Interaction between Pain, Disability, Mechanosensitivity and Cranio-Cervical Angle in Subjects with Cervicogenic Headache: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
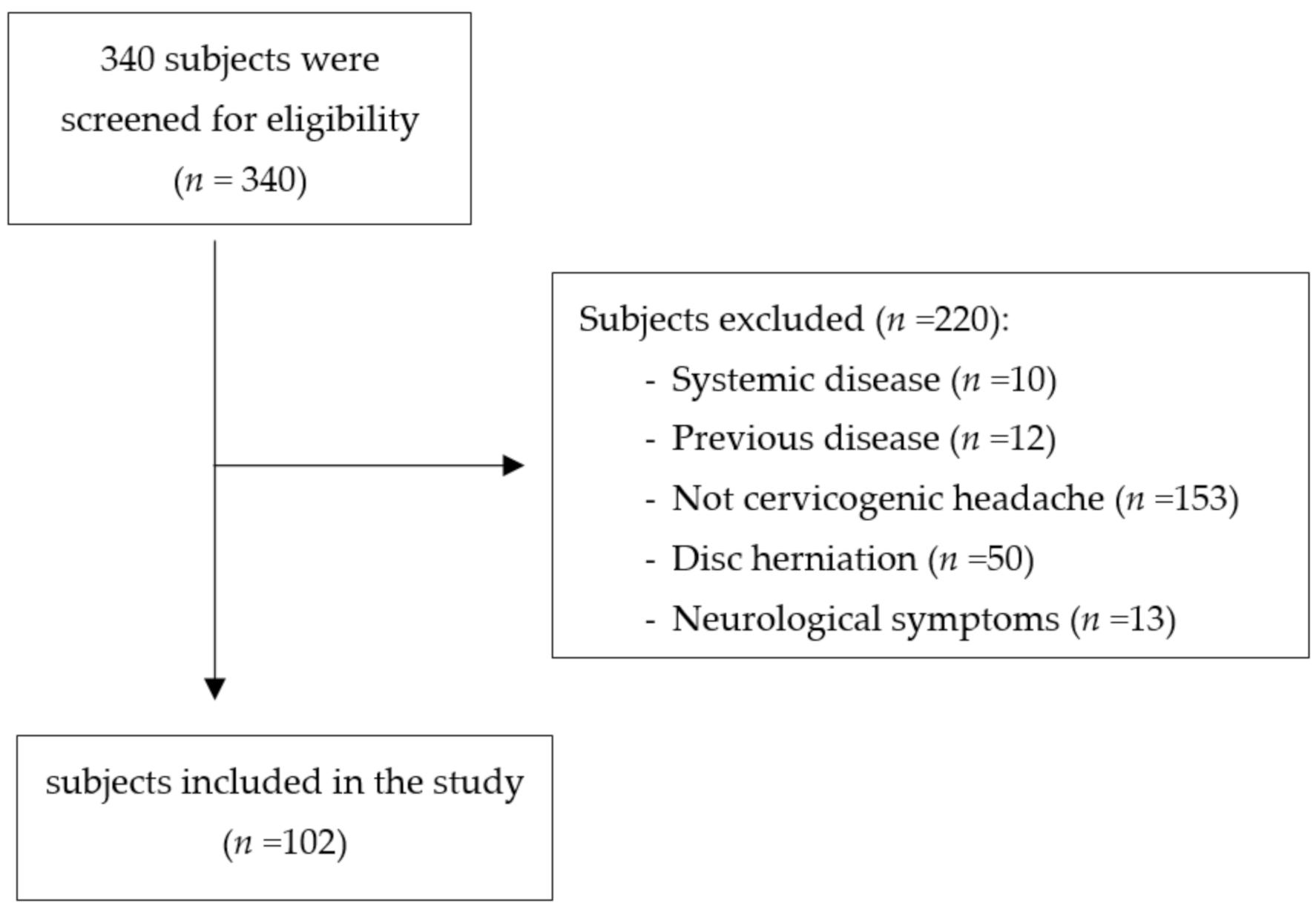
2.2. Subjects
2.3. Sample Size
2.4. Measurements
2.4.1. Pain Intensity and Disability
2.4.2. Cranio-Cervical Angle
2.4.3. Pressure Pain Threshold
2.5. Statistical Analysis
3. Results
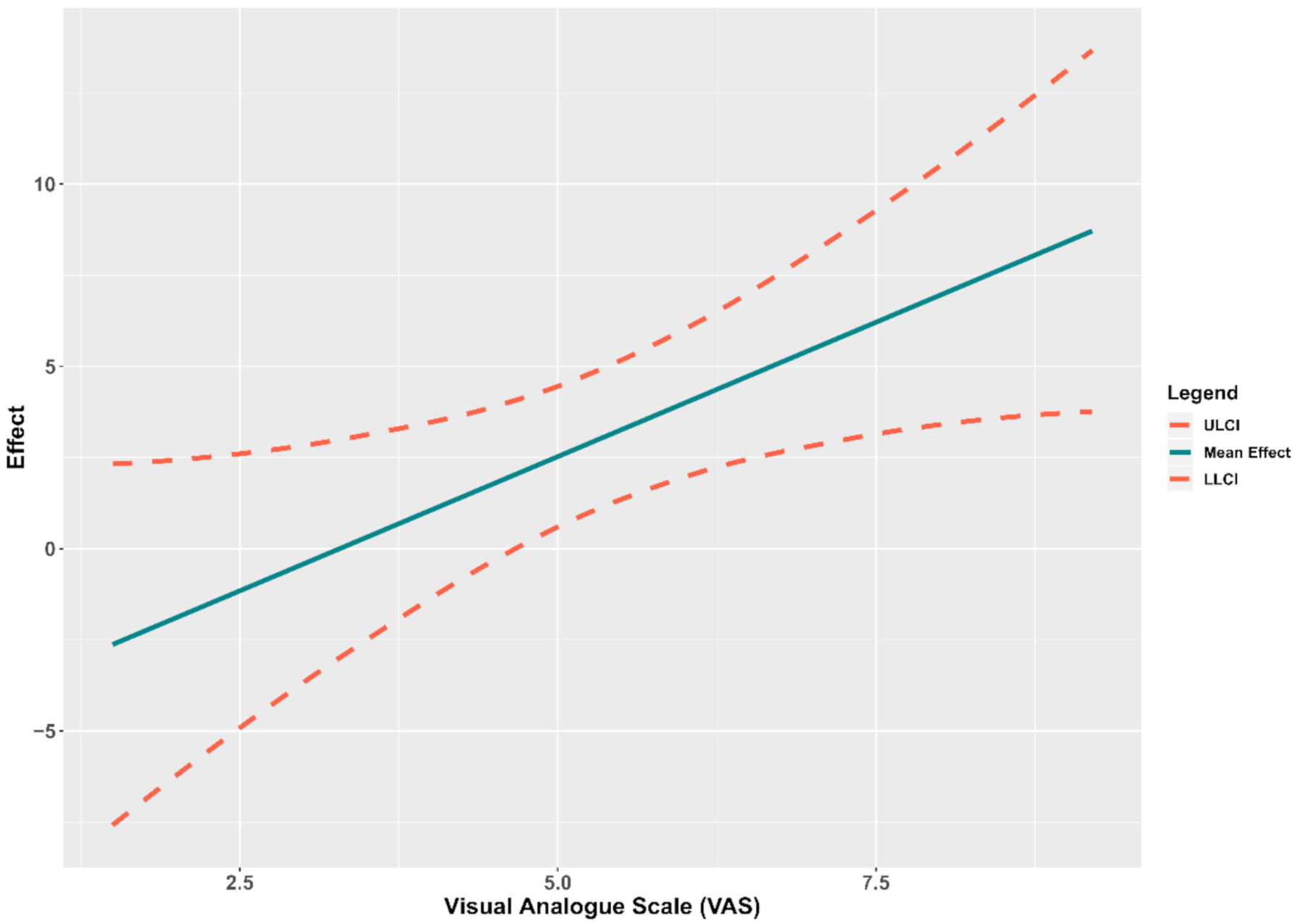
Relationship between Pressure Pain Threshold and Cranio-Cervical Angle
4. Discussion
4.1. Relationship between Pressure Pain Threshold and Cranio-Cervical Angle
4.2. Interaction between Mechanosensitivity at C2, Pain Intensity and Disability
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olesen, J. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- Pourahmadi, M.; Mohseni-Bandpei, M.A.; Keshtkar, A.; Koes, B.W.; Fernández-De-Las-Peñas, C.; Dommerholt, J.; Bahramian, M. Effectiveness of dry needling for improving pain and disability in adults with tension-type, cervicogenic, or migraine headaches: Protocol for a systematic review. Chiropr. Man. Ther. 2019, 27, 43. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, N.F.; Hassan, K.A.; Abdelmajeed, S.F.; Moustafa, I.M.; Silva, A.G. The Relationship Between Forward Head Posture and Neck Pain: A Systematic Review and Meta-Analysis. Curr. Rev. Musculoskelet. Med. 2019, 12, 562–577. [Google Scholar] [CrossRef] [PubMed]
- Narouze, S.N.; Casanova, J.; Mekhail, N. The longitudinal effectiveness of lateral atlantoaxial intra-articular steroid injection in the treatment of cervicogenic headache. Pain Med. 2007, 8, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Hoy, D.G.; Protani, M.; De, R.; Buchbinder, R. The epidemiology of neck pain. Best Pract. Res. Clin. Rheumatol. 2010, 24, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Chua, N.H.L.; van Suijlekom, H.A.; Vissers, K.C.; Arendt-Nielsen, L.; Wilder-Smith, O.H. Differences in sensory processing between chronic cervical zygapophysial joint pain patients with and without cervicogenic headache. Cephalalgia 2011, 31, 953–963. [Google Scholar] [CrossRef]
- Linde, M.; Gustavsson, A.; Stovner, L.J.; Steiner, T.J.; Barré, J.; Katsarava, Z.; Lainez, J.M.; Lampl, C.; Lantéri-Minet, M.; Rastenyte, D.; et al. The cost of headache disorders in Europe: The Eurolight project. Eur. J. Neurol. 2012, 19, 703–711. [Google Scholar] [CrossRef]
