Multi-Omics Analysis of Anti-Inflammatory Action of Alkaline Extract of the Leaves of Sasa sp.
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Cell Culture
2.3. Assay for Cytotoxic Activity
2.4. Assay for Pro-Inflammatory Substances
2.5. Processing for Metabolomic Analysis
2.6. DNA Microarray Processing
2.7. Statistical Analysis
3. Results
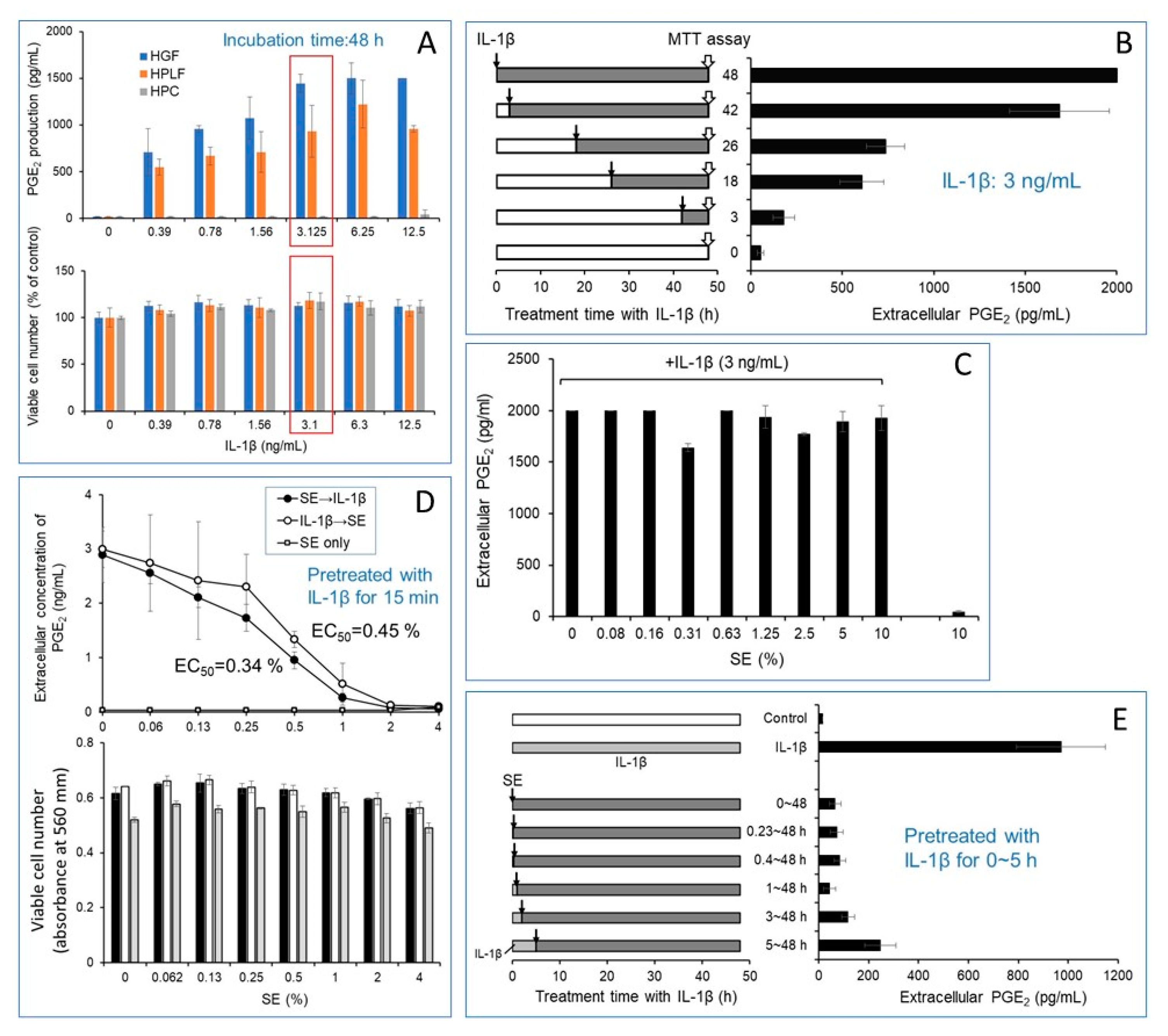
3.1. Pretreatment, Rather Than Post-Treatment, with SE More Efficiently Inhibited IL-1β-Stimulated PGE2 Production in HGF.t
3.1.1. Optimal Conditions for Induction of Inflammation by IL-1β
3.1.2. Inhibition of IL-1β-Induced Inflammation by SE
3.1.3. Mild Growth Stimulation Effect of IL-1β and SE
3.2. Metabolome Analysis
3.3. DNA Array Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ohno, H.; Miyoshi, S.; Araho, D.; Kanamoto, T.; Terakubo, S.; Nakashima, H.; Tsuda, T.; Sunaga, K.; Amano, S.; Ohkoshi, E.; et al. Efficient utilization of licorice root by alkaline extraction. In Vivo 2014, 28, 785–794. [Google Scholar]
- Sakagami, H.; Ohkoshi, E.; Amano, S.; Satoh, K.; Kanamoto, T.; Terakubo, S.; Nakashima, H.; Sunaga, K.; Otsuki, T.; Ikeda, H.; et al. Efficient utilization of plant resources by alkaline extraction. Altern. Integr. Med. 2013, 2, 1–7. [Google Scholar]
- Sakagami, H.; Iwamoto, S.; Matsuta, T.; Satoh, K.; Shimada, C.; Kanamoto, T.; Terakubo, S.; Nakashima, H.; Morita, Y.; Ohkubo, A.; et al. Comparative study of biological activity of three commercial products of Sasa senanensis Rehder leaf extract. In Vivo 2012, 26, 259–264. [Google Scholar]
- Pang, X.; Wang, Y.; Wu, J.; Zhou, Z.; Xu, T.; Jin, L.; Yu, Y.; Li, Z.; Gobin, R.; Xue, C.; et al. Yunnan Baiyao Conditioned Medium Promotes the Odonto/Osteogenic Capacity of Stem Cells from Apical Papilla via Nuclear Factor Kappa B Signaling Pathway. Biomed. Res. Int. 2019, 2019, 9327386. [Google Scholar] [CrossRef]
- Kim, H.; Kim, C.; Kim, D.U.; Chung, H.C.; Hwang, J.K. Inhibitory Effects of Boesenbergia pandurata on Age-Related Periodontal Inflammation and Alveolar Bone Loss in Fischer 344 Rats. J. Microbiol. Biotechnol. 2018, 28, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Kagermeier-Callaway, A.S.; Bredick, J.; Willershausen, B. Effect of three mouthrinses, containing amine/stannous fluoride, herbal extracts or Emser salt on the growth of oral bacteria—An in vitro study. Eur. J. Med. Res. 2000, 5, 523–529. [Google Scholar] [PubMed]
