The Effect of High-Intensity Interval Training Periods on Morning Serum Testosterone and Cortisol Levels and Physical Fitness in Men Aged 35–40 Years
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Group
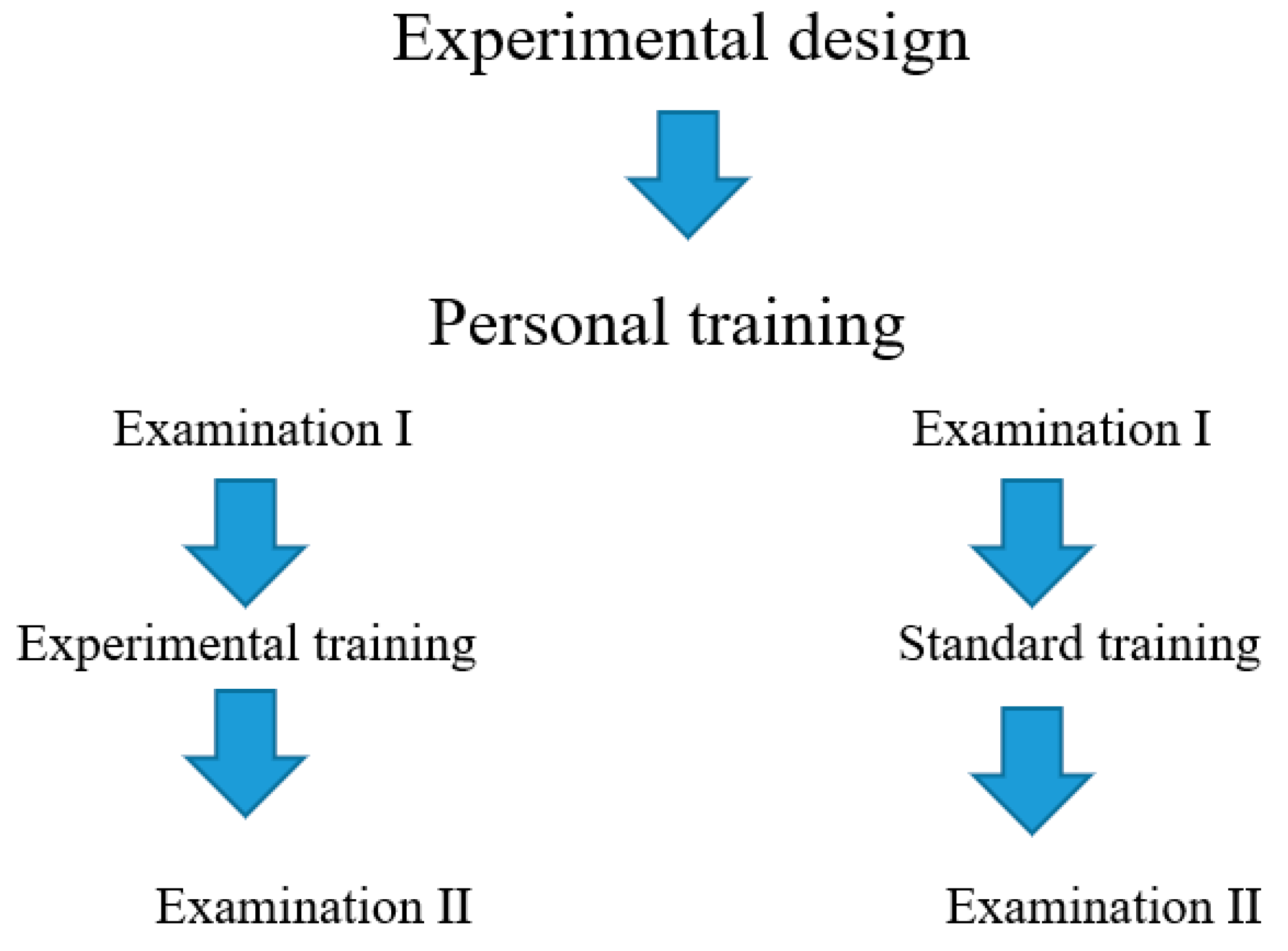
2.2. Research Program and Methodology
2.3. Measurement of Physical Fitness
- Evaluation of aerobic capacity. To assessVO2max, a running test with graded exercise intensity is performed on a treadmill (h/p/Cosmos, Nußdorf, Germany). The test begins with a 2-min recording of respiratory indices at rest, during which the participants remain in a standing position. During the first 4 min of the test, the participants run at a speed of 8 km·h−1. Next, the running speed is increased by 1 km·h−1 every 2 min. The effort is continued until the extreme fatigue of the participants, which is manifested by the inability to continue running at the set speed. During the test, the levels of cardiorespiratory indices are recorded based on the breath-by-breath method using an ergospirometer (Cosmed, Rome, Italy). The highest recorded value of minute oxygen uptake is considered to be VO2max [25].
