What Is New in Helicobacter pylori Diagnosis. An Overview
Abstract
:1. Introduction
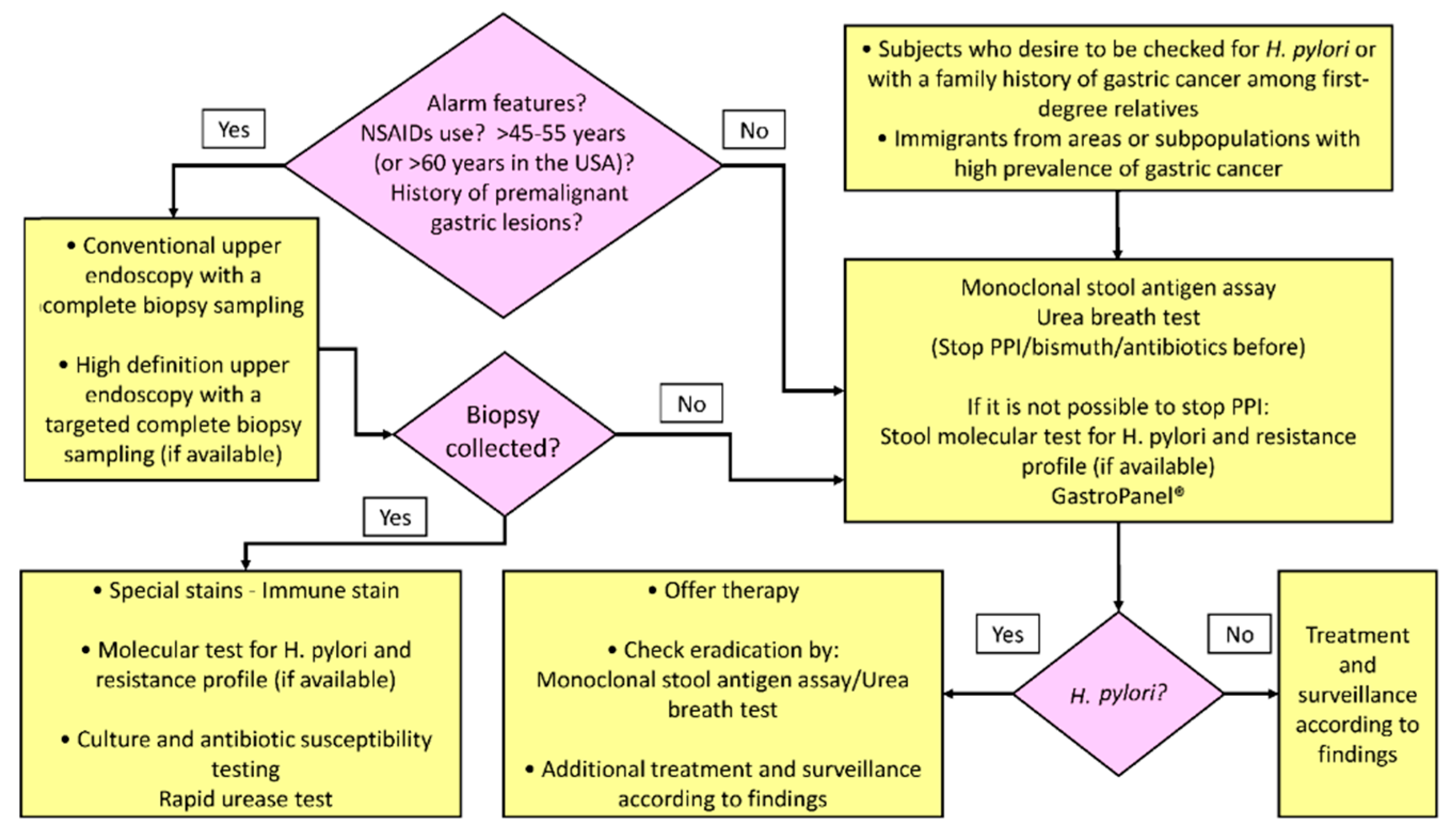
2. Invasive Tests
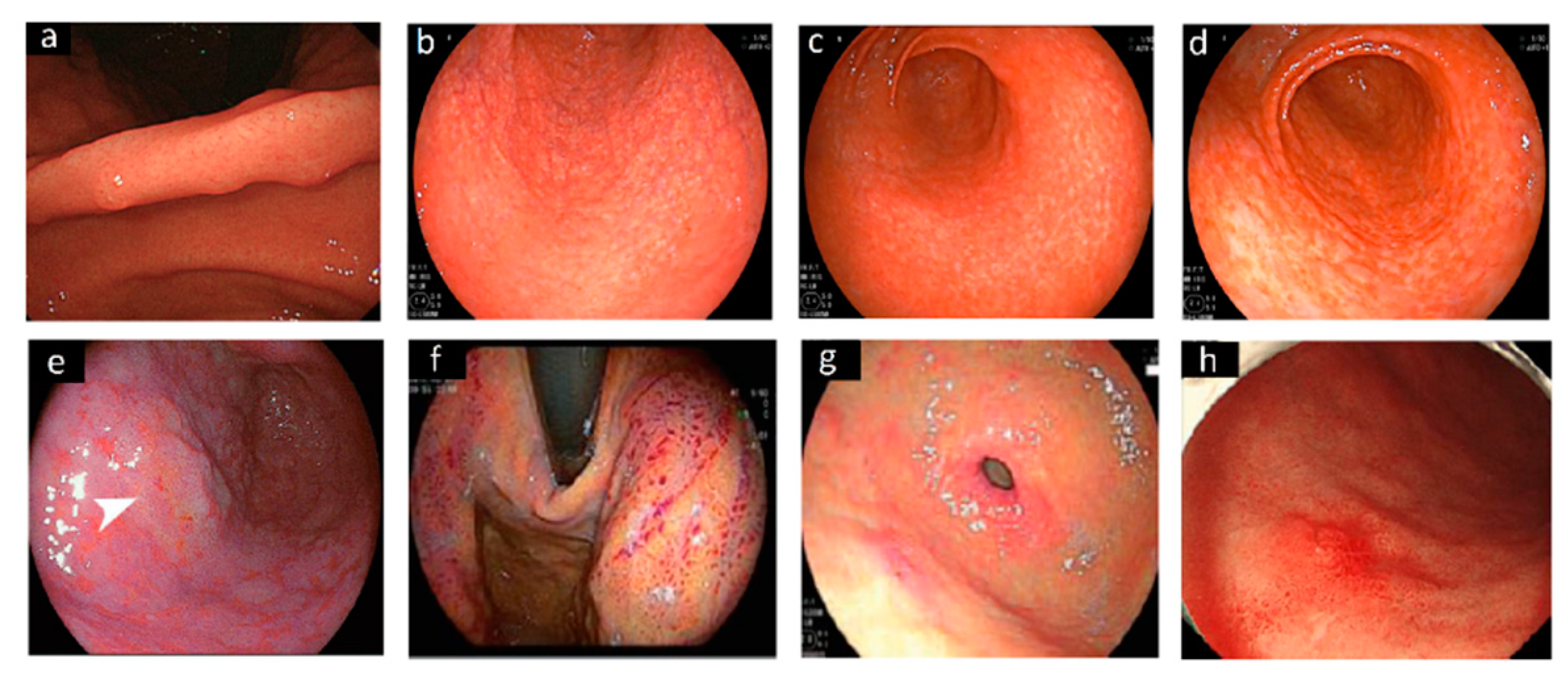
2.1. Endoscopy
2.2. Histology
2.3. Rapid Urease Test
2.4. Culture
3. Non-Invasive Tests
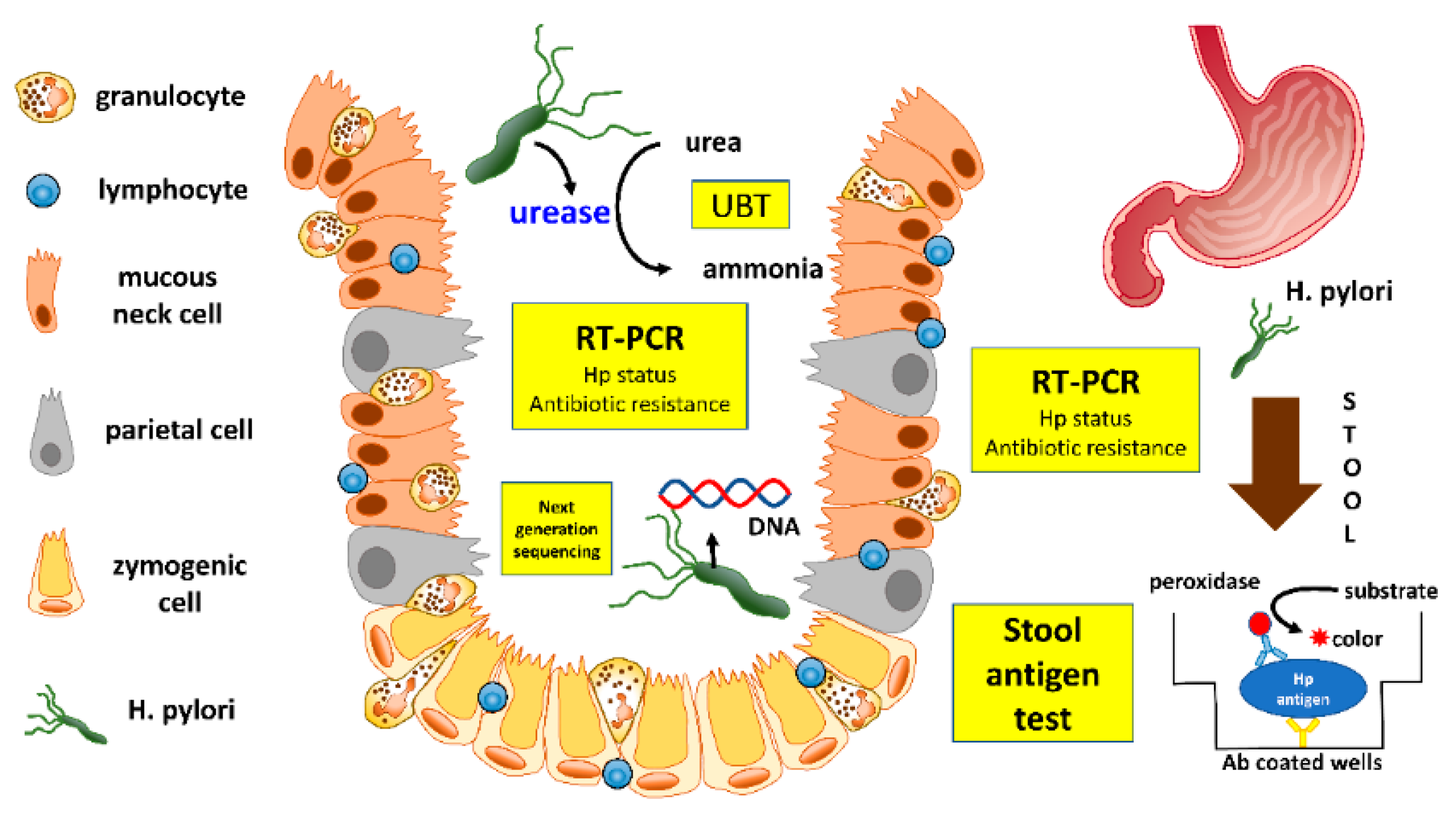
3.1. Urea Breath Test
3.2. Stool Antigen Test
3.3. Molecular Testing
3.4. Serology
3.5. Tests on Plasma, Blood, Saliva and Urine
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Talley, N.J. American Gastroenterological Association medical position statement: Evaluation of dyspepsia. Gastroenterology 2005, 129, 1753–1755. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.; Bazzoli, F.; El-Omar, E.; Graham, D.; Hunt, R.; Rokkas, T.; Vakil, N.; Kuipers, E.J. Current concepts in the management of Helicobacter pylori infection: The Maastricht III Consensus Report. Gut 2007, 56, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Moayyedi, P.; Lacy, B.E.; Andrews, C.N.; Enns, R.A.; Howden, C.W.; Vakil, N. ACG and CAG Clinical Guideline: Management of Dyspepsia. Am. J. Gastroenterol. 2017, 112, 988–1013. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Kao, J.Y.; Kanwal, F.; Gilger, M.; LoVecchio, F.; Moss, S.F.; Crowe, S.E.; Elfant, A.; Haas, T.; Hapke, R.J.; et al. Houston Consensus Conference on Testing for Helicobacter pylori Infection in the United States. Clin. Gastroenterol. Hepatol. 2018, 16, 992–1002.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dore, M.P.; Pes, G.M.; Bassotti, G.; Usai-Satta, P. Dyspepsia: When and How to Test for Helicobacter pylori Infection. Gastroenterol. Res. Pract. 2016, 2016, 8463614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loffeld, R.J. Diagnostic value of endoscopic signs of gastritis: With special emphasis to nodular antritis. Neth. J. Med. 1999, 54, 96–100. [Google Scholar] [CrossRef]
- Conti-Nibali, S.; Sferlazzas, C.; Fera, M.T.; Saitta, G.; Tedeschi, A.; Magazzu, G. Helicobacter pylori infection: A simplified diagnostic approach. Am. J. Gastroenterol. 1990, 85, 1573–1575. [Google Scholar] [PubMed]
- Luzza, F.; Pensabene, L.; Imeneo, M.; Mancuso, M.; Contaldo, A.; Giancotti, L.; La Vecchia, A.M.; Costa, M.C.; Strisciuglio, P.; Docimo, C.; et al. Antral nodularity identifies children infected with Helicobacter pylori with higher grades of gastric inflammation. Gastrointest. Endosc. 2001, 53, 60–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bah, A.; Saraga, E.; Armstrong, D.; Vouillamoz, D.; Dorta, G.; Duroux, P.; Weber, B.; Froehlich, F.; Blum, A.L.; Schnegg, J.F. Endoscopic features of Helicobacter pylori-related gastritis. Endoscopy 1995, 27, 593–596. [Google Scholar] [CrossRef]
- Laine, L.; Cohen, H.; Sloane, R.; Marin-Sorensen, M.; Weinstein, W.M. Interobserver agreement and predictive value of endoscopic findings for H. pylori and gastritis in normal volunteers. Gastrointest. Endosc. 1995, 42, 420–423. [Google Scholar] [CrossRef]
- Matrakool, L.; Tongtawee, T.; Bartpho, T.; Dechsukhum, C.; Loyd, R.A.; Kaewpitoon, S.J.; Kaewpitoon, N. Improved Detection of Helicobacter pylori Infection and Premalignant Gastric Mucosa Using Conventional White Light Source Gastroscopy. Asian Pac. J. Cancer Prev. 2016, 17, 2099–2103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redeen, S.; Petersson, F.; Jonsson, K.A.; Borch, K. Relationship of gastroscopic features to histological findings in gastritis and Helicobacter pylori infection in a general population sample. Endoscopy 2003, 35, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Hatano, Y.; Haruma, K.; Kamada, T.; Shiotani, A.; Takahari, K.; Matsumoto, M.; Uchida, O. Factors Associated with Gastric Black Spot, White Flat Elevated Mucosa, and Cobblestone-Like Mucosa: A Cross-Sectional Study. Digestion 2018, 98, 185–193. [Google Scholar] [CrossRef]
- Okamura, T.; Iwaya, Y.; Kitahara, K.; Suga, T.; Tanaka, E. Accuracy of Endoscopic Diagnosis for Mild Atrophic Gastritis Infected with Helicobacter pylori. Clin. Endosc. 2018, 51, 362–367. [Google Scholar] [CrossRef] [Green Version]
- Tongtawee, T.; Kaewpitoon, S.; Kaewpitoon, N.; Dechsukhum, C.; Loyd, R.A.; Matrakool, L. Correlation between Gastric Mucosal Morphologic Patterns and Histopathological Severity of Helicobacter pylori Associated Gastritis Using Conventional Narrow Band Imaging Gastroscopy. Biomed. Res. Int. 2015, 2015, 808505. [Google Scholar] [CrossRef]
- Tahara, T.; Tahara, S.; Tuskamoto, T.; Horiguchi, N.; Yoshida, D.; Kawamura, T.; Okubo, M.; Nagasaka, M.; Nakagawa, Y.; Urano, M.; et al. Magnifying NBI Patterns of Gastric Mucosa After Helicobacter pylori Eradication and Its Potential Link to the Gastric Cancer Risk. Dig. Dis. Sci. 2017, 62, 2421–2427. [Google Scholar] [CrossRef]