- Matsuta, T.; Sakagami, H.; Tanaka, S.; Machino, M.; Tomomura, M.; Tomomura, A.; Yasui, T.; Itoh, K.; Sugiura, T.; Kitajima, M.; et al. Pilot clinical study of Sasa senanensis Rehder leaf extract treatment on lichenoid dysplasia. In Vivo 2012, 26, 957–962. [Google Scholar] [PubMed]
- Sakagami, H. Biological activities and possible dental application of three major groups of polyphenols. J. Pharmacol. Sci. 2014, 126, 92–106. [Google Scholar] [CrossRef] [Green Version]
- Ono, M.; Kantoh, K.; Ueki, J.; Shimada, A.; Wakabayashi, H.; Matsuta, T.; Sakagami, H.; Kumada, H.; Hamada, N.; Kitajima, M.; et al. Quest for anti-inflammatory substances using IL-1β-stimulated gingival fibroblasts. In Vivo 2011, 25, 763–768. [Google Scholar]
- Fukuchi, K.; Sakagami, H.; Sugita, Y.; Takao, K.; Asai, D.; Terakubo, S.; Takemura, H.; Ohno, H.; Horiuchi, M.; Suguro, M.; et al. Quantification of the Ability of Natural Products to Prevent Herpes Virus Infection. Medicines 2020, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Sakagami, H.; Zhou, L.; Kawano, M.; Thet, M.M.; Tanaka, S.; Machino, M.; Amano, S.; Kuroshita, R.; Watanabe, S.; Chu, Q.; et al. Multiple biological complex of alkaline extract of the leaves of Sasa senanensis Rehder. In Vivo 2010, 24, 735–743. [Google Scholar]
- Huang, J.Q.; Qi, R.T.; Pang, M.R.; Liu, C.; Li, G.Y.; Zhang, Y. Isolation, chemical characterization, and immunomodulatory activity of naturally acetylated hemicelluloses from bamboo shavings. J. Zhejiang Univ. Sci. B 2017, 18, 138–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakagami, H.; Matsuta, T.; Satoh, K.; Ohtsuki, S.; Shimada, C.; Kanamoto, T.; Terakubo, S.; Nakashima, H.; Morita, Y.; Ohkubo, A.; et al. Biological activity of SE-10, granulated powder of Sasa senanensis Rehder leaf extract. In Vivo 2012, 26, 411–418. [Google Scholar] [PubMed]
- Kantoh, K.; Ono, M.; Nakamura, Y.; Nakamura, Y.; Hashimoto, K.; Sakagami, H.; Wakabayashi, H. Hormetic and anti-radiation effects of tropolone-related compounds. In Vivo 2010, 24, 843–851. [Google Scholar] [PubMed]