- Evaluation of abdominal strength (sit-ups). The tested person lies on the mattress with feet 30 cm apart and knees bent at a right angle. Hands are intertwined, resting on the neck. The participant is assisted by a partner who holds the participant’s feet so that they remain in contact with the ground. At the start signal, the participant sits up to touch their knees with their elbows and then returns to the starting position. The exercise duration is 30 s.
- Evaluation of shoulder girdle strength by the number of repetitions of pull-ups on a bar. The participant catches the bar with a pronated grip and hangs there; at the signal, the participant bends his arms at the elbow and pulls his body up so high that the chin is above the bar, and then, without a rest, returns to a simple hanging position. The exercise is repeated as many times as possible without rest; the result is the number of complete pull-ups (chin over the bar)
- Evaluation of the dynamic strength of lower limbs (long jump from a standing position). The participant stands with his feet slightly apart in front of the starting line and bends his knees and moves his arms backward at the same time; then, he performs an arm swing and jumps as far as he can. The landing occurs on both feet while maintaining the upright position. The test is performed twice.
2.4. Experimental Program
3. Results
4. Discussion
Limitation of the Study
5. Conclusions
- The strength and endurance training, performed based on high-intensity interval sessions (circuit training), increases testosterone levels in men aged 35–40 years and can be used to enhance general well-being and partly inhibit harmful age-related changes.
- It is worth using this type of training in adult men because it can positively affect their quality of life and health by physiologically increasing testosterone levels, lowering cortisol, and improving anabolic–catabolic balance and muscle strength.
- This type of physical activity can act as an alternative or support pharmacotherapy for increasing testosterone levels in men.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Logan, S.; Royce, G.H.; Owen, D.; Farley, J.; Ranjo-Bishop, M.; Sonntag, W.E.; Deepa, S.S. Accelerated decline in cognition in a mouse model of increased oxidative stress. GeroScience 2019, 41, 591–607. [Google Scholar] [CrossRef] [PubMed]
- Snyder, P.J. Effects of Age on Testicular Function and Consequences of Testosterone Treatment 1. J. Clin. Endocrinol. Metab. 2001, 86, 2369–2372. [Google Scholar] [CrossRef]
- Handelsman, D.J.; Sikaris, K.; Ly, L.P. Estimating age-specific trends in circulating testosterone and sex hormone-binding globulin in males and females across the lifespan. Ann. Clin. Biochem. 2016, 53, 377–384. [Google Scholar] [CrossRef] [Green Version]
- Feldman, H.A.; Longcope, C.; Derby, C.A.; Johannes, C.B.; Araujo, A.B.; Coviello, A.D.; Bremner, W.J.; McKinlay, J.B. Age Trends in the Level of Serum Testosterone and Other Hormones in Middle-Aged Men: Longitudinal Results from the Massachusetts Male Aging Study. J. Clin. Endocrinol. Metab. 2002, 87, 589–598. [Google Scholar] [CrossRef]
- Shiels, M.S.; Rohrmann, S.; Menke, A.; Selvin, E.; Crespo, C.J.; Rifai, N.; Dobs, A.; Feinleib, M.; Guallar, E.; Platz, E.A. Association of cigarette smoking, alcohol consumption, and physical activity with sex steroid hormone levels in US men. Cancer Causes Control 2009, 20, 877–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gooren, L.J.; Behre, H.M.; Saad, F.; Frank, A.; Schwerdt, S. Diagnosing and treating testosterone deficiency in different parts of the world. Results from global market research. Aging Male 2007, 10, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Ohl, D.A.; Quallich, S.A. Clinical hypogonadism and androgen replacement therapy: an overview. Urol. Nurs. Off. J. Am. Urol. Assoc. Allied. 2006, 26, 253–260. [Google Scholar]