- Kanemitsu, T.; Yao, K.; Nagahama, T.; Imamura, K.; Fujiwara, S.; Ueki, T.; Chuman, K.; Tanabe, H.; Atsuko, O.; Iwashita, A.; et al. Extending magnifying NBI diagnosis of intestinal metaplasia in the stomach: The white opaque substance marker. Endoscopy 2017, 49, 529–535. [Google Scholar] [CrossRef]
- White, J.R.; Sami, S.S.; Reddiar, D.; Mannath, J.; Ortiz-Fernandez-Sordo, J.; Beg, S.; Scott, R.; Thiagarajan, P.; Ahmad, S.; Parra-Blanco, A.; et al. Narrow band imaging and serology in the assessment of premalignant gastric pathology. Scand. J. Gastroenterol. 2018, 53, 1611–1618. [Google Scholar] [CrossRef]
- Rugge, M.; Kim, J.G.; Mahachai, V.; Miehlke, S.; Pennelli, G.; Russo, V.M.; Perng, C.L.; Chang, F.Y.; Tandon, R.K.; Singal, D.K.; et al. OLGA gastritis staging in young adults and country-specific gastric cancer risk. Int. J. Surg. Pathol. 2008, 16, 150–154. [Google Scholar] [CrossRef]
- Saka, A.; Yagi, K.; Nimura, S. OLGA- and OLGIM-based staging of gastritis using narrow-band imaging magnifying endoscopy. Dig. Endosc. 2015, 27, 734–741. [Google Scholar] [CrossRef]
- Kotachi, T.; Ito, M.; Boda, T.; Kiso, M.; Masuda, K.; Hata, K.; Kawamura, T.; Sanomura, Y.; Yoshihara, M.; Tanaka, S.; et al. Clinical Significance of Reddish Depressed Lesions Observed in the Gastric Mucosa after Helicobacter pylori Eradication. Digestion 2018, 98, 48–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horiguchi, N.; Tahara, T.; Kawamura, T.; Okubo, M.; Tahara, S.; Nagasaka, M.; Nakagawa, Y.; Shibata, T.; Ohmiya, N. A Comparative Study of White Light Endoscopy, Chromoendoscopy and Magnifying Endoscopy with Narrow Band Imaging in the Diagnosis of Early Gastric Cancer after Helicobacter pylori Eradication. J. Gastrointestin. Liver Dis. 2017, 26, 357–362. [Google Scholar] [CrossRef] [Green Version]
- Horiguchi, N.; Tahara, T.; Yamada, H.; Yoshida, D.; Okubo, M.; Nagasaka, M.; Nakagawa, Y.; Shibata, T.; Tsukamoto, T.; Kuroda, M.; et al. In vivo diagnosis of early-stage gastric cancer found after Helicobacter pylori eradication using probe-based confocal laser endomicroscopy. Dig. Endosc. 2018, 30, 219–227. [Google Scholar] [CrossRef]
- Nishikawa, Y.; Ikeda, Y.; Murakami, H.; Hori, S.I.; Hino, K.; Sasaki, C.; Nishikawa, M. Classification of atrophic mucosal patterns on Blue LASER Imaging for endoscopic diagnosis of Helicobacter pylori-related gastritis: A retrospective, observational study. PLoS ONE 2018, 13, e0193197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osawa, H.; Miura, Y.; Takezawa, T.; Ino, Y.; Khurelbaatar, T.; Sagara, Y.; Lefor, A.K.; Yamamoto, H. Linked Color Imaging and Blue Laser Imaging for Upper Gastrointestinal Screening. Clin. Endosc. 2018, 51, 513–526. [Google Scholar] [CrossRef]
- Jiang, Z.X.; Nong, B.; Liang, L.X.; Yan, Y.D.; Zhang, G. Differential diagnosis of Helicobacter pylori-associated gastritis with the linked-color imaging score. Dig. Liver Dis. 2019, 51, 1665–1670. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.; Dohi, O.; Yagi, N.; Sanomura, Y.; Tanaka, S.; Naito, Y.; Sakamoto, N.; Kato, M. Accuracies of Endoscopic Diagnosis of Helicobacter pylori-Gastritis: Multicenter Prospective Study Using White Light Imaging and Linked Color Imaging. Digestion 2020, 101, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lin, X.C.; Li, H.L.; Yang, X.S.; Zhang, L.; Li, X.; Bai, P.; Wang, Y.; Fan, X.; Ding, Y.M. Clinical significance and influencing factors of linked color imaging technique in real-time diagnosis of active Helicobacter pylori infection. Chin. Med. J. Engl. 2019, 132, 2395–2401. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, F.; Zhou, Y.; Xia, G.L.; Dong, L.; He, W.H.; Xiao, B. Blue laser magnifying endoscopy in the diagnosis of chronic gastritis. Exp. Ther. Med. 2019, 18, 1993–2000. [Google Scholar] [CrossRef] [Green Version]
- Sato, H.; Inoue, H.; Ikeda, H.; Sato, C.; Phlanusittepha, C.; Hayee, B.; Santi, E.G.; Kobayashi, Y.; Kudo, S.E. In vivo gastric mucosal histopathology using endocytoscopy. World J. Gastroenterol. 2015, 21, 5002–5008. [Google Scholar] [CrossRef]
- Fontenete, S.; Leite, M.; Figueiredo, C.; Cos, P.; Azevedo, N.F. Detection of Helicobacter pylori in the Gastric Mucosa by Fluorescence In Vivo Hybridization. Methods Mol. Biol. 2017, 1616, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, O.; Nishizawa, T.; Koike, K. Endoscopic Kyoto classification of Helicobacter pylori infection and gastric cancer risk diagnosis. World J. Gastroenterol. 2020, 26, 466–477. [Google Scholar] [CrossRef]
- Nakashima, H.; Kawahira, H.; Kawachi, H.; Sakaki, N. Artificial intelligence diagnosis of Helicobacter pylori infection using blue laser imaging-bright and linked color imaging: A single-center prospective study. Ann. Gastroenterol. 2018, 31, 462–468. [Google Scholar] [CrossRef]
- Bang, C.S.; Lee, J.J.; Baik, G.H. Artificial Intelligence for the Prediction of Helicobacter pylori Infection in Endoscopic Images: Systematic Review and Meta-Analysis Of Diagnostic Test Accuracy. J. Med. Internet Res. 2020, 22, e21983. [Google Scholar] [CrossRef]