- Garcia-Contreras, R.; Sugimoto, M.; Umemura, N.; Kaneko, M.; Hatakeyama, Y.; Soga, T.; Tomita, M.; Scougall-Vilchis, R.J.; Contreras-Bulnes, R.; Nakajima, H.; et al. Alteration of metabolomic profiles by titanium dioxide nanoparticles in human gingivitis model. Biomaterials 2015, 57, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Wong, D.T.; Hirayama, A.; Soga, T.; Tomita, M. Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics 2010, 6, 78–95. [Google Scholar] [CrossRef] [Green Version]
- Sakagami, H.; Shi, H.; Bandow, K.; Tomomura, M.; Tomomura, A.; Horiuchi, M.; Fujisawa, T.; Oizumi, T. Search of Neuroprotective Polyphenols Using the “Overlay” Isolation Method. Molecules 2018, 23, 1840. [Google Scholar] [CrossRef] [Green Version]
- Morrison, I.M. Structural invesiigations on the lignin-carbohydrate complexes of Lolium perenne. Biochem. J. 1974, 139, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Allerdings, E.; Ralph, J.; Steinhart, H.; Bunzel, M. Isolation and structural identification of complex feruloylated heteroxylan side-chains from maize bran. Phytochemistry 2006, 67, 1276–1286. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, W.; Shang, L.; Song, A.; Ge, S. N-WASP knockdown upregulates inflammatory cytokines expression in human gingival fibroblasts. Arch. Oral. Biol. 2020, 110, 104605. [Google Scholar] [CrossRef]
- Ara, T.; Sogawa, N. Effects of shinbuto and ninjinto on prostaglandin E(2) production in lipopolysaccharide-treated human gingival fibroblasts. PeerJ 2017, 5, e4120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, T.; Segami, N.; Sakagami, H. Anti-inflammatory Activity of Hangeshashinto in IL-1β-stimulated Gingival and Periodontal Ligament Fibroblasts. In Vivo 2016, 30, 257–263. [Google Scholar] [PubMed]
- Suzuki, S.; Kodera, Y.; Saito, T.; Fujimoto, K.; Momozono, A.; Hayashi, A.; Kamata, Y.; Shichiri, M. Methionine sulfoxides in serum proteins as potential clinical biomarkers of oxidative stress. Sci. Rep. 2016, 6, 38299. [Google Scholar] [CrossRef] [Green Version]
- Holte, K.B.; Svanteson, M.; Hanssen, K.F.; Sveen, K.A.; Seljeflot, I.; Solheim, S.; Sell, D.R.; Monnier, V.M.; Berg, T.J. Collagen methionine sulfoxide and glucuronidine/LW-1 are markers of coronary artery disease in long-term survivors with type 1 diabetes. The Dialong study. PLoS ONE 2020, 15, e0233174. [Google Scholar] [CrossRef] [PubMed]
