Real-World Experience with Bezlotoxumab for Prevention of Recurrence of Clostridioides difficile Infection
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Olsen, M.A.; Yan, Y.; Reske, K.A.; Zilberberg, M.D.; Dubberke, E.R. Recurrent Clostridium difficile infection is associated with increased mortality. Clin. Microbiol. Infect. 2015, 21, 164–170. [Google Scholar] [CrossRef]
- Eyre, D.W.; Walker, A.S.; Wyllie, D.; Dingle, K.E.; Griffiths, D.; Finney, J.; O’Connor, L.; Vaughan, A.; Crook, D.W.; Wilcox, M.H.; et al. Predictors of First Recurrence of Clostridium difficile Infection: Implications for Initial Management. Clin. Infect. Dis. 2012, 55, S77–S87. [Google Scholar] [CrossRef]
- Sheitoyan-Pesant, C.; Abou Chakra, C.N.; Pepin, J.; Marcil-Héguy, A.; Nault, V.; Valiquette, L. Clinical and Healthcare Burden of Multiple Recurrences of Clostridium difficile Infection. Clin. Infect. Dis. 2016, 62, 574–580. [Google Scholar] [CrossRef]
- Van Dorp, S.M.; Kinross, P.; Gastmeier, P.; Behnke, M.; Kola, A.; Delmée, M.; Pavelkovich, A.; Mentula, S.; Barbut, F.; Hajdu, A.; et al. Standardised surveillance of Clostridium difficile infection in European acute care hospitals: A pilot study, 2013. Eurosurveill. Eur. Cent. Dis. Prev. Control. 2016, 21, 20381. [Google Scholar]
- Louie, T.J.; Miller, M.A.; Mullane, K.M.; Weiss, K.; Lentnek, A.; Golan, Y.; Gorbach, S.; Sears, P.; Shue, Y.-K. Fidaxomicin versus vancomycin for Clostridium difficile infection. N. Engl. J. Med. 2011, 364, 422–431. [Google Scholar] [CrossRef]
- Cornely, O.A.; Crook, D.W.; Esposito, R.; Poirier, A.; Somero, M.S.; Weiss, K.; Sears, P.; Gorbach, S. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: A double-blind, non-inferiority, randomised controlled trial. Lancet Infect. Dis. 2012, 12, 281–289. [Google Scholar] [CrossRef]
- Wilcox, M.H.; Gerding, D.N.; Poxton, I.R.; Kelly, C.; Nathan, R.; Birch, T.; Cornely, O.A.; Rahav, G.; Bouza, E.; Lee, C.; et al. Bezlotoxumab for Prevention of Recurrent Clostridium difficile Infection. N. Engl. J. Med. 2017, 376, 305–317. [Google Scholar] [CrossRef]
- Oksi, J.; Aalto, A.; Saila, P.; Partanen, T.; Anttila, V.-J.; Mattila, E. Real-world efficacy of bezlotoxumab for prevention of recurrent Clostridium difficile infection: A retrospective study of 46 patients in five university hospitals in Finland. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1947–1952. [Google Scholar] [CrossRef]
