Materials and Applications for Low-Cost Ceramic Membranes
Abstract
1. Introduction
2. Materials
2.1. Natural Minerals
2.1.1. Kaolin
2.1.2. Other Clays
2.1.3. Zeolite Minerals
2.1.4. Apatite
2.1.5. Quartz Sand
2.1.6. Natural Pozzolan
2.1.7. Bauxite
2.2. Waste Materials (Ashes)
2.2.1. Fly Ash
2.2.2. Rice Husk Ash
2.2.3. Sugarcane Bagasse Ash
2.3. Cement
2.3.1. Portland Cement
2.3.2. Geopolymer Cement
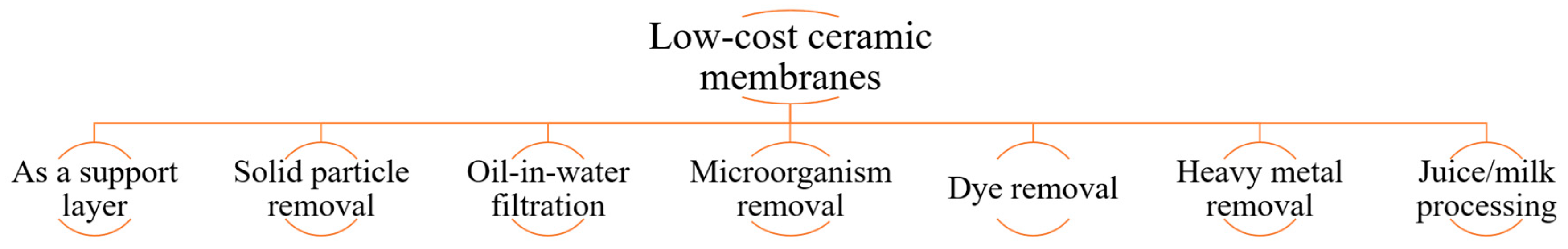
3. Application Areas of Low-Cost Inorganic Membranes
4. Cost Evaluation
5. Summary and Outlook
- The development of novel combinations of low-cost precursor materials and pore formers through iterative experimentation;
- Continued development of freeze casting methods for the structuring of pores [194];
- Safety concerns should be considered, i.e., effluents of low-cost membranes should be tested to check the presence and dissolution of radioactive elements and heavy metals [195].
- Clays, in particular, are of keen interest in the design of low-cost ceramic membranes. Kaolin-based hollow fiber membranes, in particular, offer a valuable pathway towards effective oil-water separation;
- Silicate bearing ashes derived from coal combustion or rice husks can serve as the basis for mullite membranes in which the intergrowth of mullite needles is harnessed to impart highly functional pore structures in the obtained membranes;
- Current researches show that natural quartz sand, zeolite mineral, apatites are also promising. However, more research is needed to investigate the effect of fabrications conditions and mineralogical composition;
- A sintering-free approach using self-hardening materials, such as Portland cement and geopolymer, enables the reduction of costs and environmental impacts of high-temperature sintering processes;
- Cost-benefit analyses indicate that the application of low-cost materials in membrane processes on an industrial scale would be economically and environmentally advantageous.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AFt | Calcium sulfoaluminate hydrates |
| C2S | Dicalcium silicate |
| C3S | Tricalcium silicate |
| CH | Calcium hydroxide |
| C-S-H | Calcium silicate hydrate |
| MF | Microfiltration |
| PVA | Polyvinyl alcohol |
| RH | Rice husk |
| RHA | Rice husk ash |
| SCBA | Sugarcane bagasse ash |
| SEM | Scanning electron microscope |
| SL | Support layer |
| SSF | Slow sand filter |
| UF | Ultrafiltration |
| XRD | X-ray powder diffraction |
References
- Coping with Water Scarcity: An Action Framework for Agriculture and Food Security; FAO: Rome, Italy, 2012.
- Goh, P.S.; Ismail, A.F. A review on inorganic membranes for desalination and wastewater treatment. Desalination 2018, 434, 60–80. [Google Scholar] [CrossRef]
- Mulder, M. Basic Principles of Membrane Technology, 2nd ed.; Kluwer Academic: Dordrecht, The Netherland; Boston, MA, USA, 1996. [Google Scholar]
- Marchetti, P.; Solomon, M.F.J.; Szekely, G.; Livingston, A.G. Molecular separation with organic solvent nanofiltration: A critical review. Chem. Rev. 2014, 114, 10735–10806. [Google Scholar] [CrossRef]
- Hofs, B.; Ogier, J.; Vries, D.; Beerendonk, E.F.; Cornelissen, E.R. Comparison of ceramic and polymeric membrane permeability and fouling using surface water. Sep. Purif. Technol. 2011, 79, 365–374. [Google Scholar] [CrossRef]
- Li, W.; Dong, H.; Yu, H.; Wang, D.; Yu, H. Global characteristics and trends of research on ceramic membranes from 1998 to 2016: Based on bibliometric analysis combined with information visualization analysis. Ceram. Int. 2018, 44, 6926–6934. [Google Scholar] [CrossRef]
- Wang, Y.H.; Tian, T.F.; Liu, X.Q.; Meng, G.Y. Titania membrane preparation with chemical stability for very hash environments applications. J. Membr. Sci. 2006, 280, 261–269. [Google Scholar] [CrossRef]
- DeFriend, K.A.; Wiesner, M.R.; Barron, A.R. Alumina and aluminate ultrafiltration membranes derived from alumina nanoparticles. J. Membr. Sci. 2003, 224, 11–28. [Google Scholar] [CrossRef]
- Yoshino, Y.; Suzuki, T.; Nair, B.; Taguchi, H.; Itoh, N. Development of tubular substrates, silica based membranes and membrane modules for hydrogen separation at high temperature. J. Membr. Sci. 2005, 267, 8–17. [Google Scholar] [CrossRef]
- Nandi, B.K.; Uppaluri, R.; Purkait, M.K. Preparation and characterization of low cost ceramic membranes for micro-filtration applications. Appl. Clay Sci. 2008, 42, 102–110. [Google Scholar] [CrossRef]
- Vasanth, D.; Pugazhenthi, G.; Uppaluri, R. Fabrication and properties of low cost ceramic microfiltration membranes for separation of oil and bacteria from its solution. J. Membr. Sci. 2011, 379, 154–163. [Google Scholar] [CrossRef]
- Monash, P.; Pugazhenthi, G. Development of Ceramic Supports Derived from Low-Cost Raw Materials for Membrane Applications and its Optimization Based on Sintering Temperature. Int. J. Appl. Ceram. Technol. 2011, 8, 227–238. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Matsuura, T.; Ismail, A.F.; Rahman, M.A.; Harun, Z.; Jaafar, J.; Nomura, M. Fabrications and applications of low cost ceramic membrane from kaolin: A comprehensive review. Ceram. Int. 2018, 44, 4538–4560. [Google Scholar] [CrossRef]
- Monash, P.; Pugazhenthi, G.; Saravanan, P. Various fabrication methods of porous ceramic supports for membrane applications. Rev. Chem. Eng. 2013, 29, 357–383. [Google Scholar] [CrossRef]
- Issaoui, M.; Limousy, L. Low-cost ceramic membranes: Synthesis, classifications, and applications. C. R. Chim. 2019, 22, 175–187. [Google Scholar] [CrossRef]
- Rhodes, D. Clay and Glazes for the Potter; Martino Publishing: Mansfield Centre, CT, USA, 2015. [Google Scholar]
- Zhou, J.; Zhang, X.; Wang, Y.; Larbot, A.; Hu, X. Elaboration and characterization of tubular macroporous ceramic support for membranes from kaolin and dolomite. J. Porous Mater. 2010, 17, 1–9. [Google Scholar] [CrossRef]
- Harabi, A.; Zenikheri, F.; Boudaira, B.; Bouzerara, F.; Guechi, A.; Foughali, L. A new and economic approach to fabricate resistant porous membrane supports using kaolin and CaCO3. J. Eur. Ceram. Soc. 2014, 34, 1329–1340. [Google Scholar] [CrossRef]