- Aledo, J.C.; Aledo, P. Susceptibility of Protein Methionine Oxidation in Response to Hydrogen Peroxide Treatment-Ex Vivo Versus In Vitro: A Computational Insight. Antioxidants 2020, 9, 987. [Google Scholar] [CrossRef]
- Sourdon, J.; Keceli, G.; Lindsey, M.L.; Paolocci, N. Death of an antioxidant brings heart failure with preserved ejection fraction to life: 5-oxoproline and post-ischaemic cardio-renal dysfunction. Cardiovasc. Res. 2018, 114, 1819–1821. [Google Scholar] [CrossRef] [PubMed]
- Van der Pol, A.; Gil, A.; Tromp, J.; Silljé, H.H.W.; van Veldhuisen, D.J.; Voors, A.A.; Hoendermis, E.S.; Grote Beverborg, N.; Schouten, E.M.; de Boer, R.A.; et al. OPLAH ablation leads to accumulation of 5-oxoproline, oxidative stress, fibrosis, and elevated fillings pressures: A murine model for heart failure with a preserved ejection fraction. Cardiovasc. Res. 2018, 114, 1871–1882. [Google Scholar] [CrossRef]
- Bachhawat, A.K.; Yadav, S.; Jainarayanan, A.K.; Dubey, P. Heart failure and the glutathione cycle: An integrated view. Biochem. J. 2020, 477, 3123–3130. [Google Scholar] [CrossRef] [PubMed]
- Gut, P.; Verdin, E. The nexus of chromatin regulation and intermediary metabolism. Nature 2013, 502, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Zamaraeva, M.V.; Sabirov, R.Z.; Maeno, E.; Ando-Akatsuka, Y.; Bessonova, S.V.; Okada, Y. Cells die with increased cytosolic ATP during apoptosis: A bioluminescence study with intracellular luciferase. Cell Death Differ. 2005, 12, 1390–1397. [Google Scholar] [CrossRef]
- Basso, F.G.; Pansani, T.N.; Turrioni, A.P.; Soares, D.G.; de Souza Costa, C.A.; Hebling, J. Tumor Necrosis Factor-α and Interleukin (IL)-1β, IL-6, and IL-8 Impair In Vitro Migration and Induce Apoptosis of Gingival Fibroblasts and Epithelial Cells, Delaying Wound Healing. J. Periodontol. 2016, 87, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Vardar-Sengul, S.; Arora, S.; Baylas, H.; Mercola, D. Expression profile of human gingival fibroblasts induced by interleukin-1beta reveals central role of nuclear factor-kappa B in stabilizing human gingival fibroblasts during inflammation. J. Periodontol. 2009, 80, 833–849. [Google Scholar] [CrossRef]