- Falsiroli Maistrello, L.; Rafanelli, M.; Turolla, A. Manual Therapy and Quality of Life in People with Headache: Systematic Review and Meta-analysis of Randomized Controlled Trials. Curr. Pain Headache Rep. 2019, 23, 23–25. [Google Scholar] [CrossRef]
- Liang, Z.; Galea, O.; Thomas, L.; Jull, G.; Treleaven, J. Cervical musculoskeletal impairments in migraine and tension type headache: A systematic review and meta-analysis. Musculoeskeletal Sci. Pract. 2019, 42, 67–83. [Google Scholar] [CrossRef]
- Subbarayalu, A.V.; Ameer, M.A. Relationships among head posture, pain intensity, disability and deep cervical flexor muscle performance in subjects with postural neck pain. J. Taibah Univ. Med. Sci. 2017, 12, 541–547. [Google Scholar] [CrossRef]
- Lee, M. Consistency of cervical and cervicothoracic posture in standing. Aust. J. Physiother. 1994, 40, 235–240. [Google Scholar] [CrossRef][Green Version]
- Patwardhan, A.G.; Khayatzadeh, S.; Havey, R.M.; Voronov, L.I.; Smith, Z.A.; Kalmanson, O.; Ghanayem, A.J.; Sears, W. Cervical sagittal balance: A biomechanical perspective can help clinical practice. Eur. Spine J. 2018, 27, 25–38. [Google Scholar] [CrossRef]
- Khayatzadeh, S.; Kalmanson, O.A.; Schuit, D.; Havey, R.M.; Voronov, L.I.; Ghanayem, A.J.; Patwardhan, A.G. Cervical Spine Muscle-Tendon Unit Length Differences Between Neutral and Forward Head Postures: Biomechanical Study Using Human Cadaveric Specimens. Phys. Ther. 2017, 97, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Szeto, G.P.Y.; Straker, L.M.; O’Sullivan, P.B. A comparison of symptomatic and asymptomatic office workers performing monotonous keyboard work—1: Neck and shoulder muscle recruitment patterns. Man. Ther. 2005, 10, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Merinero, P.; Lluch, E.; Gallezo-Izquierdo, T.; Pecos-Martín, D.; Plaza-Manzano, G.; Nuñez-Nagy, S.; Falla, D. The influence of a depressed scapular alignment on upper limb neural tissue mechanosensitivity and local pressure pain sensitivity. Musculoskelet. Sci. Pract. 2017, 29, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Castien, R.F.; van der Wouden, J.C.; De Hertogh, W. Pressure pain thresholds over the cranio-cervical region in headache: A systematic review and meta-analysis. J. Headache Pain 2018, 19, 9. [Google Scholar] [CrossRef]
- Martinez-Merinero, P.; Nuñez-Nagy, S.; Achalandabaso-Ochoa, A.; Fernandez-Matias, R.; Pecos-Martin, D.; Gallego-Izquierdo, T. Relationship between Forward Head Posture and Tissue Mechanosensitivity: A Cross-Sectional Study. J. Clin. Med. 2020, 9, 634. [Google Scholar] [CrossRef]