- Boudaira, B.; Harabi, A.; Bouzerara, F.; Zenikheri, F.; Foughali, L.; Guechi, A. Preparation and characterization of membrane supports for microfiltration and ultrafiltration using kaolin (DD2) and CaCO3. Desalin. Water Treat. 2016, 57, 5258–5265. [Google Scholar] [CrossRef]
- Jana, S.; Purkait, M.K.; Mohanty, K. Preparation and characterization of low-cost ceramic microfiltration membranes for the removal of chromate from aqueous solutions. Appl. Clay Sci. 2010, 47, 317–324. [Google Scholar] [CrossRef]
- Hedfi, I.; Hamdi, N.; Srasra, E.; Rodríguez, M.A. The preparation of micro-porous membrane from a Tunisian kaolin. Appl. Clay Sci. 2014, 101, 574–578. [Google Scholar] [CrossRef]
- Mohtor, N.H.; Othman, M.H.D.; Ismail, A.F.; Rahman, M.A.; Jaafar, J.; Hashim, N.A. Investigation on the effect of sintering temperature on kaolin hollow fibre membrane for dye filtration. Environ. Sci. Pollut. Res. Int. 2017, 24, 15905–15917. [Google Scholar] [CrossRef]
- Ali, M.B.; Hamdi, N.; Rodriguez, M.A.; Mahmoudi, K.; Srasra, E. Preparation and characterization of new ceramic membranes for ultrafiltration. Ceram. Int. 2018, 44, 2328–2335. [Google Scholar]
- Bellotto, M.; Gualtieri, A.; Artioli, G.; Clark, S.M. Kinetic study of the kaolinite-mullite reaction sequence. Part I: Kaolinite dihydroxylation. Phys. Chem. Miner. 1995, 22, 207–217. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Harun, Z.; Ismail, A.F.; Rahman, M.A.; Jaafar, J.; Jamil, S.M.; Mohtor, N.H. Superhydrophilic, low cost kaolin-based hollow fibre membranes for efficient oily-wastewater separation. Mater. Lett. 2017, 191, 119–122. [Google Scholar] [CrossRef]
- Zhu, Z.; Wei, Z.; Sun, W.; Hou, J.; He, B.; Dong, Y. Cost-effective utilization of mineral-based raw materials for preparation of porous mullite ceramic membranes via in-situ reaction method. Appl. Clay Sci. 2016, 120, 135–141. [Google Scholar] [CrossRef]
- Chen, G.; Ge, X.; Wang, Y.; Xing, W.; Guo, Y. Design and preparation of high permeability porous mullite support for membranes by in-situ reaction. Ceram. Int. 2015, 41, 8282–8287. [Google Scholar] [CrossRef]
- Guechi, A.; Harabi, A.; Condoum, S.; Zenikheri, F.; Boudaira, B.; Bouzerara, F.; Foughali, L. Elaboration and characterization of tubular supports for membranes filtration. Desalin. Water Treat. 2016, 57, 5246–5252. [Google Scholar] [CrossRef]
- Almandoz, M. Preparation and characterization of non-supported microfiltration membranes from aluminosilicates. J. Membr. Sci. 2004, 241, 95–103. [Google Scholar] [CrossRef]
- Emani, S.; Uppaluri, R.; Purkait, M.K. Preparation and characterization of low cost ceramic membranes for mosambi juice clarification. Desalination 2013, 317, 32–40. [Google Scholar] [CrossRef]
- Emani, S.; Uppaluri, R.; Purkait, M.K. Cross flow microfiltration of oil–water emulsions using kaolin based low cost ceramic membranes. Desalination 2014, 341, 61–71. [Google Scholar] [CrossRef]
- Eom, J.-H.; Kim, Y.-W.; Yun, S.-H.; Song, I.-H. Low-cost clay-based membranes for oily wastewater treatment. J. Ceram. Soc. Jpn. 2014, 122, 788–794. [Google Scholar] [CrossRef]
- Hedfi, I.; Hamdi, N.; Rodriguez, M.A.; Srasra, E. Preparation of macroporous membrane using natural Kaolin and Tunisian lignite as a pore-forming agent. Desalin. Water Treat. 2016, 57, 13388–13393. [Google Scholar] [CrossRef]
- Rekik, S.B.; Bouaziz, J.; Deratani, A.; Beklouti, S. Study of Ceramic Membrane from Naturally Occurring-Kaolin Clays for Microfiltration Applications. Period. Polytech. Chem. Eng. 2017, 61, 206. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Ismail, A.F.; Rahman, M.A.; Jaafar, J. A low cost hydrophobic kaolin hollow fiber membrane (h-KHFM) for arsenic removal from aqueous solution via direct contact membrane distillation. Sep. Purif. Technol. 2019, 214, 31–39. [Google Scholar] [CrossRef]
- Arzani, M.; Mahdavi, H.R.; Sheikhi, M.; Mohammadi, T.; Bakhtiari, O. Ceramic monolith as microfiltration membrane: Preparation, characterization and performance evaluation. Appl. Clay Sci. 2018, 161, 456–463. [Google Scholar] [CrossRef]
- Kumar, R.V.; Ghoshal, A.K.; Pugazhenthi, G. Elaboration of novel tubular ceramic membrane from inexpensive raw materials by extrusion method and its performance in microfiltration of synthetic oily wastewater treatment. J. Membr. Sci. 2015, 490, 92–102. [Google Scholar] [CrossRef]
- Harabi, A.; Guechi, A.; Condom, S. Production of Supports and Filtration Membranes from Algerian Kaolin and Limestone. Proc. Eng. 2012, 33, 220–224. [Google Scholar] [CrossRef]
- Das, B.; Chakrabarty, B.; Barkakati, P. Preparation and characterization of novel ceramic membranes for micro-filtration applications. Ceram. Int. 2016, 42, 14326–14333. [Google Scholar] [CrossRef]
- Kouras, N.; Harabi, A.; Bouzerara, F.; Foughali, L.; Policicchio, A.; Stelitano, S.; Galiano, F.; Figoli, A. Macro-porous ceramic supports for membranes prepared from quartz sand and calcite mixtures. J. Eur. Ceram. Soc. 2017, 37, 3159–3165. [Google Scholar] [CrossRef]
- Boudaira, B.; Harabia, A.; Bouzerara, F.; Condom, S. Preparation and characterization of microfi ltration membranes and their supports using kaolin (DD2) and CaCO3. Desalin. Water Treat. 2009, 9, 142–148. [Google Scholar] [CrossRef]
- Harabi, A.; Boudaira, B.; Bouzerara, F.; Foughali, L.; Zenikheri, F.; Guechi, A.; Ghouil, B.; Condom, S. Porous Ceramic Supports for Membranes Prepared from Kaolin (DD3) and Calcite Mixtures. Acta Phys. Pol. A 2015, 127, 1164–1166. [Google Scholar] [CrossRef]
- Bouzerara, F.; Harabi, A.; Condom, S. Porous ceramic membranes prepared from kaolin. Desalin. Water Treat. 2009, 12, 415–419. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Kumar, P.; Othman, M.H.D.; Ismail, A.F.; Rahman, M.A.; Jaafar, J. A low cost, superhydrophobic and superoleophilic hybrid kaolin-based hollow fibre membrane (KHFM) for efficient adsorption–separation of oil removal from water. RSC Adv. 2018, 8, 2986–2995. [Google Scholar] [CrossRef]