- Vingren, J.L.; Kraemer, W.J.; Ratamess, N.A.; Anderson, J.M.; Volek, J.S.; Maresh, C.M. Testosterone Physiology in Resistance Exercise and Training. Sport. Med. 2010, 40, 1037–1053. [Google Scholar] [CrossRef]
- Brown, G.A.; Vukovich, M.D.; Reifenrath, T.A.; Uhl, N.L.; Parsons, K.A.; Sharp, R.L.; King, D.S. Effects of Anabolic Precursors on Serum Testosterone Concentrations and Adaptations to Resistance Training in Young Men. Int. J. Sport Nutr. Exerc. Metab. 2000, 10, 340–359. [Google Scholar] [CrossRef]
- Cadegiani, F.A.; Kater, C.E. Basal Hormones and Biochemical Markers as Predictors of Overtraining Syndrome in Male Athletes: The EROS-BASAL Study. J. Athl. Train. 2019, 54, 906–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obmiński Z Intensified training period: when much is to too much? J. Combat Sport. Martial Arts 2016, 7, 117–125. [CrossRef]
- Ambroży, T. W poszukiwaniu związków treningu obwodowego z prozdrowotną aktywnością fizyczną. Ann. Univrsitatis Mariae Curie-Skłodowska 2007, LXII, 55–61. [Google Scholar]
- Kraemer, W.J.; Häkkinen, K.; Newton, R.U.; Nindl, B.C.; Volek, J.S.; McCormick, M.; Gotshalk, L.A.; Gordon, S.E.; Fleck, S.J.; Campbell, W.W.; et al. Effects of heavy-resistance training on hormonal response patterns in younger vs. older men. J. Appl. Physiol. 1999, 87, 982–992. [Google Scholar] [CrossRef] [Green Version]
- Gorostiaga, E.M.; Izquierdo, M.; Ruesta, M.; Iribarren, J.; González-Badillo, J.J.; Ibáñez, J. Strength training effects on physical performance and serum hormones in young soccer players. Eur. J. Appl. Physiol. 2004, 91, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Herbert, P.; Hayes, L.; Sculthorpe, N.; Grace, F. HIIT produces increases in muscle power and free testosterone in male masters athletes. Endocr. Connect. 2017, 6, 430–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vesterinen, V.; Häkkinen, K.; Laine, T.; Hynynen, E.; Mikkola, J.; Nummela, A. Predictors of individual adaptation to high-volume or high-intensity endurance training in recreational endurance runners. Scand. J. Med. Sci. Sports 2016, 26, 885–893. [Google Scholar] [CrossRef]
- Capranica, L.; Lupo, C.; Cortis, C.; Chiodo, S.; Cibelli, G.; Tessitore, A. Salivary cortisol and alpha-amylase reactivity to taekwondo competition in children. Eur. J. Appl. Physiol. 2012, 112, 647–652. [Google Scholar] [CrossRef]
- Casto, K.V.; Edwards, D.A. Testosterone, cortisol, and human competition. Horm. Behav. 2016, 82, 21–37. [Google Scholar] [CrossRef]
- Papacosta, E.; Nassis, G.P.; Gleeson, M. Salivary hormones and anxiety in winners and losers of an international judo competition. J. Sports Sci. 2016, 34, 1281–1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obmiński, Z.; Wojtkowiak, M.; Stupnicki, R.; Golec, L.; Hackney, A.C. Effect of acceleration stress on salivary cortisol and plasma cortisol and testosterone levels in cadet pilots. J. Physiol. Pharmacol 1997, 48, 193–200. [Google Scholar]
- Obmiński, Z.; Golec, L.; Stupnicki, R.; Hackney, A.C. Effect of hypobaric-hypoxia on the salivary cortisol levels of aircraft pilots. Aviat. Sp. Environ. Med. 1997, 6893, 183–186. [Google Scholar]