- Dixon, M.F.; Genta, R.M.; Yardley, J.H.; Correa, P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am. J. Surg. Pathol. 1996, 20, 1161–1181. [Google Scholar] [CrossRef] [PubMed]
- Varbanova, M.; Wex, T.; Jechorek, D.; Rohl, F.W.; Langner, C.; Selgrad, M.; Malfertheiner, P. Impact of the angulus biopsy for the detection of gastric preneoplastic conditions and gastric cancer risk assessment. J. Clin. Pathol. 2016, 69, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.Y.; Opekun, A.R.; Yamaoka, Y.; Osato, M.S.; el-Zimaity, H.M. Early events in proton pump inhibitor-associated exacerbation of corpus gastritis. Aliment. Pharmacol. Ther. 2003, 17, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Glickman, J.N.; Noffsinger, A.; Nevin, D.T.; Ray, M.; Lash, R.H.; Genta, R.M. Helicobacter infections with rare bacteria or minimal gastritis: Expecting the unexpected. Dig. Liver Dis. 2015, 47, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Snead, D.R.; Tsang, Y.W.; Meskiri, A.; Kimani, P.K.; Crossman, R.; Rajpoot, N.M.; Blessing, E.; Chen, K.; Gopalakrishnan, K.; Matthews, P.; et al. Validation of digital pathology imaging for primary histopathological diagnosis. Histopathology 2016, 68, 1063–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benoit, A.; Hoyeau, N.; Flejou, J.F. Diagnosis of Helicobacter pylori infection on gastric biopsies: Standard stain, special stain or immunohistochemistry? Ann. Pathol. 2018, 38, 363–369. [Google Scholar] [CrossRef]
- Godbole, G.; Megraud, F.; Bessede, E. Review: Diagnosis of Helicobacter pylori infection. Helicobacter 2020, 25 (Suppl. 1), e12735. [Google Scholar] [CrossRef]
- Seo, J.H.; Park, J.S.; Rhee, K.H.; Youn, H.S. Limitations of urease test in diagnosis of pediatric Helicobacter pylori infection. World J. Clin. Pediatr. 2015, 4, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Jeon, S.R.; Kim, H.G.; Jin, S.Y.; Park, S. Factors for improving the diagnostic efficiency of the rapid urease test from the gastric corpus. Scand. J. Gastroenterol. 2017, 52, 1320–1325. [Google Scholar] [CrossRef] [PubMed]
- Parihar, V.; Holleran, G.; Hall, B.; Brennan, D.; Crotty, P.; McNamara, D. A combined antral and corpus rapid urease testing protocol can increase diagnostic accuracy despite a low prevalence of Helicobacter pylori infection in patients undergoing routine gastroscopy. United Eur. Gastroenterol. J. 2015, 3, 432–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dechant, F.X.; Dechant, R.; Kandulski, A.; Selgrad, M.; Weber, F.; Reischl, U.; Wilczek, W.; Mueller, M.; Weigand, K. Accuracy of Different Rapid Urease Tests in Comparison with Histopathology in Patients with Endoscopic Signs of Gastritis. Digestion 2020, 101, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Dolak, W.; Bilgilier, C.; Stadlmann, A.; Leiner, J.; Puspok, A.; Plieschnegger, W.; Siebert, F.; Wewalka, F.; Schofl, R.; Huber-Schonauer, U.; et al. A multicenter prospective study on the diagnostic performance of a new liquid rapid urease test for the diagnosis of Helicobacter pylori infection. Gut Pathog. 2017, 9, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhns, L.G.; Benoit, S.L.; Bayyareddy, K.; Johnson, D.; Orlando, R.; Evans, A.L.; Waldrop, G.L.; Maier, R.J. Carbon Fixation Driven by Molecular Hydrogen Results in Chemolithoautotrophically Enhanced Growth of Helicobacter pylori. J. Bacteriol. 2016, 198, 1423–1428. [Google Scholar] [CrossRef] [Green Version]
- Peretz, A.; On, A.; Koifman, A.; Brodsky, D.; Isakovich, N.; Glyatman, T.; Paritsky, M. An efficiency comparison between three invasive methods for the diagnosis of Helicobacter pylori infections: Culture from stomach biopsy, rapid urease test (CUTest((R))), and histologic examination of gastric biopsy. Ann. Clin. Lab. Sci. 2015, 45, 148–151. [Google Scholar]
- Peretz, A.; Paritsky, M.; Pastukh, N.; Koifman, A.; Brodsky, D.; Glyatman, T.; On, A. Improvement and optimization of the classical gastric biopsy culture technique for Helicobacter pylori diagnosis using trypsin. J. Med. Microbiol. 2015, 64, 642–645. [Google Scholar] [CrossRef] [Green Version]
- Pohl, D.; Keller, P.M.; Bordier, V.; Wagner, K. Review of current diagnostic methods and advances in Helicobacter pylori diagnostics in the era of next generation sequencing. World J. Gastroenterol. 2019, 25, 4629–4660. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.M.; Zhang, F.; Shi, D.M.; Xiang, P.; Xiao, L.; Huang, Y.Q.; Zhang, G.S.; Bao, Z.J. Effect of posture on (13)C-urea breath test in partial gastrectomy patients. World J. Gastroenterol. 2015, 21, 12888–12895. [Google Scholar] [CrossRef] [PubMed]
- Klein, P.D.; Malaty, H.M.; Martin, R.F.; Graham, K.S.; Genta, R.M.; Graham, D.Y. Noninvasive detection of Helicobacter pylori infection in clinical practice: The 13C urea breath test. Am. J. Gastroenterol. 1996, 91, 690–694. [Google Scholar] [PubMed]