- Fritz, S.J. Ideality of Clay Membranes in Osmotic Processes: A Review. Clays Clay Miner. 1986, 34, 214–223. [Google Scholar] [CrossRef]
- Fan, B.; Wei, G.; Hao, H.; Guo, A.; Li, J. Preparation of a ceramic membrane from prevalent natural clay for the purification of phosphate wastewater. Desalin. Water Treat. 2016, 57, 17308–17321. [Google Scholar] [CrossRef]
- Zhou, J.-E.; Dong, Y.; Hampshire, S.; Meng, G. Utilization of sepiolite in the synthesis of porous cordierite ceramics. Appl. Clay Sci. 2011, 52, 328–332. [Google Scholar] [CrossRef]
- Almandoz, M.C.; Pagliero, C.L.; Ochoa, N.A.; Marchese, J. Composite ceramic membranes from natural aluminosilicates for microfiltration applications. Ceram. Int. 2015, 41, 5621–5633. [Google Scholar] [CrossRef]
- Henriques, J.D.D.; Pedrassani, M.W.; Klitzke, W.; Mariano, A.B.; Vargas, J.V.C.; Vieira, R.B. Thermal treatment of clay-based ceramic membranes for microfiltration of Acutodesmus obliquus. Appl. Clay Sci. 2017, 150, 217–224. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, D. Preparation and characterization of attapulgite-based nanofibrous membranes. Mater. Design 2017, 113, 60–67. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, D. Clay-based nanofibrous membranes reinforced by multi-walled carbon nanotubes. Ceram. Int. 2018, 44, 15873–15879. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, D. Novel clay-based nanofibrous membranes for effective oil/water emulsion separation. Ceram. Int. 2017, 43, 9465–9471. [Google Scholar] [CrossRef]
- Foorginezhad, S.; Zerafat, M.M. Microfiltration of cationic dyes using nano-clay membranes. Ceram. Int. 2017, 43, 15146–15159. [Google Scholar] [CrossRef]
- Ersoy, B.; Gunay, V. Preparation and Characterization of Sol-Gel Derived 4%La2 O3-Al2O3 Ceramic Membrane on Clay-Based Supports. Key Eng. Mater. 2004, 264–268, 403–406. [Google Scholar] [CrossRef]
- Saffaj, N.; Persin, M.; Younsi, S.A.; Albizane, A.; Cretin, M.; Larbot, A. Elaboration and characterization of microfiltration and ultrafiltration membranes deposited on raw support prepared from natural Moroccan clay: Application to filtration of solution containing dyes and salts. Appl. Clay Sci. 2006, 31, 110–119. [Google Scholar] [CrossRef]
- Saffaj, N.; Persin, M.; Younssi, S.A.; Albizane, A.; Bouhria, M.; Loukili, H.; Dach, H.; Larbot, A. Removal of salts and dyes by low ZnAl2O4–TiO2 ultrafiltration membrane deposited on support made from raw clay. Sep. Purif. Technol. 2005, 47, 36–42. [Google Scholar] [CrossRef]
- Anbri, Y.; Tijani, N.; Coronas, J.; Mateo, E.; Menéndez, M.; Bentama, J. Clay plane membranes: Development and characterization. Desalination 2008, 221, 419–424. [Google Scholar] [CrossRef]
- Palacio, L.; Bouzerdi, Y.; Ouammou, M.; Albizane, A.; Bennazha, J.; Hernández, A.; Calvo, J.I. Ceramic membranes from Moroccan natural clay and phosphate for industrial water treatment. Desalination 2009, 245, 501–507. [Google Scholar] [CrossRef]
- Baraka, N.E.; Saffaj, N.; Mamouni, R.; Laknifli, A.; Younssi, S.A.; Albizane, A.; El Haddad, M. Elaboration of a new flat membrane support from Moroccan clay. Desalin. Water Treat. 2014, 52, 1357–1361. [Google Scholar] [CrossRef]
- Elomari, H.; Achiou, B.; Ouammou, M.; Albizane, A.; Bennazha, J.; Younssi, S.A.; Elamrani, I. Elaboration and characterization of flat membrane supports from Moroccan clays. Application for the treatment of wastewater. Desalin. Water Treat. 2016, 57, 20298–20306. [Google Scholar] [CrossRef]
- Bouazizi, A.; Saja, S.; Achiou, B.; Ouammou, M.; Calvo, J.I.; Aaddane, A.; Younssi, S.A. Elaboration and characterization of a new flat ceramic MF membrane made from natural Moroccan bentonite. Application to treatment of industrial wastewater. Appl. Clay Sci. 2016, 132–133, 33–40. [Google Scholar] [CrossRef]
- Bouazizi, A.; Breida, M.; Karim, A.; Achiou, B.; Ouammou, M.; Calvo, J.I.; Aaddane, A.; Khiat, K.; Younssi, S.A. Development of a new TiO2 ultrafiltration membrane on flat ceramic support made from natural bentonite and micronized phosphate and applied for dye removal. Ceram. Int. 2017, 43, 1479–1487. [Google Scholar] [CrossRef]
- Mouiya, M.; Abourriche, A.; Bouazizi, A.; Benhammou, A.; el Hafiane, Y.; Abouliatim, Y.; Nibou, L.; Oumam, M.; Ouammou, M.; Smith, A.; et al. Flat ceramic microfiltration membrane based on natural clay and Moroccan phosphate for desalination and industrial wastewater treatment. Desalination 2018, 427, 42–50. [Google Scholar] [CrossRef]
- Misrar, W.; Loutou, M.; Saadi, L.; Mansori, M.; Waqif, M.; Favotto, C. Cordierite containing ceramic membranes from smectetic clay using natural organic wastes as pore-forming agents. J. Asian Ceram. Soc. 2017, 5, 199–208. [Google Scholar] [CrossRef]
- Eom, J.-H.; Yeom, H.-J.; Kim, Y.-W.; Song, I.-H. Ceramic Membranes Prepared from a Silicate and Clay-mineral Mixture for Treatment of Oily Wastewater. Clays Clay Miner. 2015, 63, 222–234. [Google Scholar] [CrossRef]
- Abubakar, M.; Tamin, M.N.; Saleh, M.A.; Uday, M.B.; Ahmad, N. Preparation and characterization of a nigerian mesoporous clay-based membrane for uranium removal from underground water. Ceram. Int. 2016, 42, 8212–8220. [Google Scholar] [CrossRef]
- Galán-Arboledas, R.J.; Cotes, T.; Martínez, C.; Bueno, S. Influence of waste addition on the porosity of clay-based ceramic membranes. Desalin. Water Treat. 2016, 57, 2633–2639. [Google Scholar] [CrossRef]
- Lorente-Ayza, M.-M.; Mestre, S.; Menéndez, M.; Sánchez, E. Comparison of extruded and pressed low cost ceramic supports for microfiltration membranes. J. Eur. Ceram. Soc. 2015, 35, 3681–3691. [Google Scholar] [CrossRef]
- Khemakhem, S.; Larbot, A.; Amar, R.B. Study of performances of ceramic microfiltration membrane from Tunisian clay applied to cuttlefish effluents treatment. Desalination 2006, 200, 307–309. [Google Scholar] [CrossRef]
- Khemakhem, S.; Amar, R.B.; Larbot, A. Synthesis and characterization of a new inorganic ultrafiltration membrane composed entirely of Tunisian natural illite clay. Desalination 2007, 206, 210–214. [Google Scholar] [CrossRef]