- Aldercreutz, H.; Harkonen, K.; Kuoppasalmi, H.; Naveri, H.; Huhtaniemi, H.; Timsanen, H. Effect of training on plasma anabolic and catabolic steroid hormones and their responses during physical exercise. Int. J. Sport. Med. 1986, 1, 27–28. [Google Scholar] [CrossRef]
- De Luccia, T.P. Use of the Testosterone/Cortisol Ratio Variable in Sports. Open Sports Sci. J. 2016, 9, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Rydzik, Ł.; Ambroży, T. Physical fitness and the level of technical and tactical training of kickboxers. Int. J. Environ. Res. Public Health 2021, 18, 3088. [Google Scholar] [CrossRef] [PubMed]
- Ambroży, T.; Maciejczyk, M.; Klimek, A.T.; Wiecha, S.; Stanula, A.; Snopkowski, P.; Pałka, T.; Jaworski, J.; Ambroży, D.; Rydzik, Ł.; et al. The effects of intermittent hypoxic training on anaerobic and aerobic power in boxers. Int. J. Environ. Res. Public Health 2020, 17, 9361. [Google Scholar] [CrossRef]
- Laursen, P.; Buchheit, M. Science and Application of High-Intensity Interval Training; Human Kinetics Publishers: Champaign, IL, USA, 2019; ISBN 978-1-4925-5212-3. [Google Scholar]
- Kramps, K.; Lane-Cordova, A. High-intensity interval training in cardiac rehabilitation. Sport Sci. Health 2021. [Google Scholar] [CrossRef]
- Bosch, P.R.; Holzapfel, S.D.; Nordin, K.; Ojameruaye, O.; Zubriski, M.; Angadi, S.S. High-Intensity Interval Training for Adults With Chronic Stroke: A Pilot Feasibility Study. Cardiopulm. Phys. Ther. J. 2021, 32, 20–29. [Google Scholar] [CrossRef]
- Marterer, N.; Menz, V.; Amin, S.; Faulhaber, M. 6-week High-intensity Interval Training (HIIT) of the Lower Extremities Improves VO2max of the Upper Extremities. Int. J. Sports Med. 2020, 41, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Tabata, I. Tabata training: one of the most energetically effective high-intensity intermittent training methods. J. Physiol. Sci. 2019, 69, 559–572. [Google Scholar] [CrossRef]
- Scholich, M. Circuit Training; Sport Verlag: Berlin, Germany, 1986. [Google Scholar]
- Nowak, M.; Ambroży, T. Współczesny trening obwodowy w teorii i praktyce; Fall: Kraków, Poland, 2015. [Google Scholar]
- Michael, B. Nowoczesny trening funkcionalny; Galaktyka: Łódź, Poland, 2019; ISBN 978-83-75-79-716-9. [Google Scholar]
- Muller, M.; den Tonkelaar, I.; Thijssen, J.H.; Grobbee, D.E.; van der Schouw, Y.T. Endogenous sex hormones in men aged 40–80 years. Eur. J. Endocrinol. 2003, 149, 583–589. [Google Scholar] [CrossRef] [Green Version]
- Salvador, A.; Suay, F.; González-Bono, E.; Serrano, M.A. Anticipatory cortisol, testosterone and psychological responses to judo competition in young men. Psychoneuroendocrinology 2003, 28, 364–375. [Google Scholar] [CrossRef]
- Allen, N.E.; Appleby, P.N.; Davey, G. Lifestyle and nutritional determinants of bioavailable androgens and related hormones in British men. Cancer Causes Control 2002, 13, 353–363. [Google Scholar] [CrossRef]
- Caminiti, G.; Volterrani, M.; Iellamo, F.; Marazzi, G.; Massaro, R.; Miceli, M.; Mammi, C.; Piepoli, M.; Fini, M.; Rosano, G.M. Effect of Long-Acting Testosterone Treatment on Functional Exercise Capacity, Skeletal Muscle Performance, Insulin Resistance, and Baroreflex Sensitivity in Elderly Patients With Chronic Heart Failure. J. Am. Coll. Cardiol. 2009, 54, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Shores, M.M.; Matsumoto, A.M. Testosterone, aging and survival. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 209–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraemer, W.J.; Ratamess, N.A. Hormonal Responses and Adaptations to Resistance Exercise and Training. Sport. Med. 2005, 35, 339–361. [Google Scholar] [CrossRef] [PubMed]