- Song, H.K.; Noh, E.M.; Kim, J.M.; You, Y.O.; Kwon, K.B.; Lee, Y.R. Reversine inhibits MMP-3, IL-6 and IL-8 expression through suppression of ROS and JNK/AP-1 activation in interleukin-1β-stimulated human gingival fibroblasts. Arch. Oral Biol. 2019, 108, 104530. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Ahn, S.H.; Lee, J.S.; Song, J.E.; Cho, S.H.; Jung, S.; Kim, S.K.; Kim, S.H.; Lee, K.P.; Kwon, K.S.; et al. Differential Matrix Metalloprotease (MMP) Expression Profiles Found in Aged Gingiva. PLoS ONE 2016, 11, e0158777. [Google Scholar] [CrossRef] [Green Version]
- Hosokawa, Y.; Hosokawa, I.; Ozaki, K.; Nakae, H.; Matsuo, T. Proinflammatory effects of tumour necrosis factor-like weak inducer of apoptosis (TWEAK) on human gingival fibroblasts. Clin. Exp. Immunol. 2006, 146, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, Y.; Hosokawa, I.; Ozaki, K.; Nakae, H.; Matsuo, T. Oncostatin M synergistically induces CXCL10 and ICAM-1 expression in IL-1beta-stimulated-human gingival fibroblasts. J. Cell Biochem. 2010, 111, 40–48. [Google Scholar] [CrossRef]
- Sakagami, H.; Amano, S.; Kikuchi, H.; Nakamura, Y.; Kuroshita, R.; Watanabe, S.; Satoh, K.; Hasegawa, H.; Nomura, A.; Kanamoto, T.; et al. Antiviral, antibacterial and vitamin C-synergized radical-scavenging activity of Sasa senanensis Rehder extract. In Vivo 2008, 22, 471–476. [Google Scholar]
- Sakagami, H.; Fukuchi, K.; Kanamoto, T.; Terakubo, S.; Nakashima, H.; Natori, T.; Suguro-Kitajima, M.; Oizumi, H.; Yasui, T.; Oizumi, T. Synergism of Alkaline Extract of the Leaves of Sasa senanensis Rehder and Antiviral Agents. In Vivo 2016, 30, 421–426. [Google Scholar]
- Sakagami, H.; Kushida, T.; Oizumi, T.; Nakashima, H.; Makino, T. Distribution of lignin-carbohydrate complex in plant kingdom and its functionality as alternative medicine. Pharmacol. Ther. 2010, 128, 91–105. [Google Scholar] [CrossRef]
- Sakagami, H.; Nagata, K.; Ishihama, A.; Oh-hara, T.; Kawazoe, Y. Anti-influenza virus activity of synthetically polymerized phenylpropenoids. Biochem. Biophys. Res. Commun. 1990, 172, 1267–1272. [Google Scholar] [CrossRef]
- Nakashima, H.; Murakami, T.; Yamamoto, N.; Naoe, T.; Kawazoe, Y.; Konno, K.; Sakagami, H. Lignified materials as medicinal resources. V. Anti-HIV (human immunodeficiency virus) activity of some synthetic lignins. Chem. Pharm. Bull. 1992, 40, 2102–2105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harada, H.; Sakagami, H.; Nagata, K.; Oh-hara, T.; Kawazoe, Y.; Ishihama, A.; Hata, N.; Misawa, Y.; Terada, H.; Konno, K. Possible involvement of lignin structure in anti-influenza virus activity. Antivir. Res. 1991, 15, 41–49. [Google Scholar] [CrossRef]