- Fakhfakh, S.; Baklouti, S.; Bouaziz, J. Elaboration and characterisation of low cost ceramic support membrane. Adv. Appl. Ceram. 2010, 109, 31–38. [Google Scholar] [CrossRef]
- Haden, W.L.; Schwint, I.A. Attapulgite: Its Properties and Applications. Ind. Eng. Chem. 1967, 59, 58–69. [Google Scholar] [CrossRef]
- Kallo, D. Applications of Natural Zeolites in Water and Wastewater Treatment. Rev. Mineral. Geochem. 2001, 45, 519–550. [Google Scholar] [CrossRef]
- Hay, R.L. Geologic Occurrence of Zeolites and Some Associated Minerals. Pure Appl. Chem. 1986, 58, 1339–1342. [Google Scholar] [CrossRef]
- Mumpton, F.A. La roca magica: Uses of natural zeolites in agriculture and industry. Proc. Natl. Acad. Sci. USA 1999, 96, 3463–3470. [Google Scholar] [CrossRef] [PubMed]
- Earl, D.J.; Deem, M.W. Toward a Database of Hypothetical Zeolite Structures. Ind. Eng. Chem. Res. 2006, 45, 5449–5454. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, Z.H. Characterisation and environmental application of an Australian natural zeolite for basic dye removal from aqueous solution. J. Hazard. Mater. 2006, 136, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Roque-Malherbe, R.; del Valle, W.; Marquez, F.; Duconge, J.; Goosen, M.F.A. Synthesis and Characterization of Zeolite Based Porous Ceramic Membranes. Sep. Sci. Technol. 2006, 41, 73–96. [Google Scholar] [CrossRef]
- Hristov, P.; Yoleva, A.; Djambazov, S.; Chukovska, I.; Dimitrov, D. Preparation and characterization of porous ceramic membranes for micro-filtration from natural zeolite. J. Univ. Chem. Technol. Metall. 2012, 47, 476–480. [Google Scholar]
- Dong, Y.; Chen, S.; Zhang, X.; Yang, J.; Liu, X.; Meng, G. Fabrication and characterization of low cost tubular mineral-based ceramic membranes for micro-filtration from natural zeolite. J. Membr. Sci. 2006, 281, 592–599. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, G.; Liu, X. Preparation and characterization of tubular porous ceramics from natural zeolite. J. Porous Mater. 2008, 15, 101–106. [Google Scholar] [CrossRef]
- Adam, M.R.; Salleh, N.M.; Othman, M.H.D.; Matsuura, T.; Ali, M.H.; Puteh, M.H.; Ismail, A.F.; Rahman, M.A.; Jaafar, J. The adsorptive removal of chromium (VI) in aqueous solution by novel natural zeolite based hollow fibre ceramic membrane. J. Environ. Manag. 2018, 224, 252–262. [Google Scholar] [CrossRef]
- Bernal, M.P.; Lopez-Real, J.M. Natural zeolites and sepiolite as ammonium and ammonia adsorbent materials. Bioresour. Technol. 1993, 43, 27–33. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Suzuki, T.; Arai, H. A study of equilibrium and mass transfer in processes for removal of heavy-metal ions by hydroxyapatite. J. Chem. Eng. Jpn. 1988, 21, 98–100. [Google Scholar] [CrossRef]
- Rakovan, J.F.; Pasteris, J.D. A Technological Gem: Materials, Medical, and Environmental Mineralogy of Apatite. Elements 2015, 11, 195–200. [Google Scholar] [CrossRef]
- Elliott, J.C. Structure and Chemistry of the Apatites and Other Calcium Orthophosphates; Elsevier Science: Amsterdam, The Netherland, 2013. [Google Scholar]
- Ioannidis, T.A.; Zouboulis, A.I. Detoxification of a highly toxic lead-loaded industrial solid waste by stabilization using apatites. J. Hazard. Mater. 2003, 97, 173–191. [Google Scholar] [CrossRef]
- Ma, Q.Y.; Traina, S.J.; Logan, T.J.; Ryan, J.A. In situ lead immobilization by apatite. Environ. Sci. Technol. 1993, 27, 1803–1810. [Google Scholar] [CrossRef]
- Bensalah, H.; Bekheet, M.F.; Younssi, S.A.; Ouammou, M.; Gurlo, A. Removal of cationic and anionic textile dyes with Moroccan natural phosphate. J. Environ. Chem. Eng. 2017, 5, 2189–2199. [Google Scholar] [CrossRef]
- Masmoudi, S.; Amar, R.B.; Larbot, A.; El Feki, H.; Salah, A.B.; Cot, L. Elaboration of inorganic microfiltration membranes with hydroxyapatite applied to the treatment of wastewater from sea product industry. J. Membr. Sci. 2005, 247, 1–9. [Google Scholar] [CrossRef]
- Bensalah, H.; Bekheet, M.F.; Younssi, S.A.; Ouammou, M.; Gurlo, A. Hydrothermal synthesis of nanocrystalline hydroxyapatite from phosphogypsum waste. J. Environ. Chem. Eng. 2018, 6, 1347–1352. [Google Scholar] [CrossRef]
- Masmoudi, S.; Larbot, A.; Feki, H.E.; Amar, R.B. Elaboration and characterisation of apatite based mineral supports for microfiltration and ultrafiltration membranes. Ceram. Int. 2007, 33, 337–344. [Google Scholar] [CrossRef]
- Masmoudi, S.; Larbot, A.; El Feki, H.; Amar, R.B. Elaboration and properties of new ceramic microfiltration membranes from natural and synthesised apatite. Desalination 2006, 190, 89–103. [Google Scholar] [CrossRef]
- Abo-Almaged, H.H.; Gaber, A.A. Synthesis and characterization of nano-hydroxyapatite membranes for water desalination. Mater. Today Commun. 2017, 13, 186–191. [Google Scholar] [CrossRef]
- Sahoo, G.C.; Halder, R.; Jedidi, I.; Oun, A.; Nasri, H.; Roychoudhurry, P.; Majumdar, S.; Bandyopadhyay, S.; Amar, R.B. Preparation and characterization of microfiltration apatite membrane over low cost clay-alumina support for decolorization of dye solution. Desalin. Water Treat. 2016, 997, 1–10. [Google Scholar] [CrossRef]
- Bates, R.L.; Jackson, J.A. Glossary of Geology, 3rd ed.; American Geological Institute: Alexandria, VA, USA, 1987. [Google Scholar]
- Pettijohn, F.J. Sedimentary Rocks, 3rd ed.; Harper et Row: New York, NY, USA, 1982. [Google Scholar]
- Ellis, K.V.; Wood, W.E. Slow sand filtration. Crit. Rev. Environ. Control 1985, 15, 315–354. [Google Scholar] [CrossRef]
- Clark, P.A.; Pinedo, C.A.; Fadus, M.; Capuzzi, S. Slow-sand water filter: Design, implementation, accessibility and sustainability in developing countries. Med. Sci. Monit. 2012, 18, RA105–RA117. [Google Scholar] [CrossRef]
- Ivanets, A.I.; Rat’ko, A.I.; Azarova, T.A.; Azarov, S.M.; Al-Khowaiter, S.H.; Al-Harbi, O.; Shemchonok, S.V.; Dobysh, V.A.; Tarasevich, V.A.; Agabekov, V.E.; et al. Preparation and properties of microfiltration membranes based on natural crystalline SiO2. Ceram. Int. 2014, 40, 12343–12351. [Google Scholar] [CrossRef]