- Meckel, Y.; Nemet, D.; Bar-Sela, S.; Radom-Aizik, S.; Cooper, D.M.; Sagiv, M.; Eliakim, A. Hormonal and Inflammatory Responses to Different Types of Sprint Interval Training. J. Strength Cond. Res. 2011, 25, 2161–2169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karkoulias, K.; Habeos, I.; Charokopos, N.; Tsiamita, M.; Mazarakis, A.; Pouli, A.; Spiropoulos, K. Hormonal responses to marathon running in non-elite athletes. Eur. J. Intern. Med. 2008, 19, 598–601. [Google Scholar] [CrossRef]
- Paunksnis, M.R.; Evangelista, A.L.; La Scala Teixeira, C.V.; Alegretti João, G.; Pitta, R.M.; Alonso, A.C.; Figueira, A.; Serra, A.J.; Baker, J.S.; Schoenfeld, B.J.; et al. Metabolic and hormonal responses to different resistance training systems in elderly men. Aging Male 2018, 21, 106–110. [Google Scholar] [CrossRef]
- Uchida, M.C.; Crewther, B.T.; Ugrinowitsch, C.; Bacurau, R.F.P.; Moriscot, A.S.; Aoki, M.S. Hormonal Responses to Different Resistance Exercise Schemes of Similar Total Volume. J. Strength Cond. Res. 2009, 23, 2003–2008. [Google Scholar] [CrossRef]
- Sheykhlouvand, M.; Khalili, E.; Agha-Alinejad, H.; Gharaat, M. Hormonal and Physiological Adaptations to High-Intensity Interval Training in Professional Male Canoe Polo Athletes. J. Strength Cond. Res. 2016, 30, 859–866. [Google Scholar] [CrossRef]
- Ahmadi, M.A.; Zar, A.; Krustrup, P.; Ahmadi, F. Testosterone and cortisol response to acute intermittent and continuous aerobic exercise in sedentary men. Sport Sci. Health 2018, 14, 53–60. [Google Scholar] [CrossRef]
- Kilian, Y.; Engel, F.; Wahl, P.; Achtzehn, S.; Sperlich, B.; Mester, J. Markers of biological stress in response to a single session of high-intensity interval training and high-volume training in young athletes. Eur. J. Appl. Physiol. 2016, 116, 2177–2186. [Google Scholar] [CrossRef]
- Cofré-Bolados, C.; Reuquen-López, P.; Herrera-Valenzuela, T.; Orihuela-Diaz, P.; Garcia-Hermoso, A.; Hackney, A.C. Testosterone and Cortisol Responses to HIIT and Continuous Aerobic Exercise in Active Young Men. Sustainability 2019, 11, 6069. [Google Scholar] [CrossRef] [Green Version]
- Ponholzer, A.; Plas, E.; Schatzl, G.; Struhal, G.; Brössner, C.; Mock, K.; Rauchenwald, M.; Madersbacher, S. Relationship between testosterone serum levels and lifestyle in aging men. Aging Male 2005, 8, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Hayes, L.D.; Elliott, B.T. Short-Term Exercise Training Inconsistently Influences Basal Testosterone in Older Men: A Systematic Review and Meta-Analysis. Front. Physiol. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Bacon, A.P.; Carter, R.E.; Ogle, E.A.; Joyner, M.J. VO2max Trainability and High Intensity Interval Training in Humans: A Meta-Analysis. PLoS One 2013, 8, e73182. [Google Scholar] [CrossRef]
- Astorino, T.A.; Allen, R.P.; Roberson, D.W.; Jurancich, M. Effect of High-Intensity Interval Training on Cardiovascular Function, V̇o2max, and Muscular Force. J. Strength Cond. Res. 2012, 26, 138–145. [Google Scholar] [CrossRef]
- Brown, E.C.; Hew-Butler, T.; Marks, C.R.C.; Butcher, S.J.; Choi, M.D. The Impact of Different High-Intensity Interval Training Protocols on Body Composition and Physical Fitness in Healthy Young Adult Females. Biores. Open Access 2018, 7, 177–185. [Google Scholar] [CrossRef]