- Pacheco, J.; Raimundo, J.; Santos, F.; Ferreira, M.; Lopes, T.; Ramos, L.; Silva, A.G. Forward head posture is associated with pressure pain threshold and neck pain duration in university students with subclinical neck pain. Somatosens. Mot. Res. 2018, 35, 103–108. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; Blettner, M.; et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Int. J. Surg. 2014, 12, 1500–1524. [Google Scholar] [CrossRef]
- Hall, T.; Robinson, K. The flexion-rotation test and active cervical mobility—A comparative measurement study in cervicogenic headache. Man. Ther. 2004, 9, 197–202. [Google Scholar] [CrossRef]
- Hawker, G.A.; Mian, S.; Kendzerska, T.; French, M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 bps), and measure of intermittent and constant osteoarthritis pain (icoap). Arthritis Care Res. 2011, 63, S240–S252. [Google Scholar] [CrossRef]
- González, T.; Balsa, A.; de Murieta, J.S.; Zamorano, E.; González, I.; Martin-Mola, E. Spanish version of the Northwick Park neck pain questionnaire: Reliability and validity. Clin. Exp. Rheumatol. 2001, 19, 41–46. [Google Scholar] [PubMed]
- Ruivo, R.M.; Pezarat-Correia, P.; Carita, A.I. Intrarater and Interrater Reliability of Photographic Measurement of Upper-Body Standing Posture of Adolescents. J. Manipulative Physiol. Ther. 2015, 38, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Chansirinukor, W.; Wilson, D.; Grimmer, K.; Dansie, B. Effects of backpacks on students: Measurement of cervical and shoulder posture. Aust. J. Physiother. 2001, 47, 110–116. [Google Scholar] [CrossRef]
- van Niekerk, S.-M.; Louw, Q.; Vaughan, C.; Grimmer-Somers, K.; Schreve, K. Photographic measurement of upper-body sitting posture of high school students: A reliability and validity study. BMC Musculoskelet. Disord. 2008, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Walton, D.; MacDermid, J.; Nielson, W.; Teasell, R.; Chiasson, M.; Brown, L. Reliability, Standard Error, and Minimum Detectable Change of Clinical Pressure Pain Threshold Testing in People With and Without Acute Neck Pain. J. Orthop. Sport. Phys. Ther. 2011, 41, 644–650. [Google Scholar] [CrossRef]
- Walton, D.M.; Levesque, L.; Payne, M.; Schick, J. Clinical Pressure Pain Threshold Testing in Neck Pain: Comparing Protocols, Responsiveness, and Association With Psychological Variables. Phys. Ther. 2014, 94, 827–837. [Google Scholar] [CrossRef]