- Aloulou, H.; Bouhamed, H.; Amar, R.B.; Khemakhem, S. New ceramic microfiltration membrane from Tunisian natural sand: Application for tangential waste water treatment. Desalin. Water Treat. 2017, 78, 41–48. [Google Scholar] [CrossRef]
- Ivanets, A.; Agabekov, V. Preparation and Characterization of Microfiltration Ceramic Membranes Based on Natural Quartz Sand. ChemJMold 2017, 12, 67–73. [Google Scholar] [CrossRef]
- Khemakhem, M.; Khemakhem, S.; Ayedi, S.; Cretin, M.; Amar, R.B. Development of an asymmetric ultrafiltration membrane based on phosphates industry sub-products. Ceram. Int. 2015, 41, 10343–10348. [Google Scholar] [CrossRef]
- Foughali, L.; Barama, S.; Harabi, A.; Bouzerara, F.; Guechi, A.; Boudaira, B. Effect of sodium phosphate addition on mechanical properties of porous Sigue quartz sand. Desalin. Water Treat. 2016, 57, 5286–5291. [Google Scholar] [CrossRef]
- Sánchez de Rojas Gómez, M.I.; Frías Rojas, M. Natural pozzolans in eco-efficient concrete. In Eco-Efficient Concrete; Woodhead Publishing: Cambridge, UK, 2013; pp. 83–104. [Google Scholar]
- Achiou, B.; Elomari, H.; Ouammou1, M.; Albizane1, A.; Bennazha1, J.; Aaddane1, A. Study of added starch on characteristics of flat ceramic microfiltration membrane made from natural Moroccan pozzolan. J. Mater. Environ. Sci. 2018, 9, 1013–1021. [Google Scholar]
- Achiou, B.; Elomari, H.; Bouazizi, A.; Karim, A.; Ouammou, M.; Albizane, A.; Bennazha, J.; Younssi, S.A.; El Amrani, I.E. Manufacturing of tubular ceramic microfiltration membrane based on natural pozzolan for pretreatment of seawater desalination. Desalination 2017, 419, 181–187. [Google Scholar] [CrossRef]
- Karim, A.; Achiou, B.; Bouazizi, A.; Aaddane, A.; Ouammou, M.; Bouziane, M.; Bennazha, J.; Younssi, S.A. Development of reduced graphene oxide membrane on flat Moroccan ceramic pozzolan support. Application for soluble dyes removal. J. Environ. Chem. Eng. 2018, 6, 1475–1485. [Google Scholar] [CrossRef]
- Achiou, B.; Beqqour, D.; Elomari, H.; Bouazizi, A.; Ouammou, M.; Bouhria, M.; Aaddane, A.; Khiat, K.; Younssi, S.A. Preparation of inexpensive NaA zeolite membrane on pozzolan support at low temperature for dehydration of alcohol solutions. J. Environ. Chem. Eng. 2018, 6, 4429–4437. [Google Scholar] [CrossRef]
- Yao, Z.T.; Ji, X.S.; Sarker, P.K.; Tang, J.H.; Ge, L.Q.; Xia, M.S.; Xi, Y.Q. A comprehensive review on the applications of coal fly ash. Earth-Sci. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef]
- Belviso, C. State-of-the-art applications of fly ash from coal and biomass: A focus on zeolite synthesis processes and issues. Prog. Energy Combust. Sci. 2018, 65, 109–135. [Google Scholar] [CrossRef]
- Golewski, G.L. Improvement of fracture toughness of green concrete as a result of addition of coal fly ash. Characterization of fly ash microstructure. Mater. Charact. 2017, 134, 335–346. [Google Scholar] [CrossRef]
- Ilic, M.; Cheeseman, C.; Sollars, C.; Knight, J. Mineralogy and microstructure of sintered lignite coal fly ash☆. Fuel 2003, 82, 331–336. [Google Scholar] [CrossRef]
- Koukouzas, N.; Hämäläinen, J.; Papanikolaou, D.; Tourunen, A.; Jäntti, T. Mineralogical and elemental composition of fly ash from pilot scale fluidised bed combustion of lignite, bituminous coal, wood chips and their blends. Fuel 2007, 86, 2186–2193. [Google Scholar] [CrossRef]
- Jedidi, I.; Saïdi, S.; Khemakhem, S.; Larbot, A.; Elloumi-Ammar, N.; Fourati, A.; Charfi, A.; Salah, A.B.; Amar, R.B. Elaboration of new ceramic microfiltration membranes from mineral coal fly ash applied to waste water treatment. J. Hazard. Mater. 2009, 172, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Jedidi, I.; Khemakhem, S.; Larbot, A.; Amar, R.B. Elaboration and characterisation of fly ash based mineral supports for microfiltration and ultrafiltration membranes. Ceram. Int. 2009, 35, 2747–2753. [Google Scholar] [CrossRef]
- Dong, Y.; Zhou, J.-e.; Lin, B.; Wang, Y.; Wang, S.; Miao, L.; Lang, Y.; Liu, X.; Meng, G. Reaction-sintered porous mineral-based mullite ceramic membrane supports made from recycled materials. J. Hazard. Mater. 2009, 172, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Hampshire, S.; Zhou, J.-e.; Lin, B.; Ji, Z.; Zhang, X.; Meng, G. Recycling of fly ash for preparing porous mullite membrane supports with titania addition. J. Hazard. Mater. 2010, 180, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Dong, X.; Li, L.; Dong, Y.; Hampshire, S. Recycling of waste fly ash for production of porous mullite ceramic membrane supports with increased porosity. J. Eur. Ceram. Soc. 2014, 34, 3181–3194. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, L.; Dong, Y.; Li, L.; Liu, J. Waste-to-Resource Strategy to Fabricate Highly Porous Whisker-Structured Mullite Ceramic Membrane for Simulated Oil-in-Water Emulsion Wastewater Treatment. ACS Sustain. Chem. Eng. 2016, 4, 2098–2106. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, M.; Dong, Y.; Tang, C.Y.; Huang, A.; Li, L. A low-cost mullite-titania composite ceramic hollow fiber microfiltration membrane for highly efficient separation of oil-in-water emulsion. Water Res. 2016, 90, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Dong, Y.; Hampshire, S.; Cerneaux, S.; Winnubst, L. Waste-to-resource preparation of a porous ceramic membrane support featuring elongated mullite whiskers with enhanced porosity and permeance. J. Eur. Ceram. Soc. 2015, 35, 711–721. [Google Scholar] [CrossRef]
- Zhu, L.; Dong, Y.; Li, L.; Liu, J.; You, S.-J. Coal fly ash industrial waste recycling for fabrication of mullite-whisker-structured porous ceramic membrane supports. RSC Adv. 2015, 5, 11163–11174. [Google Scholar] [CrossRef]
- Liu, J.; Dong, Y.; Dong, X.; Hampshire, S.; Zhu, L.; Zhu, Z.; Li, L. Feasible recycling of industrial waste coal fly ash for preparation of anorthite-cordierite based porous ceramic membrane supports with addition of dolomite. J. Eur. Ceram. Soc. 2016, 36, 1059–1071. [Google Scholar] [CrossRef]
- Rawat, M.; Bulasara, V.K. Synthesis and characterization of low-cost ceramic membranes from fly ash and kaolin for humic acid separation. Korean J. Chem. Eng. 2018, 35, 725–733. [Google Scholar] [CrossRef]
