis in Cancer Therapy: Moving Beyond Vascular Endothelial Growth Factor. Oncologist 2015, 20, 660–673. [Google Scholar] [CrossRef] [Green Version]
- Saut, O.; Lagaert, J.B.; Colin, T.; Fathallah-Shaykh, H.M. A Multilayer Grow-or-Go Model for GBM: Effects of Invasive Cells and Anti-Angiogenesis on Growth. Bull. Math. Biol. 2014, 76, 2306–2333. [Google Scholar] [CrossRef]
- Byrne, H.; Preziosi, L. Modelling solid tumour growth using the theory of mixtures. Math. Med. Biol. 2003, 20, 341–366. [Google Scholar] [CrossRef]
- Ambrosi, D.; Preziosi, L. on the Closure of Mass Balance Models for Tumor Growth. Math. Model. Methods Appl. Sci. 2002, 12, 737–754. [Google Scholar] [CrossRef]
- Lipkova, J.; Angelikopoulos, P.; Wu, S.; Alberts, E.; Wiestler, B.; Diehl, C.; Preibisch, C.; Pyka, T.; Combs, S.E.; Hadjidoukas, P.; et al. Personalized Radiotherapy Design for Glioblastoma: Integrating Mathematical Tumor Models, Multimodal Scans, and Bayesian Inference. IEEE Trans. Med. Imaging 2019, 38, 1875–1884. [Google Scholar] [CrossRef]
- Keller, E.F.; Segel, L.A. Model for chemotaxis. J. Theor. Biol. 1971, 30, 225–234. [Google Scholar] [CrossRef]
- Turner, S.; Sherratt, J.A. Intercellular adhesion and cancer invasion: A discrete simulation using the extended potts model. J. Theor. Biol. 2002, 216, 85–100. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, R.B.; Tranquillo, R.T. A stochastic model for adhesion-mediated cell random motility and haptotaxis. J. Math. Biol. 1993, 31, 563–600. [Google Scholar] [CrossRef]
- Frieboes, H.B.; Jin, F.; Chuang, Y.L.; Wise, S.M.; Lowengrub, J.S.; Cristini, V.V. Three-dimensional multispecies nonlinear tumor growth-II: Tumor invasion and angiogenesis. J. Theor. Biol. 2010, 264, 1254–1278. [Google Scholar] [CrossRef] [Green Version]
- Duchting, W.; Ulmer, W.; Lehrig, R.; Ginsberg, T.; Dedeleit, E. Computer simulation and modelling of tumor spheroid growth and their relevance for optimization of fractionated radiotherapy. Strahlenther. Onkol. 1992, 168, 354–360. [Google Scholar] [PubMed]
- Wasserman, R.; Acharya, R.; Sibata, C.; Shin, K.H. A patient-specific in vivo tumor model. Math. Biosci. 1996, 136, 111–140. [Google Scholar] [CrossRef]
- Kansal, A.R.; Torquato, S.; Harsh IV, G.R.; Chiocca, E.A.; Deisboeck, T.S. Simulated brain tumor growth dynamics using a three-dimensional cellular automaton. J. Theor. Biol. 2000, 203, 367–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kansal, A.R.; Torquato, S.; Harsh Iv, G.R.; Chiocca, E.A.; Deisboeck, T.S. Cellular automaton of idealized brain tumor growth dynamics. In Proceedings of the BioSystems, Perth, Australia, 2–4 February 2000; pp. 119–127. [Google Scholar]
- Dionysiou, D.D.; Stamatakos, G.S.; Uzunoglu, N.K.; Nikita, K.S.; Marioli, A. A four-dimensional simulation model of tumour response to radiotherapy in vivo: Parametric validation considering radiosensitivity, genetic profile and fractionation. J. Theor. Biol. 2004, 230, 1–20. [Google Scholar] [CrossRef]
- Dionysiou, D.D.; Peristeris, T.; Stamatakos, G.S.; Nikita, K.S.; Uzunoglu, N.K. The genetic profile of a tumor as a determinant of its response to radiotherapy: A computer simulation of two different radiotherapeutic schemes. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, New Orleans, LA, USA, 20–24 September 2004. [Google Scholar]
- Dionysiou, D.; Stamatakos, G.; Gintides, D.; Uzunoglu, N.; Kyriaki, K. Critical Parameters Determining Standard Radiotherapy Treatment Outcome for Glioblastoma Multiforme: A Computer Simulation. Open Biomed. Eng. J. 2008, 2, 43–51. [Google Scholar] [CrossRef]
- Frieboes, H.B.; Lowengrub, J.S.; Wise, S.; Zheng, X.; Macklin, P.; Bearer, E.L.; Cristini, V. Computer simulation of glioma growth and morphology. Neuroimage 2007, 37, S59–S70. [Google Scholar] [CrossRef] [Green Version]