- Best, L.M.; Takwoingi, Y.; Siddique, S.; Selladurai, A.; Gandhi, A.; Low, B.; Yaghoobi, M.; Gurusamy, K.S. Non-invasive diagnostic tests for Helicobacter pylori infection. Cochrane Database Syst. Rev. 2018, 3, CD012080. [Google Scholar] [CrossRef] [Green Version]
- Abd Rahim, M.A.; Johani, F.H.; Shah, S.A.; Hassan, M.R.; Abdul Manaf, M.R. 13C-Urea Breath Test Accuracy for Helicobacter pylori Infection in the Asian Population: A Meta-Analysis. Ann. Glob. Health 2019, 85. [Google Scholar] [CrossRef]
- Coelho, L.G.; Sant’Ana, C.R.; Oliveira, R.B.; Cezar, R.C.E.; Araujo, A.C.C.; Silva, R.; Trindade, O.R.; Coelho, M.C.; Ferrioli, E.; Bendassolli, J.A. Performance of the 13C-urea breath test for the diagnosis of H. pylori infection using a substrate synthesized in Brazil: A preliminary study. Clin. Sao Paulo 2018, 73, e16553. [Google Scholar] [CrossRef]
- Agha, A.; Opekun, A.R.; Abudayyeh, S.; Graham, D.Y. Effect of different organic acids (citric, malic and ascorbic) on intragastric urease activity. Aliment. Pharmacol. Ther. 2005, 21, 1145–1148. [Google Scholar] [CrossRef]
- Tepes, B.; Seruga, M.; Vujasinovic, M.; Urlep, D.; Ljepovic, L.; Brglez, J.N.; Forte, A.; Anita Kek, L.; Skvarc, M. Premalignant Gastric Lesions in Patients Included in National Colorectal Cancer Screening. Radiol. Oncol. 2018, 52, 7–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisdorfer, I.; Shalev, V.; Goren, S.; Chodick, G.; Muhsen, K. Sex differences in urea breath test results for the diagnosis of Helicobacter pylori infection: A large cross-sectional study. Biol. Sex Differ. 2018, 9, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, Y.H.; Kim, N.; Lee, J.Y.; Choi, Y.J.; Yoon, K.; Hwang, J.J.; Lee, H.J.; Lee, A.; Jeong, Y.S.; Oh, S.; et al. The Diagnostic Validity of Citric Acid-Free, High Dose (13)C-Urea Breath Test After Helicobacter pylori Eradication in Korea. Helicobacter 2015, 20, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Suki, M.; Leibovici Weissman, Y.; Boltin, D.; Itskoviz, D.; Tsadok Perets, T.; Comaneshter, D.; Cohen, A.; Niv, Y.; Dotan, I.; Leibovitzh, H.; et al. Helicobacter pylori infection is positively associated with an increased BMI, irrespective of socioeconomic status and other confounders: A cohort study. Eur. J. Gastroenterol. Hepatol. 2018, 30, 143–148. [Google Scholar] [CrossRef]
- Graham, D.Y.; Miftahussurur, M. Helicobacter pylori urease for diagnosis of Helicobacter pylori infection: A mini review. J. Adv. Res. 2018, 13, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.X.; Huang, L.L.; Liu, C.; Formichella, L.; Zhang, Y.; Wang, Y.M.; Zhang, L.; Ma, J.L.; Liu, W.D.; Ulm, K.; et al. Cut-off optimization for (13)C-urea breath test in a community-based trial by mathematic, histology and serology approach. Sci. Rep. 2017, 7, 2072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perets, T.T.; Gingold-Belfer, R.; Leibovitzh, H.; Itskoviz, D.; Schmilovitz-Weiss, H.; Snir, Y.; Dickman, R.; Dotan, I.; Levi, Z.; Boltin, D. Optimization of (13) C-urea breath test threshold levels for the detection of Helicobacter pylori infection in a national referral laboratory. J. Clin. Lab. Anal. 2019, 33, e22674. [Google Scholar] [CrossRef] [Green Version]
- Unler, G.K.; Ozgur, G.T.; Gokturk, H.S.; Durukan, E.; Erhamamci, S. Does the urea breath test predict eradication of Helicobacter pylori infection? Acta Gastroenterol. Belg. 2016, 79, 3–7. [Google Scholar]
- Molina-Molina, E.; Bonfrate, L.; Lorusso, M.; Shanmugam, H.; Scaccianoce, G.; Rokkas, T.; Portincasa, P. Faster Detection of Helicobacter pylori Infection by 13 C-Urea Breath Test. Comparing Short versus Standard Sampling Time. J. Gastrointestin. Liver Dis. 2019, 28, 151–161. [Google Scholar] [CrossRef]
- Richter, V.; Gonzalez, J.O.; Hazan, S.; Gottlieb, G.; Friedenberg, K.; Gatof, D.; Ganeshappa, R.; Delgado, J.S.; Abramowitz, D.; Hardi, R.; et al. The validity of breath collection bags method in detecting Helicobacter pylori using the novel BreathID ((R)) Hp Lab System: A multicenter clinical study in 257 subjects. Ther. Adv. Gastrointest. Endosc. 2019, 12, 2631774519843401. [Google Scholar] [CrossRef] [Green Version]
- Coelho, L.G.V.; Trindade, O.R.; Leao, L.A.; Ribeiro, H.G.; Freitas, I.S.; Coelho, M.C.F. Prospective Study for Validation of a Single Protocol for the 13c-Urea Breath Test Using Two Different Devices in the Diagnosis of H. Pylori Infection. Arq. Gastroenterol. 2019, 56, 197–201. [Google Scholar] [CrossRef]
- Opekun, A.R.; Abdalla, N.; Sutton, F.M.; Hammoud, F.; Kuo, G.M.; Torres, E.; Steinbauer, J.; Graham, D.Y. Urea breath testing and analysis in the primary care office. J. Fam. Pract. 2002, 51, 1030–1032. [Google Scholar]