- Sterling, M.; Treleaven, J.; Edwards, S.; Jull, G. Pressure pain thresholds of upper limb peripheral nerve trunks in asymptomatic subjects. Physiother. Res. Int. 2000, 5, 220–229. [Google Scholar] [CrossRef]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach, 2nd ed.; Little, T.D., Ed.; Guilford Press: London, UK, 2018. [Google Scholar]
- Hayes, A.F.; Rockwood, N.J. Regression-based statistical mediation and moderation analysis in clinical research: Observations, recommendations, and implementation. Behav. Res. Ther. 2017, 98, 39–57. [Google Scholar] [CrossRef]
- Kalmanson, O.A.; Khayatzadeh, S.; Germanwala, A.; Scott-Young, M.; Havey, R.M.; Voronov, L.I.; Patwardhan, A.G. Anatomic considerations in headaches associated with cervical sagittal imbalance: A cadaveric biomechanical study. J. Clin. Neurosci. 2019, 65, 140–144. [Google Scholar] [CrossRef]
- McDougall, J.J. Arthritis and pain. Neurogenic origin of joint pain. Arthritis Res. Ther. 2006, 8, 220. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Jull, G.; Bullock-Saxton, J.; Darnell, R.; Lander, C. Cervical musculoskeletal impairment in frequent intermittent headache. Part 2: Subjects with concurrent headache types. Cephalalgia 2007, 27, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Kocur, P.; Wilski, M.; Goliwąs, M.; Lewandowski, J.; Łochyński, D. Influence of Forward Head Posture on Myotonometric Measurements of Superficial Neck Muscle Tone, Elasticity, and Stiffness in Asymptomatic Individuals With Sedentary Jobs. J. Manipulative Physiol. Ther. 2019, 42, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Huber, J.; Lisiński, P.; Polowczyk, A. Reinvestigation of the dysfunction in neck and shoulder girdle muscles as the reason of cervicogenic headache among office workers. Disabil. Rehabil. 2013, 35, 793–802. [Google Scholar] [CrossRef]
- Bovim, G. Cervicogenic headache, migraine, and tension-type headache. Pressure-pain threshold measurements. Pain 1992, 51, 169–173. [Google Scholar] [CrossRef]
- Zito, G.; Jull, G.; Story, I. Clinical tests of musculoskeletal dysfunction in the diagnosis of cervicogenic headache. Man. Ther. 2006, 11, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Brantingham, J.W.; Cassa, T.K.; Bonnefin, D.; Pribicevic, M.; Robb, A.; Pollard, H.; Tong, V.; Korporaal, C. Manipulative and multimodal therapy for upper extremity and temporomandibular disorders: A systematic review. J. Manipulative Physiol. Ther. 2013, 36, 143–201. [Google Scholar] [CrossRef]