- Oraiopoulou, M.E.; Tzamali, E.; Tzedakis, G.; Liapis, E.; Zacharakis, G.; Vakis, A.; Papamatheakis, J.; Sakkalis, V. Integrating in vitro experiments with in silico approaches for Glioblastoma invasion: The role of cell-to-cell adhesion heterogeneity. Sci. Rep. 2018, 8, 16200. [Google Scholar] [CrossRef]
- Zheng, X.; Wise, S.M.; Cristini, V. Nonlinear simulation of tumor necrosis, neo-vascularization and tissue invasion via an adaptive finite-element/level-set method. Bull. Math. Biol. 2005, 67, 211–259. [Google Scholar] [CrossRef]
- Cristini, V.; Frieboes, H.B.; Gatenby, R.; Caserta, S.; Ferrari, M.; Sinek, J. Morphologic instability and cancer invasion. Clin. Cancer Res. 2005, 11, 6772–6779. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Roh, S. A hybrid model for cell proliferation and migration in glioblastoma. In Discrete and Continuous Dynamical Systems—Series B; Missouri State University: Springfield, MO, USA, 2013. [Google Scholar]
- Angeli, S.; Emblem, K.E.; Due-Tonnessen, P.; Stylianopoulos, T. Towards patient-specific modeling of brain tumor growth and formation of secondary nodes guided by DTI-MRI. NeuroImage Clin. 2018, 20, 664–673. [Google Scholar] [CrossRef]
- Gallaher, J.A.; Massey, S.C.; Hawkins-Daarud, A.; Noticewala, S.S.; Rockne, R.C.; Johnston, S.K.; GonzalezCuyar, L.; Juliano, J.; Gil, O.; Swanson, K.R.; et al. From cells to tissue: How cell scale heterogeneity impacts glioblastoma growth and treatment response. PLoS Comput. Biol. 2020, 16, e1007672. [Google Scholar] [CrossRef] [Green Version]
- Phillips, H.S.; Kharbanda, S.; Chen, R.; Forrest, W.F.; Soriano, R.H.; Wu, T.D.; Misra, A.; Nigro, J.M.; Colman, H.; Soroceanu, L.; et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 2006, 9, 157–173. [Google Scholar] [CrossRef] [Green Version]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [Green Version]
- Gatenby, R.A.; Maini, P.K. Mathematical oncology: Cancer summed up. Nat. Cell Biol. 2003, 421, 321. [Google Scholar] [CrossRef] [Green Version]
- Jagiella, N.; Müller, B.; Müller, M.; Vignon-Clementel, I.E.; Drasdo, D. Inferring Growth Control Mechanisms in Growing Multi-cellular Spheroids of NSCLC Cells from Spatial-Temporal Image Data. PLoS Comput. Biol. 2016, 12, e1004412. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.M.; Suki, D.; Hess, K.; Sawaya, R. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: Can we do better than gross-total resection? J. Neurosurg. 2016, 124, 977–988. [Google Scholar] [CrossRef] [Green Version]
- Wijnenga, M.M.J.; French, P.J.; Dubbink, H.J.; DInjens, W.N.M.; Atmodimedjo, P.N.; Kros, J.M.; Smits, M.; Gahrmann, R.; Rutten, G.J.; Verheul, J.B.; et al. The impact of surgery in molecularly defined low-grade glioma: An integrated clinical, radiological, and molecular analysis. Neuro Oncol. 2018, 20, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Hollon, T.C.; Pandian, B.; Adapa, A.R.; Urias, E.; Save, A.V.; Khalsa, S.S.S.; Eichberg, D.G.; D’Amico, R.S.; Farooq, Z.U.; Lewis, S.; et al. Near real-time intraoperative brain tumor diagnosis using stimulated Raman histology and deep neural networks. Nat. Med. 2020, 26, 52–58. [Google Scholar] [CrossRef]
- Tsao, M.N.; Mehta, M.P.; Whelan, T.J.; Morris, D.E.; Hayman, J.A.; Flickinger, J.C.; Mills, M.; Rogers, C.L.; Souhami, L. The American Society for Therapeutic Radiology and Oncology (ASTRO) evidence-based review of the role of radiosurgery for malignant glioma. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 47–55. [Google Scholar] [CrossRef]
- Thomas, A.A.; Brennan, C.W.; DeAngelis, L.M.; Omuro, A.M. Emerging therapies for glioblastoma. JAMA Neurol. 2014, 71, 1437–1444. [Google Scholar] [CrossRef]
- Westphal, M.; Hilt, D.C.; Bortey, E.; Delavault, P.; Olivares, R.; Warnke, P.C.; Whittle, I.R.; Jääskeläinen, J.; Ram, Z. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003, 5, 79–88. [Google Scholar] [CrossRef]