- Kwon, Y.H.; Kim, N.; Yoon, H.; Shin, C.M.; Park, Y.S.; Lee, D.H. Effect of Citric Acid on Accuracy of (13)C-Urea Breath Test after Helicobacter pylori Eradication Therapy in a Region with a High Prevalence of Atrophic Gastritis. Gut Liver 2019, 13, 506–514. [Google Scholar] [CrossRef]
- Ramirez-Lazaro, M.J.; Lite, J.; Lario, S.; Perez-Jove, P.; Montserrat, A.; Quilez, M.E.; Martinez-Bauer, E.; Calvet, X. Good diagnostic accuracy of a chemiluminescent immunoassay in stool samples for diagnosis of Helicobacter pylori infection in patients with dyspepsia. J. Investig. Med. 2016, 64, 388–391. [Google Scholar] [CrossRef]
- Dore, M.P.; Osato, M.S.; Malaty, H.M.; Graham, D.Y. Characterization of a culture method to recover Helicobacter pylori from the feces of infected patients. Helicobacter 2000, 5, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef] [Green Version]
- Vaira, D.; Malfertheiner, P.; Megraud, F.; Axon, A.T.; Deltenre, M.; Hirschl, A.M.; Gasbarrini, G.; O’Morain, C.; Garcia, J.M.; Quina, M.; et al. Diagnosis of Helicobacter pylori infection with a new non-invasive antigen-based assay. HpSA European study group. Lancet 1999, 354, 30–33. [Google Scholar] [CrossRef]
- Korkmaz, H.; Findik, D.; Ugurluoglu, C.; Terzi, Y. Reliability of stool antigen tests: Investigation of the diagnostic value of a new immunochromatographic Helicobacter pylori approach in dyspeptic patients. Asian Pac. J. Cancer Prev. 2015, 16, 657–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lario, S.; Ramirez-Lazaro, M.J.; Montserrat, A.; Quilez, M.E.; Junquera, F.; Martinez-Bauer, E.; Sanfeliu, I.; Brullet, E.; Campo, R.; Segura, F.; et al. Diagnostic accuracy of three monoclonal stool tests in a large series of untreated Helicobacter pylori infected patients. Clin. Biochem. 2016, 49, 682–687. [Google Scholar] [CrossRef]
- Moubri, M.; Burucoa, C.; Kalach, N.; Larras, R.R.; Nouar, N.; Mouffok, F.; Arrada, Z. Performances of the IDEIA HpStAR Stool Antigen Test in Detection of Helicobacter pylori Infection Before and After Eradication Treatment in Algerian Children. J. Trop Pediatr. 2019, 65, 210–216. [Google Scholar] [CrossRef]
- Kakiuchi, T.; Okuda, M.; Hashiguchi, K.; Imamura, I.; Nakayama, A.; Matsuo, M. Evaluation of a Novel Stool Antigen Rapid Test Kit for Detection of Helicobacter pylori Infection. J. Clin. Microbiol. 2019, 57. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.J.; Chen, M.J.; Chen, C.C.; Lee, J.Y.; Yang, T.H.; Yu, C.C.; Chiu, M.C.; Kuo, C.C.; Weng, Y.J.; Bair, M.J.; et al. Accuracy of rapid Helicobacter pylori antigen tests for the surveillance of the updated prevalence of H. pylori in Taiwan. J. Formos Med. Assoc. 2020, 119, 1626–1633. [Google Scholar] [CrossRef]
- Dore, M.P.; Negrini, R.; Tadeu, V.; Marras, L.; Maragkoudakis, E.; Nieddu, S.; Simula, L.; Cherchi, G.B.; Massarelli, G.; Realdi, G. Novel monoclonal antibody-based Helicobacter pylori stool antigen test. Helicobacter 2004, 9, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; de la Morena, F.; Abraira, V. Accuracy of monoclonal stool antigen test for the diagnosis of H. pylori infection: A systematic review and meta-analysis. Am. J. Gastroenterol. 2006, 101, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Guarner, J.; Kalach, N.; Elitsur, Y.; Koletzko, S. Helicobacter pylori diagnostic tests in children: Review of the literature from 1999 to 2009. Eur. J. Pediatr. 2010, 169, 15–25. [Google Scholar] [CrossRef]
- Bénéjat, L.; Ducournau, A.; Lehours, P.; Megraud, F. Real-time PCR for Helicobacter pylori diagnosis. The best tools available. Helicobacter 2018, 23, e12512. [Google Scholar] [CrossRef]
- Redondo, J.J.; Keller, P.M.; Zbinden, R.; Wagner, K. A novel RT-PCR for the detection of Helicobacter pylori and identification of clarithromycin resistance mediated by mutations in the 23S rRNA gene. Diagn Microbiol. Infect Dis. 2018, 90, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talarico, S.; Safaeian, M.; Gonzalez, P.; Hildesheim, A.; Herrero, R.; Porras, C.; Cortes, B.; Larson, A.; Fang, F.C.; Salama, N.R. Quantitative Detection and Genotyping of Helicobacter pylori from Stool using Droplet Digital PCR Reveals Variation in Bacterial Loads that Correlates with cagA Virulence Gene Carriage. Helicobacter 2016, 21, 325–333. [Google Scholar] [CrossRef] [Green Version]
- Bazin, T.; Nchare Mfondi, A.; Julie, C.; Emile, J.F.; Raymond, J.; Lamarque, D. Contribution of genetic amplification by PCR for the diagnosis of Helicobacter pylori infection in patients receiving proton pump inhibitors. United Eur. Gastroenterol. J. 2018, 6, 1267–1273. [Google Scholar] [CrossRef] [Green Version]
- Morilla, A.; Melon, S.; Alvarez-Arguelles, M.E.; Armesto, E.; Villar, H.; de Ona, M. Utility of normalized genome quantification of Helicobacter pylori in gastric mucosa using an in-house real-time polymerase chain reaction. PLoS ONE 2017, 12, e0178674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalach, N.; Gosset, P.; Dehecq, E.; Decoster, A.; Spyckerelle, C.; Papadopolos, S.; Dupont, C.; Raymond, J. Usefulness of Gastric Biopsy-Based Real-Time Polymerase Chain Reaction for the Diagnosis of Helicobacter pylori Infection in Children. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 307–312. [Google Scholar] [CrossRef]