- Ozudogru Celik, T.; Duyur Cakit, B.; Nacir, B.; Genc, H.; Cakit, M.O.; Karagoz, A. Neurodynamic evaluation and nerve conduction studies in patients with forward head posture. Acta Neurol. Belg. 2020, 120, 621–628. [Google Scholar] [CrossRef]
- Julius, A.; Lees, R.; Dilley, A.; Lynn, B. Shoulder posture and median nerve sliding. BMC Musculoskelet. Disord. 2004, 5, 23. [Google Scholar] [CrossRef]
- McClure, P.W.; Michener, L.A. Staged Approach for Rehabilitation Classification: Shoulder Disorders (STAR-Shoulder). Phys. Ther. 2015, 95, 791–800. [Google Scholar] [CrossRef]
- Lewis, J.; McCreesh, K.; Roy, J.-S.; Ginn, K. Rotator Cuff Tendinopathy: Navigating the Diagnosis-Management Conundrum. J. Orthop. Sports Phys. Ther. 2015, 45, 923–937. [Google Scholar] [CrossRef] [PubMed]
- McLean, S.M.; Moffett, J.K.; Sharp, D.M.; Gardiner, E. An investigation to determine the association between neck pain and upper limb disability for patients with non-specific neck pain: A secondary analysis. Man. Ther. 2011, 16, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Yip, C.H.T.; Chiu, T.T.W.; Poon, A.T.K. The relationship between head posture and severity and disability of patients with neck pain. Man. Ther. 2008, 13, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Ghamkhar, L.; Kahlaee, A.H. Is forward head posture relevant to cervical muscles performance and neck pain? A case–control study. Brazilian J. Phys. Ther. 2019, 23, 346–354. [Google Scholar] [CrossRef]
- Jull, G.A.; Stanton, W.R. Predictors of responsiveness to physiotherapy management of cervicogenic headache. Cephalalgia 2005, 25, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Uthaikhup, S.; Sterling, M.; Jull, G. Cervical musculoskeletal impairment is common in elders with headache. Man. Ther. 2009, 14, 636–641. [Google Scholar] [CrossRef]
| Variable | Mean ± SE (Minimum–Maximum) |
|---|---|
| Age, years | 21.92 ± 0.35 (18.00–33.00) |
| Height, cm | 169.18 ± 0.97 (153.00–196.00) |
| Weight, kg | 62.60 ± 1.21 (43.00–106.40) |
| BMI, kg/m2 | 21.65 ± 0.26 (15.70–32.12) |
| Sex, n (%) | |
| Women | 73 (72.3) |
| Men | 28 (27.7) |
| CCA, degrees | 50.29 ± 0.52 (38.00–63.00) |
| VAS, cm | 5.03 ± 0.16 (1.50–9.20) |
| NPQ | 16.59 ± 0.78 (2.77–38.00) |
| PPT, kg/cm2 | |
| Upper trapezius | 2.47 ± 0.07 (1.40–4.18) |
| Splenius capitis | 2.07 ± 0.05 (1.15–4.15) |
| Median nerve | 1.66 ± 0.04 (1.00–2.95) |
| C2 | 2.20 ± 0.05 (1.20–3.60) |
| Variable | B * (SE) | 95% CI |