- Shibahara, I.; Miyasaka, K.; Sekiguchi, A.; Ishiyama, H.; Inukai, M.; Yasui, Y.; Watanabe, T.; Sato, S.; Hide, T.; Kumabe, T. Long-term follow-up after BCNU wafer implantation in patients with newly diagnosed glioblastoma. J. Clin. Neurosci. 2021, 86, 202–210. [Google Scholar] [CrossRef]
- Xiao, Z.Z.; Wang, Z.F.; Lan, T.; Huang, W.H.; Zhao, Y.H.; Ma, C.; Li, Z.Q. Carmustine as a Supplementary Therapeutic Option for Glioblastoma: A Systematic Review and Meta-Analysis. Front. Neurol. 2020, 11. [Google Scholar] [CrossRef]
- Plate, K.H.; Scholz, A.; Dumont, D.J. Tumor angiogenesis and anti-angiogenic therapy in malignant gliomas revisited. Acta Neuropathol. 2012, 124, 763–775. [Google Scholar] [CrossRef] [Green Version]
- Arvold, N.D.; Reardon, D.A. Treatment options and outcomes for glioblastoma in the elderly patient. Clin. Interv. Aging 2014, 9, 357–367. [Google Scholar]
- Cao, J.Q.; Bauman, G.S.; Fisher, B.J.; Macdonald, D.R.; Megyesi, J.F.; Watling, C.J. Hypofractionated radiotherapy (XRT) plus concurrent and adjuvant versus salvage temozolomide (TMZ) in elderly patients with glioblastoma multiforme: A review of ten-year single institutional experience. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, S167. [Google Scholar] [CrossRef]
- Parsons, D.W.; Jones, S.; Zhang, X.; Lin, J.C.H.; Leary, R.J.; Angenendt, P.; Mankoo, P.; Carter, H.; Siu, I.M.; Gallia, G.L.; et al. An integrated genomic analysis of human glioblastoma multiforme. Science 2008, 321, 1807–1812. [Google Scholar] [CrossRef] [Green Version]
- Falco, J.; Cavallo, C.; Vetrano, I.G.; de Laurentis, C.; Siozos, L.; Schiariti, M.; Broggi, M.; Ferroli, P.; Acerbi, F. Fluorescein Application in Cranial and Spinal Tumors Enhancing at Preoperative MRI and Operated With a Dedicated Filter on the Surgical Microscope: Preliminary Results in 279 Patients Enrolled in the FLUOCERTUM Prospective Study. Front. Surg. 2019, 6, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegi, M.E.; Diserens, A.-C.; Gorlia, T.; Hamou, M.-F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT Gene Silencing and Benefit from Temozolomide in Glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef] [Green Version]
- Agosti, A.; Cattaneo, C.; Giverso, C.; Ambrosi, D.; Ciarletta, P. A computational framework for the personalized clinical treatment of glioblastoma multiforme. ZAMM J. Appl. Math. Mech./Zeitschrift Angew. Math. Mech. 2018, 98, 2307–2327. [Google Scholar] [CrossRef]
- Byrne, H.M.; King, J.R.; McElwain, D.L.S.; Preziosi, L. A two-phase model of solid tumour growth. Appl. Math. Lett. 2003, 16, 567–573. [Google Scholar] [CrossRef]
- Agosti, A.; Antonietti, P.F.; Ciarletta, P.; Grasselli, M.; Verani, M. A Cahn-Hilliard-type equation with application to tumor growth dynamics. Math. Methods Appl. Sci. 2017, 40, 7598–7626. [Google Scholar] [CrossRef]
- Kikinis, R.; Pieper, S. 3D Slicer as a tool for interactive brain tumor segmentation. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011. [Google Scholar]
- Antiga, L.; Piccinelli, M.; Botti, L.; Ene-Iordache, B.; Remuzzi, A.; Steinman, D.A. An image-based modeling framework for patient-specific computational hemodynamics. Med. Biol. Eng. Comput. 2008, 46, 1097–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Si, H. TetGen, a delaunay-based quality tetrahedral mesh generator. ACM Trans. Math. Softw. 2015, 41, 1–36. [Google Scholar] [CrossRef]
- Schroeder, W.J.; Martin, K.M. The visualization toolkit. In Visualization Handbook; Elsevier: Amsterdam, The Netherlands, 2005; ISBN 9780123875822. [Google Scholar]
- Hedouin, R.; Commowick, O.; Bannier, E.; Scherrer, B.; Taquet, M.; Warfield, S.K.; Barillot, C. Block-Matching Distortion Correction of Echo-Planar Images with Opposite Phase Encoding Directions. IEEE Trans. Med. Imaging 2017, 36, 1106–1115. [Google Scholar] [CrossRef] [Green Version]