- Menz, V.; Marterer, N.; Amin, S.B.; Faulhaber, M.; Hansen, A.B.; Lawley, J.S. Functional Vs. Running Low-Volume High-Intensity Interval Training: Effects on VO2max and Muscular Endurance. J. Sports Sci. Med. 2019, 18, 497–504. [Google Scholar]
| Group | N | Variable | Mean | SD | Min–Max | 95%Cl |
|---|---|---|---|---|---|---|
| Experimental | Age (years) | 37.7 | 1.9 | 35–40 | 36.81–38.78 | |
| 15 | Stature (cm) | 180.0 | 5.6 | 174–195 | 177.68–183.92 | |
| BM (kg) | 90.6 | 13.1 | 65.2–112.0 | 83.36–97.94 | ||
| BMI (kg/m2) | 27.6 | 3.9 | 21.2–36.3 | 25.40–29.71 | ||
| Control | Age (years) | 37.8 | 1.7 | 35–40 | 36.88–38.17 | |
| 15 | Stature (cm) | 175.0 | 5.6 | 163–186 | 172.29–178.51 | |
| BM (kg) | 84.8 | 14.2 | 65.4–121.2 | 76.96–92.63 | ||
| BMI (kg/m2) | 27.6 | 3.3 | 22.9–34.9 | 25.74–29.44 |
| Interval Training 2:1 (HIIT) | Strength Circuit with the Example of Resistance Training with a Kettlebell |
|---|---|
| At the beginning of the training, participants had to do warm-up and adaptation exercises with minimal external resistance under the supervision of a personal trainer (duration 10–15 min). The participant performed a form of activity that involved high-intensity exercise alternated with rest (60 s/30 s). It consisted of 10 exercises that were performed one after the other to form a circuit. 1. Push-ups on dumbbells with the dumbbell pulled alternately to the chest at the moment of straightening the arms. 2. Squat with a dumbbell held with both hands at chest height. 3. Standing kettlebell side bends. 4. Jumps with a change of legs (from the forward lunge position). 5. Standing dumbbell press. 6. Half squat with a dumbbell held between legs with both hands. 7. Dumbbell weighted sit-ups. 8. Dumbbell pull (dumbbell row). 9. Push-ups. 10. Dumbbell reverse lunges. | The participant performed a small circuit of 5 exercises with a kettlebell (25 repetitions each). During one training session, they performed from 3 to 5 circuits, according to the principle of a gradual increase in loads. The rest between circuits was 1 to 2 min. 1. Kettlebell swing. 2. Standing one-handed press. 3. Squat with a kettlebell held with both hands in front of the body. 4. Kettlebell clean. 5. Kettlebell snatch. The duration of a training session was up to 30 min. The first session was devoted to learning and mastering the correct technique of the exercises. The participant ended each training session with stretching for about 6 min. |
| Testosterone nmol/L | Experimental Group | Control Group | Between Groups | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | Min | Max | SD | Mean | Median | Min | Max | SD | t1 | p | Cohen’s d | |
| Pre | 14.85 | 14.23 | 8.05 | 24.05 | 4.09 | 16.66 | 16.76 | 8.02 | 27.62 | 5.10 | −1.09 | 0.285 | 0.391 |
| Post | 20.30 | 18.60 | 13.85 | 25.85 | 4.23 | 17.66 | 18.46 | 8.05 | 26.82 | 5.45 | 1.48 | 0.150 | 0.541 |
| differences | 5.45 | 5.48 | 5.79 | 1.79 | 2.40 | 1.00 | 0.69 | 0.03 | −0.80 | 2.32 | 5.05 | 0.001 | 1.89 |
| Between examinations | t2 = −9.08, p < 0.001, Cohen’s d = 2.27 | t2 = −1.53, p = 0.149, Cohen’s d = 0.435 | |||||||||||
| Cortisol nmol/L | Experimental Group | Control Group | Between Groups | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | Min | Max | SD | Mean | Median | Min | Max | SD | t1 | p | Cohen’s d | |
| Pre | 460 | 428 | 334 | 666 | 93 | 390 | 406 | 201 | 593 | 113 | 2.07 | 0.045 | 0.681 |
| Post | 405 | 424 | 274 | 527 | 85 | 385 | 378 | 166 | 702 | 159 | 0.47 | 0.640 | 0.171 |
| differences | −55 | −4 | −60 | −139 | 118 | −5 | −28 | −35 | 109 | 137 | −1.21 | 0.235 | 0.391 |