|---|---|---|
| Age | 0.11 (0.15) | −0.19 to 0.41 |
| BMI | 0.07 (0.17) | −0.28 to 0.40 |
| Sex | 0.57 (1.33) | −2.04 to 3.16 |
| PPT C2 | −3.38 (4.06) | −11.51 to 4.44 |
| VAS | −4.38 † (1.76) | −7.87 to −1.02 |
| NPQ | 0.44 (0.26) | −0.04 to 1.00 |
| Interaction 1 | 1.79 † (0.70) | 0.45 to 3.21 |
| Interaction 2 | −0.18 (0.11) | −0.42 to 0.02 |
| Variable | B * (SE) | 95% CI |
|---|---|---|
| Age | 0.10 (0.16) | −0.22 to 0.40 |
| BMI | 0.09 (0.17) | −0.26 to 0.43 |
| Sex | 0.77 (1.32) | −1.82 to 3.33 |
| PPT C2 | −4.99 (3.91) | −12.62 to 2.68 |
| VAS | −3.70 † (1.76) | −7.33 to −0.43 |
| NPQ | 0.03 (0.06) | −0.09 to 0.16 |
| Interaction | 1.52 † (0.70) | 0.22 to 2.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Merinero, P.; Aneiros Tarancón, F.; Montañez-Aguilera, J.; Nuñez-Nagy, S.; Pecos-Martín, D.; Fernández-Matías, R.; Achalandabaso-Ochoa, A.; Fernández-Carnero, S.; Gallego-Izquierdo, T. Interaction between Pain, Disability, Mechanosensitivity and Cranio-Cervical Angle in Subjects with Cervicogenic Headache: A Cross-Sectional Study. J. Clin. Med. 2021, 10, 159. https://doi.org/10.3390/jcm10010159
Martínez-Merinero P, Aneiros Tarancón F, Montañez-Aguilera J, Nuñez-Nagy S, Pecos-Martín D, Fernández-Matías R, Achalandabaso-Ochoa A, Fernández-Carnero S, Gallego-Izquierdo T. Interaction between Pain, Disability, Mechanosensitivity and Cranio-Cervical Angle in Subjects with Cervicogenic Headache: A Cross-Sectional Study. Journal of Clinical Medicine. 2021; 10(1):159. https://doi.org/10.3390/jcm10010159
Chicago/Turabian StyleMartínez-Merinero, Patricia, Fernando Aneiros Tarancón, Javier Montañez-Aguilera, Susana Nuñez-Nagy, Daniel Pecos-Martín, Rubén Fernández-Matías, Alexander Achalandabaso-Ochoa, Samuel Fernández-Carnero, and Tomás Gallego-Izquierdo. 2021. "Interaction between Pain, Disability, Mechanosensitivity and Cranio-Cervical Angle in Subjects with Cervicogenic Headache: A Cross-Sectional Study" Journal of Clinical Medicine 10, no. 1: 159. https://doi.org/10.3390/jcm10010159
APA StyleMartínez-Merinero, P., Aneiros Tarancón, F., Montañez-Aguilera, J., Nuñez-Nagy, S., Pecos-Martín, D., Fernández-Matías, R., Achalandabaso-Ochoa, A., Fernández-Carnero, S., & Gallego-Izquierdo, T. (2021). Interaction between Pain, Disability, Mechanosensitivity and Cranio-Cervical Angle in Subjects with Cervicogenic Headache: A Cross-Sectional Study. Journal of Clinical Medicine, 10(1), 159. https://doi.org/10.3390/jcm10010159