- Hengel, R.L.; Ritter, T.E.; Nathan, R.V.; Van Anglen, L.J.; Schroeder, C.P.; Dillon, R.J.; Marcella, S.W.; Garey, K.W. Real-world Experience of Bezlotoxumab for Prevention of Clostridioides difficile Infection: A Retrospective Multicenter Cohort Study. Open Forum Infect. Dis. 2020, 7, ofaa097. [Google Scholar] [CrossRef]
- Alonso, C.D.; Mahoney, M.V. Bezlotoxumab for the prevention of Clostridium difficile infection: A review of current evidence and safety profile. IDR 2019, 12, 1–9. [Google Scholar] [CrossRef]
- Sherman, R.E.; Anderson, S.A.; Dal Pan, G.J.; Gray, G.W.; Gross, T.; Hunter, N.L.; LaVange, L.; Marinac-Dabic, D.; Marks, P.W.; Robb, M.A.; et al. Real-World Evidence—What Is It and What Can It Tell Us? N. Engl. J. Med. 2016, 375, 2293–2297. [Google Scholar] [CrossRef]
- McDonald, L.C.; Gerding, D.N.; Johnson, S.; Bakken, J.S.; Carroll, K.C.; Coffin, S.E.; Dubberke, E.R.; Garey, K.W.; Gould, C.V.; Kelly, C.; et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 2018, 66, e1–e48. [Google Scholar] [CrossRef] [PubMed]
- Zar, F.A.; Bakkanagari, S.R.; Moorthi, K.M.L.S.T.; Davis, M.B. A Comparison of Vancomycin and Metronidazole for the Treatment of Clostridium difficile Associated Diarrhea, Stratified by Disease Severity. Clin. Infect. Dis. 2007, 45, 302–307. [Google Scholar] [CrossRef]
- Debast, S.B.; Bauer, M.P.; Kuijper, E.J. European Society of Clinical Microbiology and Infectious Diseases: Update of the Treatment Guidance Document for Clostridium difficile Infection. Clin. Microbiol. Infect. 2014, 20, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Phatharacharukul, P.; Thongprayoon, C.; Cheungpasitporn, W.; Edmonds, P.J.; Mahaparn, P.; Bruminhent, J. The Risks of Incident and Recurrent Clostridium difficile Associated Diarrhea in Chronic Kidney Disease and End-Stage Kidney Disease Patients: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2015, 60, 2913–2922. [Google Scholar] [CrossRef]
- Deshpande, A.; Pasupuleti, V.; Thota, P.; Pant, C.; Rolston, D.D.K.; Hernandez, A.V.; Donskey, C.J.; Fraser, T.G. Risk Factors for Recurrent Clostridium difficile Infection: A Systematic Review and Meta-Analysis. Infect. Control. Hosp. Epidemiol. 2015, 36, 452–460. [Google Scholar] [CrossRef]