- Singh, G.; Bulasara, V.K. Preparation of low-cost microfiltration membranes from fly ash. Desalin. Water Treat. 2013, 267, 1–9. [Google Scholar] [CrossRef]
- Wei, Z.; Hou, J.; Zhu, Z. High-aluminum fly ash recycling for fabrication of cost-effective ceramic membrane supports. J. Alloys Compd. 2016, 683, 474–480. [Google Scholar] [CrossRef]
- Suresh, K.; Pugazhenthi, G. Development of ceramic membranes from low-cost clays for the separation of oil–water emulsion. Desalin. Water Treat. 2016, 57, 1927–1939. [Google Scholar] [CrossRef]
- Suresh, K.; Pugazhenthi, G.; Uppaluri, R. Fly ash based ceramic microfiltration membranes for oil-water emulsion treatment: Parametric optimization using response surface methodology. J. Water Process Eng. 2016, 13, 27–43. [Google Scholar] [CrossRef]
- Dong, Y.; Hampshire, S.; Zhou, J.-e.; Ji, Z.; Wang, J.; Meng, G. Sintering and characterization of flyash-based mullite with MgO addition. J. Eur. Ceram. Soc. 2011, 31, 687–695. [Google Scholar] [CrossRef]
- Zhu, L.; Ji, J.; Wang, S.; Xu, C.; Yang, K.; Xu, M. Removal of Pb(II) from wastewater using Al2O3-NaA zeolite composite hollow fiber membranes synthesized from solid waste coal fly ash. Chemosphere 2018, 206, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Qin, G.; Wei, W.; Zhao, X. Preparation and characterization of tubular supported ceramic microfiltration membranes from fly ash. Sep. Purif. Technol. 2011, 80, 585–591. [Google Scholar] [CrossRef]
- Zou, D.; Qiu, M.; Chen, X.; Drioli, E.; Fan, Y. One step co-sintering process for low-cost fly ash based ceramic microfiltration membrane in oil-in-water emulsion treatment. Sep. Purif. Technol. 2019, 210, 511–520. [Google Scholar] [CrossRef]
- Rice | Statista. Available online: https://www.statista.com/study/14593/rice-statista-dossier/ (accessed on 29 January 2019).
- Hossain, S.S.K.; Mathur, L.; Roy, P.K. Rice husk/rice husk ash as an alternative source of silica in ceramics: A review. J. Asian Ceram. Soc. 2018, 6, 299–313. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.; Gupta, V.K. Rice Husk and Its Ash as Low-Cost Adsorbents in Water and Wastewater Treatment. Ind. Eng. Chem. Res. 2011, 50, 13589–13613. [Google Scholar] [CrossRef]
- Bhavornthanayod, C.; Rungrojchaipon, P. Synthesis of zeolite A membrane from rice husk ash. J. Met. Mater. Miner. 2017, 19, 79–83. [Google Scholar]
- Hubadillah, S.K.; Othman, M.H.D.; Harun, Z.; Ismail, A.F.; Rahman, M.A.; Jaafar, J. A novel green ceramic hollow fiber membrane (CHFM) derived from rice husk ash as combined adsorbent-separator for efficient heavy metals removal. Ceram. Int. 2017, 43, 4716–4720. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Ismail, A.F.; Rahman, M.A.; Jaafar, J.; Iwamoto, Y.; Honda, S.; Dzahir, M.I.H.M.; Yusop, M.Z.M. Fabrication of low cost, green silica based ceramic hollow fibre membrane prepared from waste rice husk for water filtration application. Ceram. Int. 2018, 44, 10498–10509. [Google Scholar] [CrossRef]
- Verma, D.; Gope, P.C.; Maheshwari, M.K.; Sharma, R.K. Bagasse Fiber Composites—A Review. J. Mater. Environ. Sci. 2012, 3, 1079–1092. [Google Scholar]
- Souza, A.E.; Teixeira, S.R.; Santos, G.T.A.; Costa, F.B.; Longo, E. Reuse of sugarcane bagasse ash (SCBA) to produce ceramic materials. J. Environ. Manag. 2011, 92, 2774–2780. [Google Scholar] [CrossRef] [PubMed]
- Faria, K.C.P.; Gurgel, R.F.; Holanda, J.N.F. Recycling of sugarcane bagasse ash waste in the production of clay bricks. J. Environ. Manag. 2012, 101, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Jamalludin, M.R.; Harun, Z.; Othman, M.H.D.; Hubadillah, S.K.; Yunos, M.Z.; Ismail, A.F. Morphology and property study of green ceramic hollow fiber membrane derived from waste sugarcane bagasse ash (WSBA). Ceram. Int. 2018, 44, 18450–18461. [Google Scholar] [CrossRef]
- Jamalludin, M.R.; Hubadillah, S.K.; Harun, Z.; Othman, M.H.D.; Yunos, M.Z. Novel superhydrophobic and superoleophilic sugarcane green ceramic hollow fibre membrane as hybrid oil sorbent-separator of real oil and water mixture. Mater. Lett. 2019, 240, 136–139. [Google Scholar] [CrossRef]
- Jamalludin, M.R.; Hubadillah, S.K.; Harun, Z.; Othman, M.H.D.; Yunos, M.Z.; Ismail, A.F.; Salleh, W.N.W. Facile fabrication of superhydrophobic and superoleophilic green ceramic hollow fiber membrane derived from waste sugarcane bagasse ash for oil/water separation. Arab. J. Chem. 2018. [Google Scholar] [CrossRef]
- Bouzerara, F.; Harabi, A.; Achour, S.; Larbot, A. Porous ceramic supports for membranes prepared from kaolin and doloma mixtures. J. Eur. Ceram. Soc. 2006, 26, 1663–1671. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry; Thomas Telford: Heron Quays, London, UK, 1997. [Google Scholar]
- Lea, F.M.; Hewlett, P.C. Lea’s Chemistry of Cement and Concrete, 4th ed.; Elservier Butterworth- Heinemann: Oxford, MA, USA, 2008. [Google Scholar]
- Bullard, J.W.; Jennings, H.M.; Livingston, R.A.; Nonat, A.; Scherer, G.W.; Schweitzer, J.S.; Scrivener, K.L.; Thomas, J.J. Mechanisms of cement hydration. Cem. Concr. Res. 2011, 41, 1208–1223. [Google Scholar] [CrossRef]
- Hover, K.C. The influence of water on the performance of concrete. Constr. Build. Mater. 2011, 25, 3003–3013. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Z.; Chang, J.; Shen, J.; Kang, J.; Yang, L.; Chen, Q. A novel cementitious microfiltration membrane: Mechanisms of pore formation and properties for water permeation. RSC Adv. 2015, 5, 99–108. [Google Scholar] [CrossRef]
- Vočka, R.; Gallé, C.; Dubois, M.; Lovera, P. Mercury intrusion porosimetry and hierarchical structure of cement pastes. Cem. Concr. Res. 2000, 30, 521–527. [Google Scholar] [CrossRef]
- Honorio, T.; Bary, B.; Benboudjema, F. Thermal properties of cement-based materials: Multiscale estimations at early-age. Cem. Concr. Compos. 2018, 87, 205–219. [Google Scholar] [CrossRef]
- Kumar, L.R.; Karthikeyan, M.; Raghu, R. Influence of Water-Cement Ratio on Compressive Strength of burnt bricks. IOSR JMCE 2017, 14, 91–94. [Google Scholar] [CrossRef]