- Coupe, P.; Yger, P.; Prima, S.; Hellier, P.; Kervrann, C.; Barillot, C. An optimized blockwise nonlocal means denoising filter for 3-D magnetic resonance images. IEEE Trans. Med. Imaging 2008, 27, 425–441. [Google Scholar] [CrossRef] [Green Version]
- Commowick, O.; Wiest-Daesslé, N.; Prima, S. Automated diffeomorphic registration of anatomical structures with rigid parts: Application to dynamic cervical MRI. In Lecture Notes in Computer Science (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics); Springer: Cham, Switzerland, 2012. [Google Scholar]
- Basser, P.J.; Mattiello, J.; LeBihan, D. MR diffusion tensor spectroscopy and imaging. Biophys. J. 1994, 66, 259–267. [Google Scholar] [CrossRef] [Green Version]
- Metzler-Baddeley, C.; O’Sullivan, M.J.; Bells, S.; Pasternak, O.; Jones, D.K. How and how not to correct for CSF-contamination in diffusion MRI. Neuroimage 2012, 59, 1394–1403. [Google Scholar] [CrossRef]
- Commowick, O.; Wiest-Daessle, N.; Prima, S. Block-matching strategies for rigid registration of multimodal medical images. In Proceedings of the International Symposium on Biomedical Imaging, Barcelona, Spain, 30 April–5 May 2012. [Google Scholar]
- Scianna, M.; Preziosi, L.; Wolf, K. A cellular potts model simulating cell migration on and in matrix environments. In Mathematical Biosciences and Engineering; Arizona State University: Tempe, AZ, USA, 2013. [Google Scholar]
- Agosti, A. Error analysis of a finite element approximation of a degenerate Cahn-Hilliard equation. ESAIM Math. Model. Numer. Anal. 2018, 52, 827–867. [Google Scholar] [CrossRef] [Green Version]
- Barrett, J.W.; Blowey, J.F.; Garcke, H. On fully practical finite element approximations of degenerate Cahn-Hilliard systems. Math. Model. Numer. Anal. 2001, 35, 713–748. [Google Scholar] [CrossRef] [Green Version]

| Authors | Key Features | Prediction |
|---|---|---|
| Owen LN (1969) [28] | Relation between cell kinetics and growth of the gross tumor | Tumor growth and cell production vs. cell loss |
| Swanson KR (2002) [29] | Quantification of the spatio-temporal growth and invasion of gliomas in three dimensions | Tumor growth and microscopic invasion |
| Swanson KR (2000) [5] | Augmented diffusion rates of malignant cells in white matter as compared to grey matter | Pattern of microscopic and submicroscopic invasion of the brain by glioma cells |
| Jbabdi S (2005) [30] | Implementation of modeled glioma diffusion by means of introduction of brain anisotropy, as detectable with diffusion tensor imaging | Pattern of glioma cells migration |
| Cristini V (2009) [31] | Role of microenvironment vasculature and chemotaxis in glioma invasive behavior | Pattern of tumor invasiveness |
| Macklin P (2007) [32] | Implementation the role the properties of microenvironment in detecting cancer morphology | Prediction of tumor 3D morphology and malignant properties |
| Harpold HLP (2007) [21] | Analyzing the relation between tumor growth velocity and cellular proliferation rate | Survival time |
| Swanson KR (2008) [20] | Analyzing tumor spreading velocity starting from patient-specific MRI | Survival time |
| Wang CH (2009) [33] | Quantification of patient-specific kinetic rate of malignant cell proliferation since serial preoperative MRI | Diffusion rate and development of GBM for each patient |
| Rockne R (2010) [34] | Incorporating the effect of radiation therapy in mathematical model of glioma growth | Tumor dimension after RT protocol |
| Unkelbach J (2014) [35] | Analysis of malignant cell infiltration by means of FLAIR images and prediction of RT response | Optimization of patient-specific radiation therapy and dosing of fall-off rate |
| Zhao Y (2015) [36] | Role of angiogenesis in tumor development and aggressiveness | Effect of antiangiogenic drugs |