- Origüen, J.; Corbella, L.; Orellana, M.Á.; Fernández-Ruiz, M.; Lopez-Medrano, F.; San Juan, R.; Lizasoain, M.; Ruiz-Merlo, T.; Morales-Cartagena, A.; Maestro1et, G.; et al. Comparison of the clinical course of Clostridium difficile infection in glutamate dehydrogenase-positive toxin-negative patients diagnosed by PCR to those with a positive toxin test. Clin. Microbiol. Infect. 2018, 24, 414–421. [Google Scholar] [CrossRef]
- Guh, A.Y.; Hatfield, K.M.; Winston, L.G.; Martin, B.; Johnston, H.; Brousseau, G.; Farley, M.M.; Wilson, L.; Perlmutter, R.; Phipps, E.C.; et al. Toxin Enzyme Immunoassays Detect Clostridioides difficile Infection with Greater Severity and Higher Recurrence Rates. Clin. Infect. Dis. 2019, 69, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Surawicz, C.M.; Brandt, L.J.; Binion, D.G.; Ananthakrishnan, A.N.; Curry, S.R.; Gilligan, P.H.; McFarland, L.V.; Mellow, M.; Zuckerbraun, B.S. Guidelines for Diagnosis, Treatment, and Prevention of Clostridium difficile Infections. Am. J. Gastroenterol. 2013, 108, 478–498. [Google Scholar] [CrossRef] [PubMed]

| Anti-C. difficile Treatment | Patients | Duration of Treatment | Follow-up after the End of Anti-C. difficile Treatment | Bezlotoxumab Infusion Time from the Start of Treatment |
|---|---|---|---|---|
| Vancomycin + metronidazole | 5 | 10 (10–10) | 76 (75–79) | 2 (1–5) |
| Vancomycin | 40 | 11 (10–14) | 82 (77.5–86) | 6.5 (3–10) |
| Vancomycin (tapered) | 32 | 42 (35.5–55.5) | 62 (45–73) | 14 (3.5–29.5) |
| Fidaxomicin | 9 | 11 (10–13) | 79 (70.5–82) | 5 (2–8) |
| Fidaxomicin (extend regimen) | 4 | 24.5 (23–26.2) | 79 (71–88) | 12.5 (1.5–22) |
| FMT (after vancomycin) | 1 | 9 | 79 | 12 |
| Cohort | Recurrence | No Recurrence | p | 95% CI | |
|---|---|---|---|---|---|
| Number of patients | 91 | 13 | 78 | ||
| Men | 46 (50.5) | 5 (38.5) | 41 (52.6) | 0.35 | 0.53–5.9 |
| Age (years) * | 71 (59–82) | 68 (57–80) | 72 (60–82) | 0.96 | 0.96–1.04 |
| Age > 65 | 61 (66.3) | 8 (61.5) | 53 (68.0) | 0.65 | 0.22–2.54 |
| Age > 85 | 17 (18.7) | 3 (23.1) | 14 (18.0) | 0.66 | 0.33–5.64 |
| Charlson index * | 4 (2–6) | 3 (2–5) | 4 (2–6) | 0.22 | 0.64–1.11 |