- Matsumoto, H.; Shiotani, A.; Nishibayashi, H.; Kamada, T.; Kimura, T.; Fujimura, Y.; Nakato, R.; Murao, T.; Fujita, M.; Haruma, K. Molecular Detection of H. pylori Using Adherent Gastric Mucous to Biopsy Forceps. Helicobacter 2016, 21, 548–553. [Google Scholar] [CrossRef]
- Chung, W.C.; Jeon, E.J.; Oh, J.H.; Park, J.M.; Kim, T.H.; Cheung, D.Y.; Kim, B.W.; Kim, S.S.; Kim, J.I. Dual-priming oligonucleotide-based multiplex PCR using tissue samples from the rapid urease test kit for the detection of Helicobacter pylori in bleeding peptic ulcers. Dig. Liver Dis. 2016, 48, 899–903. [Google Scholar] [CrossRef]
- Hsieh, M.S.; Liu, C.J.; Hsu, W.H.; Li, C.J.; Tsai, P.Y.; Hu, H.M.; Shih, H.Y.; Lu, C.Y.; Yu, F.J.; Kuo, F.C.; et al. Gastric juice-based PCR assay: An alternative testing method to aid in the management of previously treated Helicobacter pylori infection. Helicobacter 2019, 24, e12568. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Song, Z.; He, L.; Lin, S.; Gong, Y.; Sun, L.; Zhao, F.; Gu, Y.; You, Y.; Zhou, L.; et al. Gastric Juice-Based Real-Time PCR for Tailored Helicobacter pylori Treatment: A Practical Approach. Int. J. Med. Sci. 2017, 14, 595–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piroozmand, A.; Soltani, B.; Razavizadeh, M.; Matini, A.H.; Moosavi, G.A.; Salehi, M.; Soltani, S. Comparison of gastric juice soluble triggering receptor expressed on myeloid cells and C-reactive protein for detection of Helicobacter pylori infection. Electron. Physician 2017, 9, 6111–6119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talarico, S.; Korson, A.S.; Leverich, C.K.; Park, S.; Jalikis, F.G.; Upton, M.P.; Broussard, E.; Salama, N.R. High prevalence of Helicobacter pylori clarithromycin resistance mutations among Seattle patients measured by droplet digital PCR. Helicobacter 2018, 23, e12472. [Google Scholar] [CrossRef] [PubMed]
- Nezami, B.G.; Jani, M.; Alouani, D.; Rhoads, D.D.; Sadri, N. Helicobacter pylori Mutations Detected by Next-Generation Sequencing in Formalin-Fixed, Paraffin-Embedded Gastric Biopsy Specimens Are Associated with Treatment Failure. J. Clin. Microbiol. 2019, 57. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Zhao, F.; Hu, B.; Fang, Y.; Miao, Y.; Huang, Y.; Ji, D.; Zhang, J.; Xu, L.; Zhang, Y.; et al. A Creative Helicobacter pylori Diagnosis Scheme Based on Multiple Genetic Analysis System: Qualification and Quantitation. Helicobacter 2015, 20, 343–352. [Google Scholar] [CrossRef]
- Trespalacios, A.A.; Rimbara, E.; Otero, W.; Reddy, R.; Graham, D.Y. Improved allele-specific PCR assays for detection of clarithromycin and fluoroquinolone resistant of Helicobacter pylori in gastric biopsies: Identification of N87I mutation in GyrA. Diagn. Microbiol. Infect. Dis. 2015, 81, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Yari, F.; Abiri, R.; Aryan, E.; Ahmadi Jouybari, T.; Navabi, J.; Alvandi, A. Loop-Mediated Isothermal Amplification as a Fast Noninvasive Method of Helicobacter pylori Diagnosis. J. Clin. Lab. Anal. 2016, 30, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Beckman, E.; Saracino, I.; Fiorini, G.; Clark, C.; Slepnev, V.; Patel, D.; Gomez, C.; Ponaka, R.; Elagin, V.; Vaira, D. A Novel Stool PCR Test for Helicobacter pylori May Predict Clarithromycin Resistance and Eradication of Infection at a High Rate. J. Clin. Microbiol. 2017, 55, 2400–2405. [Google Scholar] [CrossRef] [Green Version]
- Iannone, A.; Giorgio, F.; Russo, F.; Riezzo, G.; Girardi, B.; Pricci, M.; Palmer, S.C.; Barone, M.; Principi, M.; Strippoli, G.F.; et al. New fecal test for non-invasive Helicobacter pylori detection: A diagnostic accuracy study. World J. Gastroenterol. 2018, 24, 3021–3029. [Google Scholar] [CrossRef]
- Clines, N.; Beckman, E. Development of a high throughput human stool specimen processing method for a molecular Helicobacter pylori clarithromycin resistance assay. PLoS ONE 2019, 14, e0224356. [Google Scholar] [CrossRef] [Green Version]
- Pichon, M.; Pichard, B.; Barrioz, T.; Plouzeau, C.; Croquet, V.; Fotsing, G.; Cheron, A.; Vuillemin, E.; Wangermez, M.; Haineaux, P.A.; et al. Diagnostic Accuracy of a Noninvasive Test for Detection of Helicobacter pylori and Resistance to Clarithromycin in Stool by the Amplidiag H. pylori+ClariR Real-Time PCR Assay. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef] [PubMed]
- Butt, J.; Blot, W.J.; Shrubsole, M.J.; Varga, M.G.; Hendrix, L.H.; Crankshaw, S.; Waterboer, T.; Pawlita, M.; Epplein, M. Performance of multiplex serology in discriminating active vs past Helicobacter pylori infection in a primarily African American population in the southeastern United States. Helicobacter 2020, 25, e12671. [Google Scholar] [CrossRef] [PubMed]