| Between examinations | t2 = −2.00, p < 0.061, Cohen’s d = 0.466 | t2 = −0.13, p = 0.902, Cohen’s d = 0.037 | |||||||||||
| T/C × 100 | Experimental Group | Control Group | Between Groups | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | Min | Max | SD | Mean | Median | Min | Max | SD | t1 | p | Cohen’s d | |
| Pre | 3.17 | 2.97 | 2.16 | 4.18 | 0.79 | 4.63 | 4.12 | 2.39 | 8.33 | 1.71 | −3.37 | 0.002 | 1.09 |
| Post | 5.05 | 4.91 | 3.55 | 7.61 | 1.13 | 5.39 | 5.00 | 1.77 | 13.18 | 2.73 | −0.49 | 0.626 | 1.64 |
| differences | 1.88 | 1.94 | 1.39 | 3.43 | 1.19 | 0.76 | 0.88 | −0.62 | 4.85 | 1.98 | 2.11 | 0.042 | 0.686 |
| Between examinations | t2 = −6.90, p ≤ 0.001, Cohen’s d = 1.58 | t2 = −0.198, p = 0.111, Cohen’s d = 0.384 | |||||||||||
| VO2max (mL/kg/min) | Experimental Group | Control Group | BetweenGroups | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | Min | Max | SD | Mean | Median | Min | Max | SD | t1 | p | Cohen’s d | |
| Pre | 32.2 | 32.0 | 27.4 | 38.1 | 3.6 | 32.8 | 22.7 | 26.3 | 41.2 | 4.3 | 1.08 | 0.288 | 0.151 |
| Post | 36.0 | 36.5 | 31.0 | 40.2 | 3.2 | 33.0 | 33.0 | 26.8 | 41.8 | 3.6 | 1.43 | 0.160 | 0.881 |
| differences | 3.8 | 3.5 | 0.5 | 10.9 | −0.4 | 0.2 | 10.3 | 0.5 | 0.6 | −0.7 | 0.17 | 0.869 | 0.315 |
| Between examinations | t2 = 0.872, p = 0.395, Cohen’s d = 9.50 | t2 = −0.13, p = 0.902, Cohen’s d = 0.286 | |||||||||||
| Regression Equation | Correlation (r) | p-Value |
|---|---|---|
| ΔVO2max = 0.887 + 1.425 × ΔT/C × 102 | 0.047 | 0.0047 |
| Groups | Testing | Sit-Ups n/30 s | Pull-Ups | Standing Long Jump cm |
|---|---|---|---|---|
| Experimental Group | pre | 20.6 ± 4.9 | 3.7 ± 4.2 | 208 ± 28 |
| post | 26.4 ± 3.1 | 7.1 ± 4.3 | 222 ± 25 | |
| difference | t = −7.60, p = ≤0.001 Cohen’s d = 1.93 | t = −8.13, p = ≤0.001 Cohen’s d = 2.09 | t = −6.54, p = ≤0.001 Cohen’s d = 1.69 | |
| Control Group | pre | 20.4 ± 4.8 | 2.7 ± 3.7 | 201 ± 16 |
| post | 21.3 ± 4.7 | 3.1 ± 3.9 | 205 ± 18 | |
| difference | t = −2.22, p = 0.043 Cohen’s d = 0.574 | t = −2.44, p = 0.028 Cohen’s d = 0.634 | t = −1.38, p = 0.195 Cohen’s d = 0.355 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambroży, T.; Rydzik, Ł.; Obmiński, Z.; Błach, W.; Serafin, N.; Błach, B.; Jaszczur-Nowicki, J.; Ozimek, M. The Effect of High-Intensity Interval Training Periods on Morning Serum Testosterone and Cortisol Levels and Physical Fitness in Men Aged 35–40 Years. J. Clin. Med. 2021, 10, 2143. https://doi.org/10.3390/jcm10102143
Ambroży T, Rydzik Ł, Obmiński Z, Błach W, Serafin N, Błach B, Jaszczur-Nowicki J, Ozimek M. The Effect of High-Intensity Interval Training Periods on Morning Serum Testosterone and Cortisol Levels and Physical Fitness in Men Aged 35–40 Years. Journal of Clinical Medicine. 2021; 10(10):2143. https://doi.org/10.3390/jcm10102143
Chicago/Turabian StyleAmbroży, Tadeusz, Łukasz Rydzik, Zbigniew Obmiński, Wiesław Błach, Natalia Serafin, Blanka Błach, Jarosław Jaszczur-Nowicki, and Mariusz Ozimek. 2021. "The Effect of High-Intensity Interval Training Periods on Morning Serum Testosterone and Cortisol Levels and Physical Fitness in Men Aged 35–40 Years" Journal of Clinical Medicine 10, no. 10: 2143. https://doi.org/10.3390/jcm10102143
APA StyleAmbroży, T., Rydzik, Ł., Obmiński, Z., Błach, W., Serafin, N., Błach, B., Jaszczur-Nowicki, J., & Ozimek, M. (2021). The Effect of High-Intensity Interval Training Periods on Morning Serum Testosterone and Cortisol Levels and Physical Fitness in Men Aged 35–40 Years. Journal of Clinical Medicine, 10(10), 2143. https://doi.org/10.3390/jcm10102143