- Fukuchi, K.; Sakagami, H.; Ikeda, M.; Kawazoe, Y.; Oh-Hara, T.; Konno, K.; Ichikawa, S.; Hata, N.; Kondo, H.; Nonoyama, M. Inhibition of herpes simplex virus infection by pine cone antitumor substances. Anticancer Res. 1989, 9, 313–317. [Google Scholar] [PubMed]
- Fukuchi, K.; Sakagami, H.; Okuda, T.; Hatano, T.; Tanuma, S.; Kitajima, K.; Inoue, Y.; Inoue, S.; Ichikawa, S.; Nonoyama, M.; et al. Inhibition of herpes simplex virus infection by tannins and related compounds. Antivir. Res. 1989, 11, 285–297. [Google Scholar] [CrossRef]
- Sakagami, H.; Satoh, K.; Ida, Y.; Koyama, N.; Premanathan, M.; Arakaki, R.; Nakashima, H.; Hatano, T.; Okuda, T.; Yoshida, T. Induction of apoptosis and anti-HIV activity by tannin- and lignin-related substances. Basic Life Sci. 1999, 66, 595–611. [Google Scholar] [CrossRef] [PubMed]
- Fukai, T.; Sakagami, H.; Toguchi, M.; Takayama, F.; Iwakura, I.; Atsumi, T.; Ueha, T.; Nakashima, H.; Nomura, T. Cytotoxic activity of low molecular weight polyphenols against human oral tumor cell lines. Anticancer Res. 2000, 20, 2525–2536. [Google Scholar]
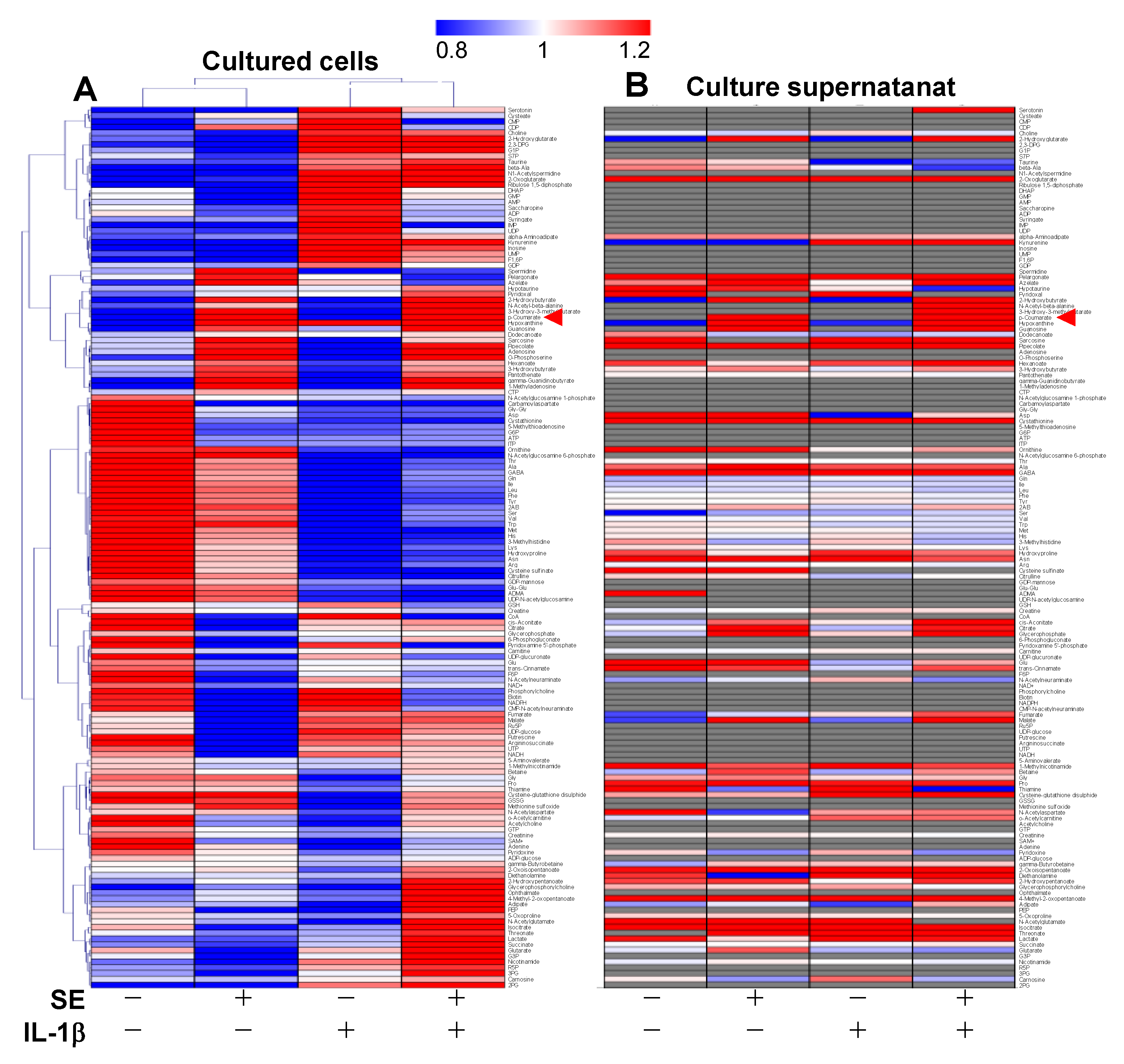
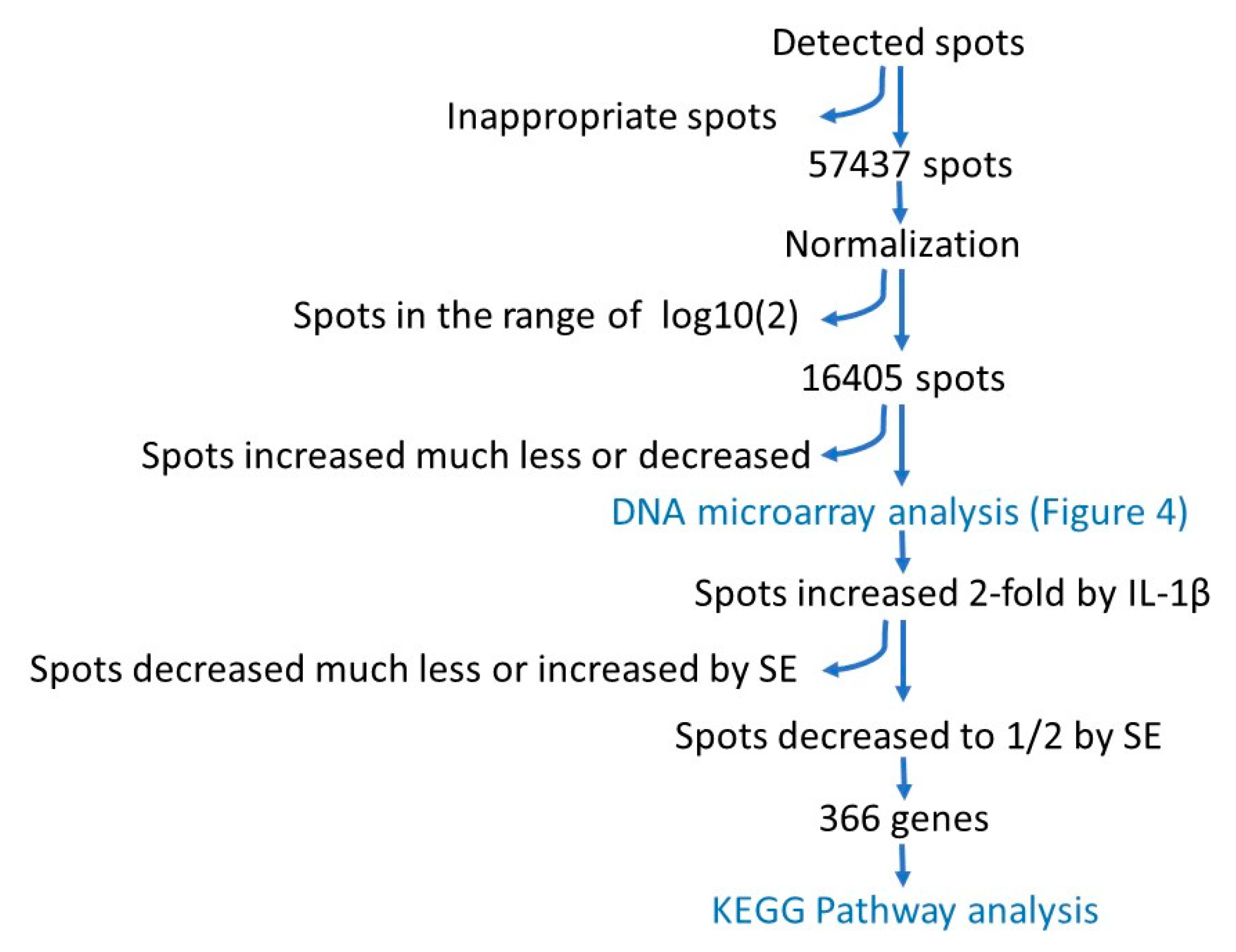


| Metabolites (Amol/Cell) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Control | (%) | IL-1β | (%) | SE | (%) | SE + IL-1β | (%) | |
| Amino acids | ||||||||
| Gly | 211,177 | 100 | 146,168 | 69 | 211,841 | 100 | 185,667 | 88 |
| Ala | 60,888 | 100 | 33,272 | 55 | 51,251 | 84 | 40,898 | 67 |
| Arg | 9902 | 100 | 6464 | 65 | 8236 | 83 | 7077 | 71 |
| Asp | 26,445 | 100 | 17,409 | 66 | 19,456 | 74 | 17,654 | 67 |
| Asn | 15,192 | 100 | 8635 | 57 | 11,970 | 79 | 9718 | 64 |
| Gln | 337,154 | 100 | 197,771 | 59 | 283,383 | 84 | 241,164 | 72 |
| Glu | 287,270 | 100 | 258,709 | 90 | 239,064 | 83 | 255,679 | 89 |
| His | 13,301 | 100 | 7407 | 56 | 10,625 | 80 | 8041 | 60 |
| Ile | 35,810 | 100 | 22,531 | 63 | 32,087 | 90 | 25,843 | 72 |
| Leu | 37,506 | 100 | 22,611 | 60 | 33,730 | 90 | 26,286 | 70 |
| Lys | 24,974 | 100 | 15,917 | 64 | 20,350 | 81 | 17,365 | 70 |
| Met | 15,489 | 100 | 7274 | 47 | 11,717 | 76 | 8548 | 55 |
| Phe | 29,015 | 100 | 16,943 | 58 | 24,954 | 86 | 19,252 | 66 |