- Shafaie, E.; Saberi, S.; Esmaeili, M.; Karimi, Z.; Najafi, S.; Tashakoripoor, M.; Abdirad, A.; Hosseini, M.E.; Mohagheghi, M.A.; Khalaj, V.; et al. Multiplex serology of Helicobacter pylori antigens in detection of current infection and atrophic gastritis—A simple and cost-efficient method. Microb. Pathog. 2018, 119, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Kawai, S.; Arai, K.; Lin, Y.; Nishiyama, T.; Sasakabe, T.; Wang, C.; Miwa, H.; Kikuchi, S. Comparison of the detection of Helicobacter pylori infection by commercially available serological testing kits and the (13)C-urea breath test. J. Infect. Chemother. 2019, 25, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Miftahussurur, M.; Yamaoka, Y. Diagnostic Methods of Helicobacter pylori Infection for Epidemiological Studies: Critical Importance of Indirect Test Validation. Biomed. Res. Int. 2016, 2016, 4819423. [Google Scholar] [CrossRef] [Green Version]
- Miwa, H.; Kikuchi, S.; Ohtaka, K.; Kobayashi, O.; Ogihara, A.; Hojo, M.; Nagahara, A.; Sato, N. Insufficient diagnostic accuracy of imported serological kits for Helicobacter pylori infection in Japanese population. Diagn. Microbiol. Infect. Dis. 2000, 36, 95–99. [Google Scholar] [CrossRef]
- Theel, E.S.; Johnson, R.D.; Plumhoff, E.; Hanson, C.A. Use of the Optum Labs Data Warehouse to assess test ordering patterns for diagnosis of Helicobacter pylori infection in the United States. J. Clin. Microbiol. 2015, 53, 1358–1360. [Google Scholar] [CrossRef] [Green Version]
- Toyoshima, O.; Nishizawa, T.; Sakitani, K.; Yamakawa, T.; Takahashi, Y.; Yamamichi, N.; Hata, K.; Seto, Y.; Koike, K.; Watanabe, H.; et al. Serum anti-Helicobacter pylori antibody titer and its association with gastric nodularity, atrophy, and age: A cross-sectional study. World J. Gastroenterol. 2018, 24, 4061–4068. [Google Scholar] [CrossRef]
- Epplein, M.; Butt, J.; Zhang, Y.; Hendrix, L.H.; Abnet, C.C.; Murphy, G.; Zheng, W.; Shu, X.O.; Tsugane, S.; Qiao, Y.L.; et al. Validation of a Blood Biomarker for Identification of Individuals at High Risk for Gastric Cancer. Cancer Epidemiol. Biomark. Prev. 2018, 27, 1472–1479. [Google Scholar] [CrossRef] [Green Version]
- MÄki, M.; SÖderstrÖm, D.; Paloheimo, L.; Hendolin, P.; Suovaniemi, O.; Syrjänen, K. Helicobacter pylori (Hp) IgG ELISA of the New-Generation GastroPanel(R) Is Highly Accurate in Diagnosis of Hp-Infection in Gastroscopy Referral Patients. Anticancer Res. 2020, 40, 6387–6398. [Google Scholar] [CrossRef]
- Lee, S.P.; Lee, S.Y.; Kim, J.H.; Sung, I.K.; Park, H.S.; Shim, C.S. Link between Serum Pepsinogen Concentrations and Upper Gastrointestinal Endoscopic Findings. J. Korean Med. Sci. 2017, 32, 796–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paloheimo, L.; Tiusanen, T.; Suovaniemi, O.; SyrjAnen, K. Serological Biomarker Test (GastroPanel((R))) in the Diagnosis of Functional Gastric Disorders, Helicobacter pylori and Atrophic Gastritis in Patients Examined for Dyspeptic Symptoms. Anticancer Res. 2021, 41, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Syrjänen, K.; Eskelinen, M.; Peetsalu, A.; Sillakivi, T.; Sipponen, P.; Harkonen, M.; Paloheimo, L.; Maki, M.; Tiusanen, T.; Suovaniemi, O.; et al. GastroPanel(R) Biomarker Assay: The Most Comprehensive Test for Helicobacter pylori Infection and Its Clinical Sequelae. A Critical Review. Anticancer Res. 2019, 39, 1091–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjomina, O.; Pavlova, J.; Daugule, I.; Janovic, P.; Kikuste, I.; Vanags, A.; Tolmanis, I.; Rudzite, D.; Polaka, I.; Kojalo, I.; et al. Pepsinogen test for the evaluation of precancerous changes in gastric mucosa: A population-based study. J. Gastrointestin. Liver Dis. 2018, 27, 11–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, J.; Lei, H.; Shi, W.; Sun, X.; Tang, Y.; Ren, C. High Serum Pepsinogen I and beta Helicobacter pylori Infection Are Risk Factors for Aspirin-Induced Gastroduodenal Injury. Dig. Dis. 2018, 36, 66–71. [Google Scholar] [CrossRef]
- Chinda, D.; Shimoyama, T.; Mikami, T.; Arai, T.; Chiba, D.; Sasaki, Y.; Komai, K.; Sawada, Y.; Saito, Y.; Chiba, H.; et al. Serum pepsinogen levels indicate the requirement of upper gastrointestinal endoscopy among Group A subjects of ABC classification: A multicenter study. J. Gastroenterol. 2018, 53, 924–931. [Google Scholar] [CrossRef]