- Alawad, O.A.; Alhozaimy, A.; Jaafar, M.S.; Aziz, F.N.A.; Al-Negheimish, A. Effect of Autoclave Curing on the Microstructure of Blended Cement Mixture Incorporating Ground Dune Sand and Ground Granulated Blast Furnace Slag. Int. J. Concr. Struct. Mater. 2015, 9, 381–390. [Google Scholar] [CrossRef]
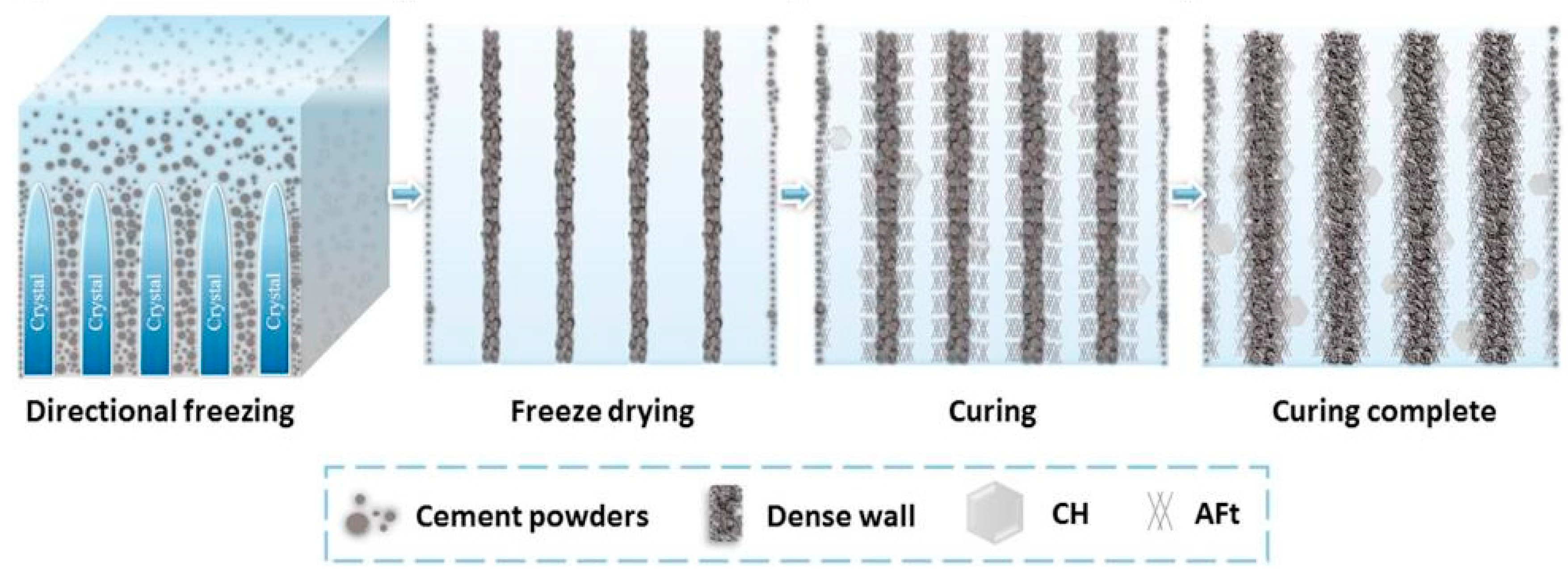
- Dong, S.; Zhu, W.; Gao, X.; Wang, Z.; Wang, L.; Wang, X.; Gao, C. Preparation of tubular hierarchically porous silicate cement compacts via a tert-butyl alcohol (TBA)-based freeze casting method. Chem. Eng. J. 2016, 295, 530–541. [Google Scholar] [CrossRef]
- Scotti, K.L.; Dunand, D.C. Freeze casting—A review of processing, microstructure and properties via the open data repository, FreezeCasting.net. Prog. Mater. Sci. 2018, 94, 243–305. [Google Scholar] [CrossRef]
- Dong, S.; Wang, L.; Gao, X.; Zhu, W.; Wang, Z.; Ma, Z.; Gao, C. Freeze casting of novel porous silicate cement supports using tert-butyl alcohol-water binary crystals as template: Microstructure, strength and permeability. J. Membr. Sci. 2017, 541, 143–152. [Google Scholar] [CrossRef]
- Dong, S.; Gao, X.; Ma, Z.; Wang, X.; Gao, C. Ice-templated porous silicate cement with hierarchical porosity. Mater. Lett. 2018, 217, 292–295. [Google Scholar] [CrossRef]
- Vaitkevičius, V.; Vaičiukynienė, D.; Kantautas, A.; Kartovickis, A.; Rudžionis, Ž. Blended Cements Produced with Synthetic Zeolite Made from Industrial By-Product. Mater. Sci. 2015, 21, 136–142. [Google Scholar] [CrossRef][Green Version]
- C09 Committee. Specification for Ready-Mixed Concrete; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- Davidovits, J. Geopolymers. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Davidovits, J. (Ed.) Geopolymer Chemistry and Applications, 4th ed.; Institut Géopolymère: Saint-Quentin, France, 2015. [Google Scholar]
- Villaquirán-Caicedo, M.A.; de Gutiérrez, R.M. Synthesis of ceramic materials from ecofriendly geopolymer precursors. Mater. Lett. 2018, 230, 300–304. [Google Scholar] [CrossRef]
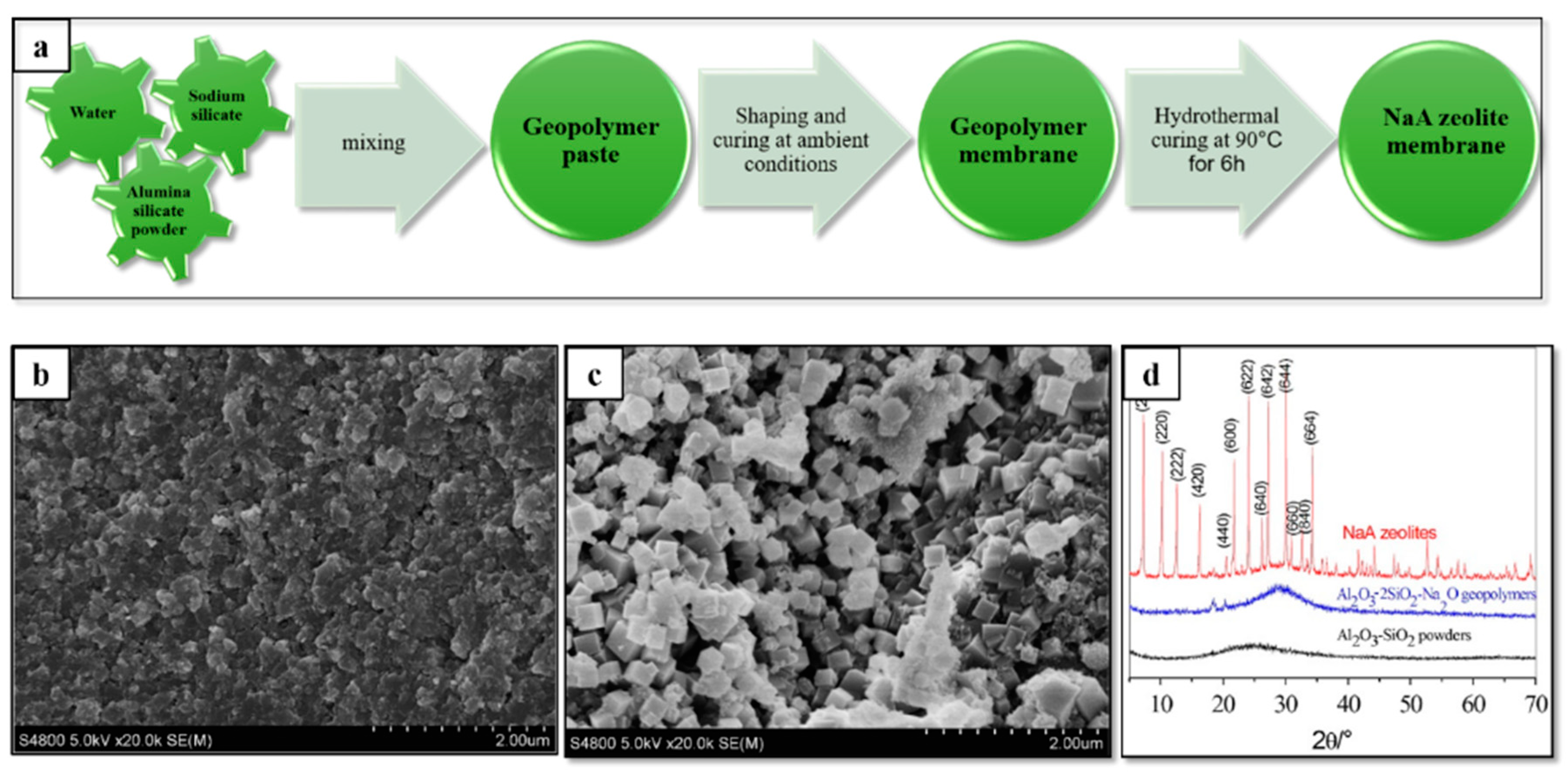
- Xu, M.-X.; He, Y.; Wang, C.-Q.; He, X.-F.; He, X.-Q.; Liu, J.; Cui, X.-M. Preparation and characterization of a self-supporting inorganic membrane based on metakaolin-based geopolymers. Appl. Clay Sci. 2015, 115, 254–259. [Google Scholar] [CrossRef]
- He, Y.; Cui, X.-M.; Liu, X.-D.; Wang, Y.-P.; Zhang, J.; Liu, K. Preparation of self-supporting NaA zeolite membranes using geopolymers. J. Membr. Sci. 2013, 447, 66–72. [Google Scholar] [CrossRef]
- Ge, Y.; Yuan, Y.; Wang, K.; He, Y.; Cui, X. Preparation of geopolymer-based inorganic membrane for removing Ni(2+) from wastewater. J. Hazard. Mater. 2015, 299, 711–718. [Google Scholar] [CrossRef]
- Zhang, P.; Zheng, Y.; Wang, K.; Zhang, J. A review on properties of fresh and hardened geopolymer mortar. Compos. Part B Eng. 2018, 152, 79–95. [Google Scholar] [CrossRef]
- Human Development Indices and Indicators 2018; United Nations: New York, NY, USA, 2018.
- Low-cost ceramic membranes: A research opportunity for industrial application. J. Eur. Ceram. Soc. 2019, 39, 3392–3407. [CrossRef]
- Zhu, L.; Rakesh, K.P.; Xu, M.; Dong, Y. Ceramic-Based Composite Membrane with a Porous Network Surface Featuring a Highly Stable Flux for Drinking Water Purification. Membranes 2019, 9, 5. [Google Scholar] [CrossRef]
- Dong, Y.; Ma, L.; Tang, C.Y.; Yang, F.; Quan, X.; Jassby, D.; Zaworotko, M.J.; Guiver, M.D. Stable Superhydrophobic Ceramic-Based Carbon Nanotube Composite Desalination Membranes. Nano Lett. 2018, 18, 5514–5521. [Google Scholar] [CrossRef]
- Das, B.; Chakrabarty, B.; Barkakati, P. Separation of oil from oily wastewater using low cost ceramic membrane. Korean J. Chem. Eng. 2017, 34, 2559–2569. [Google Scholar] [CrossRef]
- Bensadok, K.; Belkacem, M.; Nezzal, G. Treatment of cutting oil/water emulsion by coupling coagulation and dissolved air flotation. Desalination 2007, 206, 440–448. [Google Scholar] [CrossRef]
- Abadi, S.R.H.; Sebzari, M.R.; Hemati, M.; Rekabdar, F.; Mohammadi, T. Ceramic membrane performance in microfiltration of oily wastewater. Desalination 2011, 265, 222–228. [Google Scholar] [CrossRef]
- Chang, Q.; Zhou, J.-E.; Wang, Y.; Liang, J.; Zhang, X.; Cerneaux, S.; Wang, X.; Zhu, Z.; Dong, Y. Application of ceramic microfiltration membrane modified by nano-TiO2 coating in separation of a stable oil-in-water emulsion. J. Membr. Sci. 2014, 456, 128–133. [Google Scholar] [CrossRef]
- Koutsonikolas, D.E.; Pantoleontos, G.; Kaldis, S.P.; Zaspalis, V.T.; Sakellaropoulos, G.P. Preparation and Characterization of Novel Titania-Modified Ceramic Membranes. Procedia Eng. 2012, 44, 908–909. [Google Scholar] [CrossRef][Green Version]
- Vasanth, D.; Suresh, K.; Pugazhenthi, G. Fabrication of circular shaped ceramic membrane using mixed clays by uniaxial compaction method for the treatment of oily wastewater. IJNBM 2014, 5, 75. [Google Scholar] [CrossRef]
- Zhu, L.; Dong, X.; Xu, M.; Yang, F.; Guiver, M.D.; Dong, Y. Fabrication of mullite ceramic-supported carbon nanotube composite membranes with enhanced performance in direct separation of high-temperature emulsified oil droplets. J. Membr. Sci. 2019, 582, 140–150. [Google Scholar] [CrossRef]
- Hua, F.L.; Tsang, Y.F.; Wang, Y.J.; Chan, S.Y.; Chua, H.; Sin, S.N. Performance study of ceramic microfiltration membrane for oily wastewater treatment. Chem. Eng. J. 2007, 128, 169–175. [Google Scholar] [CrossRef]
- Zhou, J.-E.; Chang, Q.; Wang, Y.; Wang, J.; Meng, G. Separation of stable oil–water emulsion by the hydrophilic nano-sized ZrO2 modified Al2O3 microfiltration membrane. Sep. Purif. Technol. 2010, 75, 243–248. [Google Scholar] [CrossRef]
- Suresh, K.; Pugazhenthi, G. Cross flow microfiltration of oil-water emulsions using clay based ceramic membrane support and TiO2 composite membrane. Egyp. J. Petrol. 2017, 26, 679–694. [Google Scholar] [CrossRef]
- Zielinski, R.A.; Finkelman, R.B. Radioactive Elements in Coal and Fly Ash: Abundance, Forms, and Environmental Significance; US Geological Survey No. 163-97; US Geological Survey: Reston, VA, USA, 1997.