| Pro | 62,148 | 100 | 49,904 | 80 | 61,787 | 99 | 56,773 | 91 |
| Ser | 47,587 | 100 | 29,061 | 61 | 42,219 | 89 | 35,215 | 74 |
| Thr | 140,117 | 100 | 73,931 | 53 | 113,094 | 81 | 91,622 | 65 |
| Trp | 6312 | 100 | 3447 | 55 | 6001 | 95 | 4418 | 70 |
| Tyr | 30,049 | 100 | 17,177 | 57 | 25,465 | 85 | 19,564 | 65 |
| Val | 29,763 | 100 | 19,547 | 66 | 28,292 | 95 | 22,768 | 76 |
| Total | 1,420,101 | 100 | 954,178 | 62 | 1235,525 | 86 | 1093,552 | 71 |
| Glutathiones | ||||||||
| GSH | 41,743 | 100 | 45,315 | 109 | 40,377 | 97 | 36,927 | 88 |
| GSSG | 19,334 | 100 | 6905 | 36 | 17,109 | 88 | 15,965 | 83 |
| Cys-GSH disulfide | 461 | 100 | 27 | 6 | 394 | 85 | 329 | 71 |
| Total | 61,538 | 100 | 52,248 | 85 | 57,880 | 94 | 53,221 | 86 |
| Others | ||||||||
| Met-sulfoxide | 283 | 100 | 175 | 41 | 316 | 74 | 297 | 149 |
| 5-Oxoproline | 8806 | 100 | 7801 | 89 | 8281 | 94 | 9507 | 108 |
| SAM+ | 713 | 100 | 324 | 45 | 426 | 60 | 443 | 62 |
| ATP/GTP utilization | ||||||||
| ATP | 64,581 | 100 | 46,778 | 72 | 46,906 | 73 | 46,787 | 72 |
| ADP | 4074 | 100 | 4734 | 116 | 3456 | 85 | 3729 | 92 |
| AMP | 425 | 100 | 581 | 136 | 333 | 78 | 423 | 99 |
| AMP/ATP | 0.0066 | 100 | 0.0124 | 188 | 0.0071 | 108 | 0.0090 | 137 |
| ADP/ATP | 0.0631 | 100 | 0.1012 | 160 | 0.0737 | 117 | 0.0797 | 126 |
| GTP | 14,959 | 100 | 12,730 | 85 | 13,880 | 93 | 14,106 | 94 |
| GDP | 747 | 100 | 861 | 115 | 713 | 95 | 776 | 104 |
| GMP | 162 | 100 | 198 | 123 | 123 | 76 | 165 | 102 |
| GMP/GTP | 0.0108 | 100 | 0.0156 | 144 | 0.0089 | 82 | 0.0117 | 109 |
| GDP/GTP | 0.0499 | 100 | 0.0676 | 135 | 0.0514 | 103 | 0.0550 | 110 |
| Expression (2n-Fold Increase) | ||||
|---|---|---|---|---|
| Control | SE | IL-1β | SE + IL-1β | |
| Btk | 0.000 | 0.000 | 1.108 | 0.000 |
| calpain | 0.000 | 0.000 | 2.139 | 0.000 |
| calpain 13 | 0.000 | 0.000 | 2.139 | 0.000 |
| collagenase-IV | 0.000 | 0.000 | 3.232 | 0.000 |
| Lefty | 0.000 | 0.000 | 3.521 | 0.000 |
| Lefty2 | 0.000 | 0.000 | 3.521 | 0.000 |
| MMP | 0.000 | 0.000 | 3.232 | 0.000 |
| MMP-2 | 0.000 | 0.000 | 3.232 | 0.000 |
| Nbeta | 0.000 | 0.000 | 1.121 | 0.000 |
| NEXT | 0.000 | 0.000 | 1.121 | 0.000 |
| NICD | 0.000 | 0.000 | 1.121 | 0.000 |
| Notch | 0.000 | 0.000 | 1.121 | 0.000 |
| Notch2 | 0.000 | 0.000 | 1.121 | 0.000 |
| NRPTP | 0.000 | 0.000 | 2.017 | 0.000 |
| PKC | 0.000 | 0.000 | 1.676 | 0.000 |