| Kidney failure | 32 (35.2) | 4 (30.8) | 28 (35.9) | 0.72 | 0.22–2.81 |
| Cancer | 20 (22.0) | 3 (23.1) | 17 (21.8) | 0.92 | 0.27–4.36 |
| Leukaemia/Lymphoma | 17 (18.7) | 1 (7.7) | 16 (20.5) | 0.29 | 0.04–2.67 |
| Any neoplasm | 33 (36.3) | 3 (23.1) | 30 (38.5) | 0.29 | 0.12–1.89 |
| Liver disease | 9 (9.9) | 2 (15.4) | 7 (9.0) | 0.71 | 0.34–10.04 |
| Intestinal inflammatory disease | 6 (6.6) | 1 (7.7) | 5 (6.4) | 0.86 | 0.13–11.34 |
| Immunosuppression: | 56 (61.5) | 7 (53.9) | 48 (62.8) | 0.54 | 0.21–2.25 |
| Chemotherapy | 13 (14.3) | 2 (15.4) | 11 (14.1) | 0.90 | 0.22–5.68 |
| Steroids | 14 (15.4) | 1 (7.7) | 13 (16.7) | 0.42 | 0.05–3.49 |
| Immunosuppressive drugs (not steroids) | 16 (17.6) | 1 (7.7) | 15 (19.2) | 0.33 | 0.04–2.91 |
| Solid organ transplant | 20 (22.0) | 3 (23.1) | 17 (21.8) | 0.92 | 0.27–4.36 |
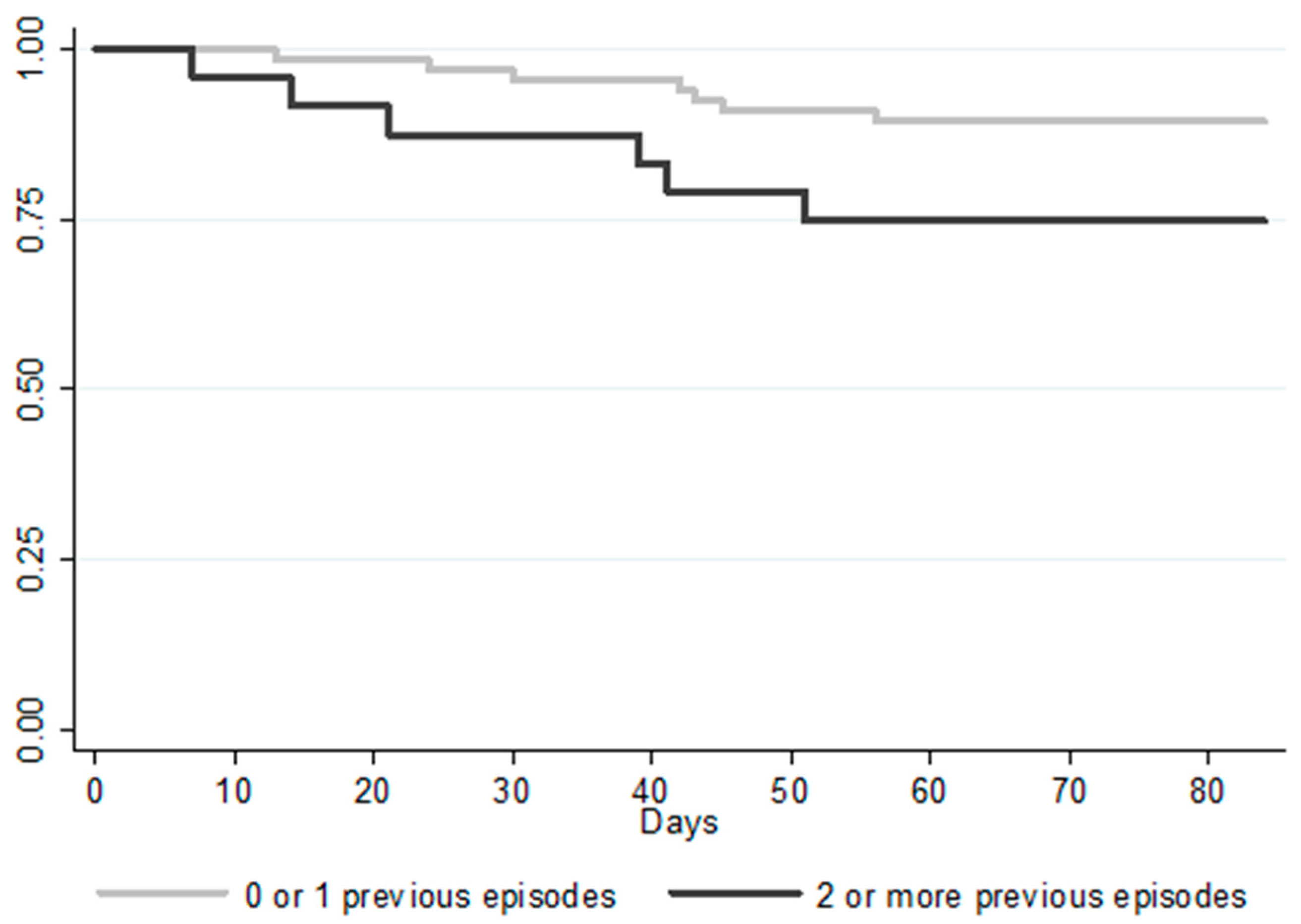
| Previous CDI episodes: | |||||
| 0 | 39 (42.9) | 5 (38.5) | 35 (44.9) | 0.73 | 0.24–2.70 |
| 1 | 28 (30.8) | 2 (15.4) | 26 (33.3) | 0.21 | 0.08–1.76 |
| ≥2 | 24 (26.4) | 6 (46.2) | 18 (23.1) | 0.09 | 0.85–9.59 |
| Proton pump inhibitor use | 59 (64.8) | 8 (61.5) | 51 (65.4) | 0.79 | 0.25–2.84 |
| Previous antibiotic treatment | 79 (86.8) | 10 (76.9) | 69 (88.5) | 0.27 | 0.10–1.88 |
| Classification of CDI episodes: | |||||
| CA | 11 (12.1) | 1 (7.7) | 10 (12.8) | 0.60 | 0.07–4.84 |
| CO-HCFA | 35 (38.5) | 3 (23.1) | 32 (41.0) | 0.23 | 0.11–1.69 |
| HO-HCFA | 39 (42.9) | 7 (53.9) | 32 (41.0) | 0.39 | 0.52–5.46 |
| Indeterminate | 6 (6.6) | 2 (15.4) | 4 (5.1) | 0.19 | 0.55–20.59 |
| Toxin positive | 66 (72.5) | 8 (61.5) | 58 (74.4) | 0.34 | 0.16–1.88 |
| NAAT positive/toxin negative | 25 (27.5) | 5 (38.5)) | 20 (25.6) | 0.34 | 0.16–1.88 |
| IDSA severe or fulminant colitis | 35 (38.5) | 5 (38.5) | 30 (38.5) | 1.00 | 0.30–3.34 |
| Severe (Zar) | 41 (45.1) | 7 (53.9) | 34 (43.6) | 0.49 | 0.46–4.91 |
| Admitted to ICU | 11 (12.1) | 1 (7.7) | 10 (12.8) | 0.60 | 0.07–4.84 |
| 027 ribotype (based on 48 patients) | 10 (20.8) | 4 (44.4) | 6 (15.4) | 0.07 | 0.91–21.29 |
| Concomitant antibiotics | 25 (27.5) | 1 (7.7) | 24 (30.8) | 0.12 | 0.02–1.52 |
| Anti-C. difficile treatment: | |||||
| Vancomycin | 40 (44.0) | 5 (38.5) | 35 (44.9) | 0.67 | 0.23–2.56 |
| Fidaxomicin | 9 (9.9) | 2 (15.4) | 7 (9.0) | 0.48 | 0.34–10.04 |