| Saut O (2014) [37] | Role of hypoxia in tumor development and invasion | Prediction of tumor behavior (proliferative vs. invasive phenotype) |
| Colombo MC (2015) [23] | Analyzing patient-specific preoperative DTI in revealing personal heterogeneity and anisotropy of brain tissue | Tumor growth |
| Lipkova J (2019) [38] | Integration complementary information from MRI and FET-PET to infer tumor cell density in GBM patient to tailor radiotherapy | Individual response to RT |
| Acerbi F (2021) [24] | Introducing in a continuous mechanical model, the heterogeneity and the anisotropicity of the brain bundles from patient-specific DTI | Tumor growth, invasion and recurrence |
| Authors | Key Features | Prediction |
|---|---|---|
| Duchting W (1992) [52] | Development of a 3D spheroid tumor model analyzing cellular cycle phases | Tumor response to different RT fractionation schemes |
| Wasserman R (1996) [53] | Integrating patient-specific mechanical properties of the tumor, as derived from personal MRI | Tumor growth and neoplastic proliferation |
| Kansal AR (2000) [54,55] | Detecting tumor behavior using a three-dimensional cellular automaton model | Tumor growth |
| Dionysiou DD (2004) [56] | Integration in a single four-dimensional simulation model several groups of cells in different phases of the cell cycle | Tumor growth and response to RT |
| Dionysiou DD (2008) [57] | Incorporation of genetic and molecular factors affecting radiosensitivity | Tumor growth and response to adjuvant therapies |
| Zheng X (2005) [58] | Analyzing the relation among neovascularization (tumor angiogenesis) and cellular invasiveness using an adaptive, unstructured finite element mesh | Tumor response to RT and antiangiogenic drugs |
| Frieboes HB (2007) [59] | Combination of analytical and stochastic models linking cellular properties and microenvironment vascularization | Tumor growth |
| Kim Y (2013) [60] | Analyzing the relation between metabolic stress and biophysical interactions with microenvironment | Experimenting target therapies |
| Angeli S (2018) [61] | Combination of cellular events which cause tumor proliferation and migration with biomechanical response at tissue level | Tumor infiltration and distant invasion |
| Gallaher JA (2020) [62] | Combination of MRI data to estimate the role of microenvironment with biopsy data to detect molecular cell properties | Prediction of tumor recurrence and effect of adjuvant therapies |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falco, J.; Agosti, A.; Vetrano, I.G.; Bizzi, A.; Restelli, F.; Broggi, M.; Schiariti, M.; DiMeco, F.; Ferroli, P.; Ciarletta, P.; et al. In Silico Mathematical Modelling for Glioblastoma: A Critical Review and a Patient-Specific Case. J. Clin. Med. 2021, 10, 2169. https://doi.org/10.3390/jcm10102169
Falco J, Agosti A, Vetrano IG, Bizzi A, Restelli F, Broggi M, Schiariti M, DiMeco F, Ferroli P, Ciarletta P, et al. In Silico Mathematical Modelling for Glioblastoma: A Critical Review and a Patient-Specific Case. Journal of Clinical Medicine. 2021; 10(10):2169. https://doi.org/10.3390/jcm10102169
Chicago/Turabian StyleFalco, Jacopo, Abramo Agosti, Ignazio G. Vetrano, Alberto Bizzi, Francesco Restelli, Morgan Broggi, Marco Schiariti, Francesco DiMeco, Paolo Ferroli, Pasquale Ciarletta, and et al. 2021. "In Silico Mathematical Modelling for Glioblastoma: A Critical Review and a Patient-Specific Case" Journal of Clinical Medicine 10, no. 10: 2169. https://doi.org/10.3390/jcm10102169
APA StyleFalco, J., Agosti, A., Vetrano, I. G., Bizzi, A., Restelli, F., Broggi, M., Schiariti, M., DiMeco, F., Ferroli, P., Ciarletta, P., & Acerbi, F. (2021). In Silico Mathematical Modelling for Glioblastoma: A Critical Review and a Patient-Specific Case. Journal of Clinical Medicine, 10(10), 2169. https://doi.org/10.3390/jcm10102169