| PKCd | 0.000 | 0.000 | 1.676 | 0.000 |
| Shp1 | 0.000 | 0.000 | 2.017 | 0.000 |
| TGFb | 0.000 | 0.000 | 3.521 | 0.000 |
| TLR | 0.000 | 0.000 | 2.478 | 0.000 |
| TLR9 | 0.000 | 0.000 | 2.478 | 0.000 |
| TNF | 0.000 | 0.000 | 1.439 | 0.255 |
| TNFa | 0.000 | 0.000 | 1.439 | 0.255 |
| AKT | 0.000 | −0.189 | 2.320 | −0.309 |
| AKT2 | 0.000 | −0.189 | 2.320 | −0.309 |
| caspase | 0.000 | −0.705 | 1.072 | −0.151 |
| caspase-3 | 0.000 | −0.705 | 1.072 | −0.151 |
| CXCL3 | 0.000 | 0.110 | 8.826 | 7.756 |
| CXCL5 | 0.000 | −0.301 | 3.038 | 1.990 |
| CXCL10 | 0.000 | 0.000 | 3.254 | 2.252 |
| CCL7 | 0.000 | 0.000 | 4.148 | 3.141 |
| + IL-1β | +SE+ IL-1β | ||
|---|---|---|---|
| Metabolomic analysis | |||
| 19 Amino acids (except Cys and Glu) | ↓ | → | ↑ |
| Total glutathione (GSH, GSSG, Cys-GSH disulfide) | ↓ | → | ↑ |
| Met-sulfoxide | ↓ | → | ↑ |
| 5-Oxoproline | ↓ | → | ↑ |
| SAM | ↓ | → | ↑ |
| DNA array analysis | |||
| AKT (Cell survival) | ↑ | → | ↓ |
| CASP3 (Apoptosis) | ↑ | → | ↓ |
| CXCL3 (Leukocyte recruitment) | ↑ | → | ↓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakagami, H.; Nakatani, S.; Enomoto, A.; Ota, S.; Kaneko, M.; Sugimoto, M.; Horiuchi, M.; Toeda, K.; Oizumi, T. Multi-Omics Analysis of Anti-Inflammatory Action of Alkaline Extract of the Leaves of Sasa sp. J. Clin. Med. 2021, 10, 2100. https://doi.org/10.3390/jcm10102100
Sakagami H, Nakatani S, Enomoto A, Ota S, Kaneko M, Sugimoto M, Horiuchi M, Toeda K, Oizumi T. Multi-Omics Analysis of Anti-Inflammatory Action of Alkaline Extract of the Leaves of Sasa sp. Journal of Clinical Medicine. 2021; 10(10):2100. https://doi.org/10.3390/jcm10102100
Chicago/Turabian StyleSakagami, Hiroshi, Sachie Nakatani, Ayame Enomoto, Sana Ota, Miku Kaneko, Masahiro Sugimoto, Misaki Horiuchi, Kazuki Toeda, and Takaaki Oizumi. 2021. "Multi-Omics Analysis of Anti-Inflammatory Action of Alkaline Extract of the Leaves of Sasa sp." Journal of Clinical Medicine 10, no. 10: 2100. https://doi.org/10.3390/jcm10102100
APA StyleSakagami, H., Nakatani, S., Enomoto, A., Ota, S., Kaneko, M., Sugimoto, M., Horiuchi, M., Toeda, K., & Oizumi, T. (2021). Multi-Omics Analysis of Anti-Inflammatory Action of Alkaline Extract of the Leaves of Sasa sp. Journal of Clinical Medicine, 10(10), 2100. https://doi.org/10.3390/jcm10102100