| Vancomycin/metronidazole | 5 (5.5) | 1 (7.7) | 4 (5.1) | 0.71 | 0.16–14.99 |
| Vancomycin (tapered) | 32 (35.2) | 4 (30.8) | 28 (35.9) | 0.72 | 0.22–2.81 |
| Fidaxomicin extended–pulsed | 4 (4.4) | 0 | 4 (5.1) | 0.40 | - |
| Faecal microbiota transplant | 1 (6.0) | 1 (7.7) | 0 | 0.01 | - |
| Extended/pulsed–tapered treatments | 36 (39.6) | 4 (30.8) | 32 (41.0) | 0.49 | 0.18–2.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escudero-Sánchez, R.; Ruiz-Ruigómez, M.; Fernández-Fradejas, J.; García Fernández, S.; Olmedo Samperio, M.; Cano Yuste, A.; Valencia Alijo, A.; Díaz-Pollán, B.; Rodríguez Hernández, M.J.; Merino De Lucas, E.; et al. Real-World Experience with Bezlotoxumab for Prevention of Recurrence of Clostridioides difficile Infection. J. Clin. Med. 2021, 10, 2. https://doi.org/10.3390/jcm10010002
Escudero-Sánchez R, Ruiz-Ruigómez M, Fernández-Fradejas J, García Fernández S, Olmedo Samperio M, Cano Yuste A, Valencia Alijo A, Díaz-Pollán B, Rodríguez Hernández MJ, Merino De Lucas E, et al. Real-World Experience with Bezlotoxumab for Prevention of Recurrence of Clostridioides difficile Infection. Journal of Clinical Medicine. 2021; 10(1):2. https://doi.org/10.3390/jcm10010002
Chicago/Turabian StyleEscudero-Sánchez, Rosa, María Ruiz-Ruigómez, Jorge Fernández-Fradejas, Sergio García Fernández, María Olmedo Samperio, Angela Cano Yuste, Angela Valencia Alijo, Beatriz Díaz-Pollán, María Jesús Rodríguez Hernández, Esperanza Merino De Lucas, and et al. 2021. "Real-World Experience with Bezlotoxumab for Prevention of Recurrence of Clostridioides difficile Infection" Journal of Clinical Medicine 10, no. 1: 2. https://doi.org/10.3390/jcm10010002
APA StyleEscudero-Sánchez, R., Ruiz-Ruigómez, M., Fernández-Fradejas, J., García Fernández, S., Olmedo Samperio, M., Cano Yuste, A., Valencia Alijo, A., Díaz-Pollán, B., Rodríguez Hernández, M. J., Merino De Lucas, E., Martín Segarra, O., Sáez Bejar, C., Armiñanzas Castillo, C., Gutiérrez-Gutiérrez, B., Rodríguez-Pardo, D., Ramos-Martínez, A., Torre-Cisneros, J., López-Medrano, F., & Cobo Reinoso, J. (2021). Real-World Experience with Bezlotoxumab for Prevention of Recurrence of Clostridioides difficile Infection. Journal of Clinical Medicine, 10(1), 2. https://doi.org/10.3390/jcm10010002