- Yu, J.; Xu, Q.; Zhang, X.; Zhu, M. Circulating microRNA signatures serve as potential diagnostic biomarkers for Helicobacter pylori infection. J. Cell Biochem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Skrebinska, S.; Daugule, I.; Santare, D.; Isajevs, S.; Liepniece-Karele, I.; Rudzite, D.; Kikuste, I.; Vanags, A.; Tolmanis, I.; Atstupens, J.; et al. Accuracy of two plasma antibody tests and faecal antigen test for non-invasive detection of H. pylori in middle-aged Caucasian general population sample. Scand. J. Gastroenterol. 2018, 53, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Mhatre, S.; Dikshit, R. Utility of dried blood spots in detecting Helicobacter pylori infection. Indian J. Med. Microbiol. 2019, 37, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Okuda, M.; Mabe, K.; Lin, Y.; Chaochen, W.; Taniguchi, Y.; Kato, M.; Kikuchi, S. Rapid urine antibody test for Helicobacter pylori infection in adolescents. Pediatr. Int. 2017, 59, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Piroozmand, A.; Soltani, B.; Razavizadeh, M.; Matini, A.H.; Gilasi, H.R.; Zavareh, A.N.; Soltani, S. Comparison of the serum and salivary antibodies to detect gastric Helicobacter pylori infection in Kashan (Iran). Electron. Physician 2017, 9, 6129–6134. [Google Scholar] [CrossRef] [Green Version]
- Aumpan, N.; Vilaichone, R.K.; Chotivitayatarakorn, P.; Pornthisarn, B.; Cholprasertsuk, S.; Bhanthumkomol, P.; Kanokwanvimol, A.; Siramolpiwat, S.; Mahachai, V. High Efficacy of Rapid Urine Test for Diagnosis of Helicobacter pylori Infection in Thai People. Asian Pac. J. Cancer Prev. 2019, 20, 1525–1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aksit Bicak, D.; Akyuz, S.; Kiratli, B.; Usta, M.; Urganci, N.; Alev, B.; Yarat, A.; Sahin, F. The investigation of Helicobacter pylori in the dental biofilm and saliva samples of children with dyspeptic complaints. BMC Oral Health 2017, 17, 67. [Google Scholar] [CrossRef] [Green Version]
- Dore, M.P.; Cipolli, A.; Ruggiu, M.W.; Manca, A.; Bassotti, G.; Pes, G.M. Helicobacter pylori eradication may influence timing of endoscopic surveillance for gastric cancer in patients with gastric precancerous lesions: A retrospective study. Medicine (Baltimore) 2018, 97, e9734. [Google Scholar] [CrossRef]
| Brand | Based on | Sensitivity | Specificity | Reference |
|---|---|---|---|---|
| LIAISON H. pylori SA assay (DiaSorin, Saluggia, Italy) | chemiluminescent immunoassay | 90.1 95.5 | 92.4 97.6 | Ramirez-Lazaro et al., 2016 [70] |
| Genx H. pylori card test (Genx Bioresearch, Kocaeli, Turkey) | monoclonal immunochromatographic assay | 51.6 | 96.0 | Korkmaz et al., 2015 [74] |
| Uni-Gold™ H. pylori Antigen (Trinity Biotech, Bray, Ireland) | monoclonal lateral flow immunochromatographic assays | 83.2 | 87–89.3 | Lario et al., 2016 [75] |
| RAPID Hp StAR (Oxoid Ltd., Hampshire, UK) | monoclonal lateral flow immunochromatographic assays | 94–95 | 77.1–84.7 | Lario et al., 2016 [75] |
| ImmunoCard STAT! HpSA (Meridian Diagnostics, Cincinnati, OH, USA) | monoclonal lateral flow immunochromatographic assays | 79–81.5 | 90.8–91.6 | Lario et al., 2016 [75] |
| IDEIA HpStAR®; (ThermoFisher Sc., Waltham, MA, USA) | monoclonal antibodies and the ELISA technique | Before Hp treatment 93.6 After Hp treatment 100 | Before Hp treatment 100 After Hp treatment 92.8 | Moubri et al., 2018 [76] |
| Quick Chaser H. pylori®, QCP, Misuho Medy, Tosu, Japan) | immunochromatography | 92.3 | Kakiuchi et al., 2019 [77] | |
| Vstrip®HpSA (Meridian) | immunochromatography | 91% | 97% | Fang et al., 2020 [78] |
| ImmunoCard STAT!® Campy (Meridian) | immunochromatography | 76.9% | 97% | Fang et al., 2020 [78] |
| Molecular Test | H. pylori DNA Target | Reference |
|---|---|---|
| multiple genetic analysis system (MGAS) | 16S rDNA and ureC | Zhou et al., 2015 [95] |
| allele-specific PCR | N87I mutation in the gyrA | Trespalacios et al., 2015 [96] |
| droplet-digital PCR (ddPCR) | cagA and its EPIYA phosphorylation motifs | Talarico et al., 2016 [84] |
| loop-mediated isothermal amplification (LAMP) | ureC gene | Yari et al., 2016 [97] |
| TaqMan RT-PCR | A2142C, A2142G and A2143G mutations | Beckman et al., 2017 [98] |
| droplet-digital PCR (ddPCR) | 16S rDNA | Talarico et al., 2018 [93] |
| real-time PCR (THD fecal test®) | 23S ribosomal RNA | Iannone et al., 2018 [99] |
| MagNA Pure 96 (Roche) | DNA | Clines et al., 2019 [100] |
| Amplidiag® H. pylori + ClariR | H. pylori and CLA resistance mutations | Pichon et al., 2020 [101] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dore, M.P.; Pes, G.M. What Is New in Helicobacter pylori Diagnosis. An Overview. J. Clin. Med. 2021, 10, 2091. https://doi.org/10.3390/jcm10102091
Dore MP, Pes GM. What Is New in Helicobacter pylori Diagnosis. An Overview. Journal of Clinical Medicine. 2021; 10(10):2091. https://doi.org/10.3390/jcm10102091
Chicago/Turabian StyleDore, Maria Pina, and Giovanni Mario Pes. 2021. "What Is New in Helicobacter pylori Diagnosis. An Overview" Journal of Clinical Medicine 10, no. 10: 2091. https://doi.org/10.3390/jcm10102091
APA StyleDore, M. P., & Pes, G. M. (2021). What Is New in Helicobacter pylori Diagnosis. An Overview. Journal of Clinical Medicine, 10(10), 2091. https://doi.org/10.3390/jcm10102091