- Dahl, O.; Pöykiö, R.; Nurmesniemi, H. Concentrations of heavy metals in fly ash from a coal-fired power plant with respect to the new Finnish limit values. J. Mater. Cycles Waste Manag. 2008, 10, 87–92. [Google Scholar] [CrossRef]
- Alharbi, W.R.; El-Taher, A. Elemental Analysis and Natural Radioactivity Levels of Clay by Gamma Ray Spectrometer and Instrumental Neutron Activation Analysis. Sci. Technol. Nucl. Install. 2016, 2016, 1–5. [Google Scholar] [CrossRef]
- Chen, Y.-M.; Gao, J.-B.; Yuan, Y.-Q.; Ma, J.; Yu, S. Relationship between heavy metal contents and clay mineral properties in surface sediments: Implications for metal pollution assessment. Cont. Shelf Res. 2016, 124, 125–133. [Google Scholar] [CrossRef]
- Alkhomashi, N.; Almasoud, F.I.; Alhorayess, O.; Alajayan, T.M.; Alsalamah, A.S.; Alssalim, Y.A.; Ababneh, Z.Q. Assessment of radioactivity and trace elements of cement produced in Saudi Arabia. Environ. Earth Sci. 2017, 76, 21. [Google Scholar] [CrossRef]
- Dong, Y.; Feng, X.; Dong, D.; Wang, S.; Yang, J.; Gao, J.; Liu, X.; Meng, G. Elaboration and chemical corrosion resistance of tubular macro-porous cordierite ceramic membrane supports. J. Membr. Sci. 2007, 304, 65–75. [Google Scholar] [CrossRef]
- Koros, W.J.; Mahajan, R. Pushing the limits on possibilities for large scale gas separation: Which strategies? J. Membr. Sci. 2000, 175, 181–196. [Google Scholar] [CrossRef]
- Ghosh, D.; Sinha, M.K.; Purkait, M.K. A comparative analysis of low-cost ceramic membrane preparation for effective fluoride removal using hybrid technique. Desalination 2013, 327, 2–13. [Google Scholar] [CrossRef]
- Bose, S.; Das, C. Role of Binder and Preparation Pressure in Tubular Ceramic Membrane Processing: Design and Optimization Study Using Response Surface Methodology (RSM). Ind. Eng. Chem. Res. 2014, 53, 12319–12329. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Harun, Z.; Ismail, A.F.; Iwamoto, Y.; Honda, S.; Rahman, M.A.; Jaafar, J.; Gani, P.; Sokri, M.N.M. Effect of fabrication parameters on physical properties of metakaolin-based ceramic hollow fibre membrane (CHFM). Ceram. Int. 2016, 42, 15547–15558. [Google Scholar] [CrossRef]
- White, M.A.; Conrad, J.; Chen, R.; Romao, C.; Pereira, A.; Hill, I. Applications of ice-templated ceramics. Int. J. Appl. Ceram. Technol. 2018, 15, 1075–1083. [Google Scholar] [CrossRef]
- Low, Z.-X.; Chua, Y.T.; Ray, B.M.; Mattia, D.; Metcalfe, I.S.; Patterson, D.A. Perspective on 3D printing of separation membranes and comparison to related unconventional fabrication techniques. J. Membr. Sci. 2017, 523, 596–613. [Google Scholar] [CrossRef]
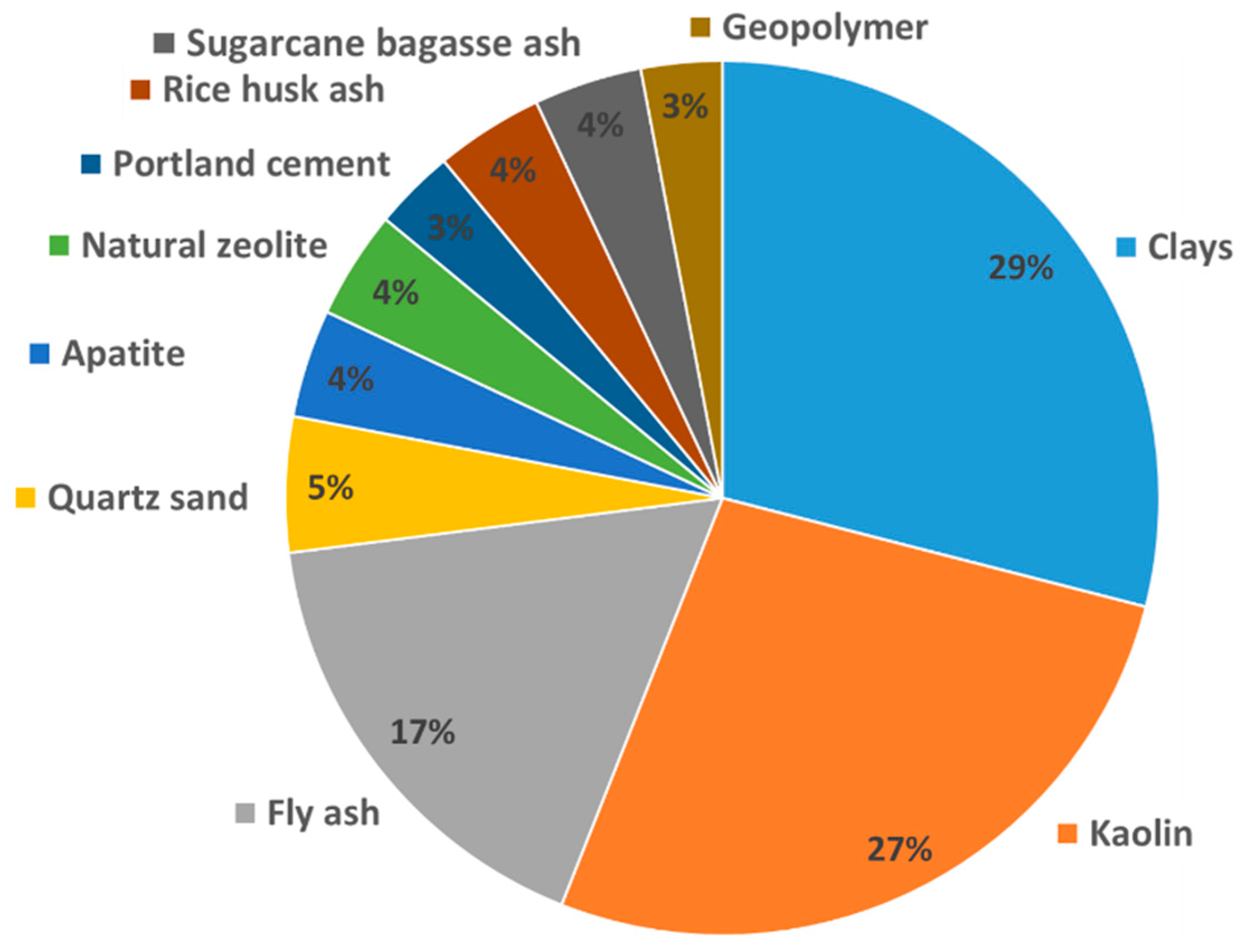
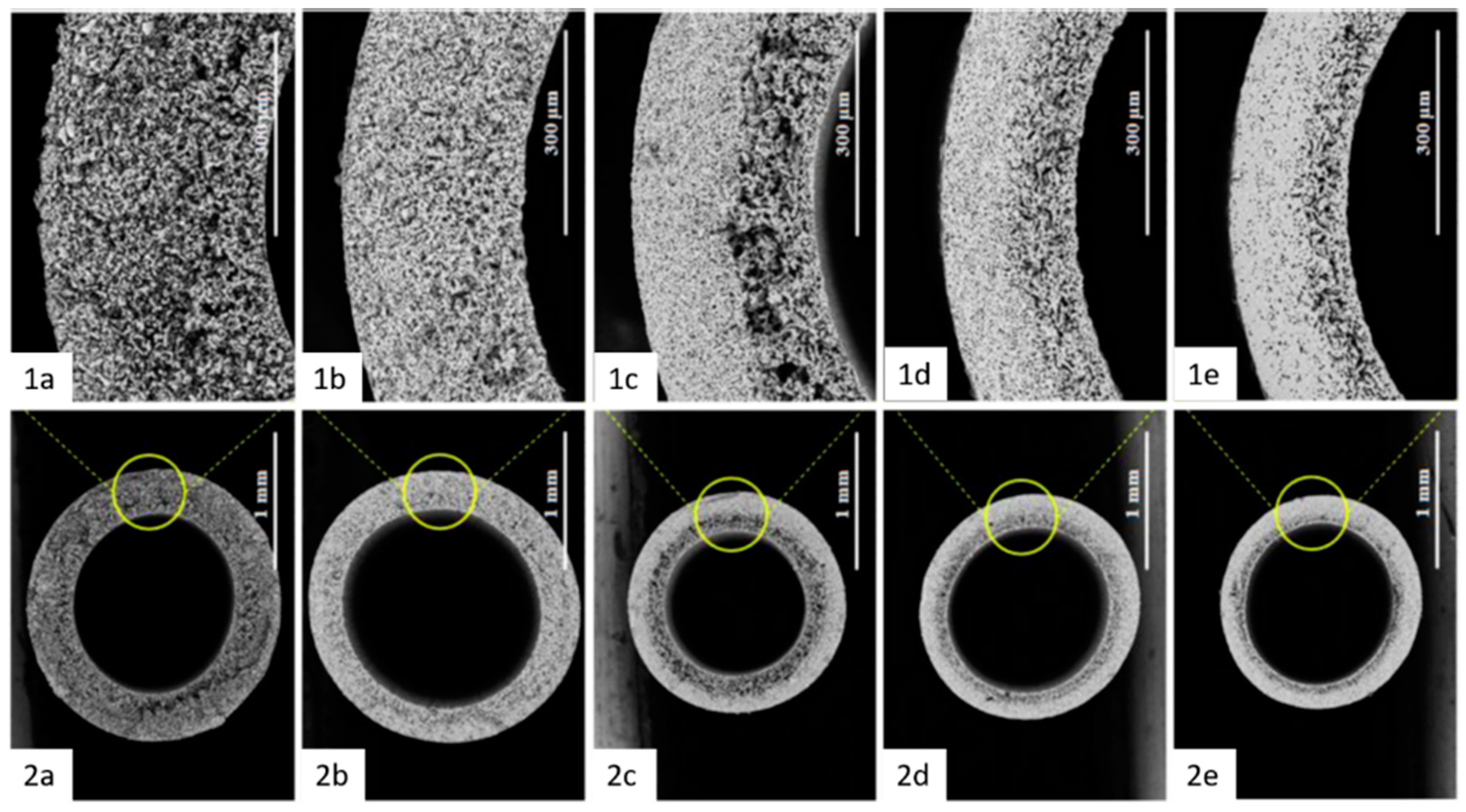
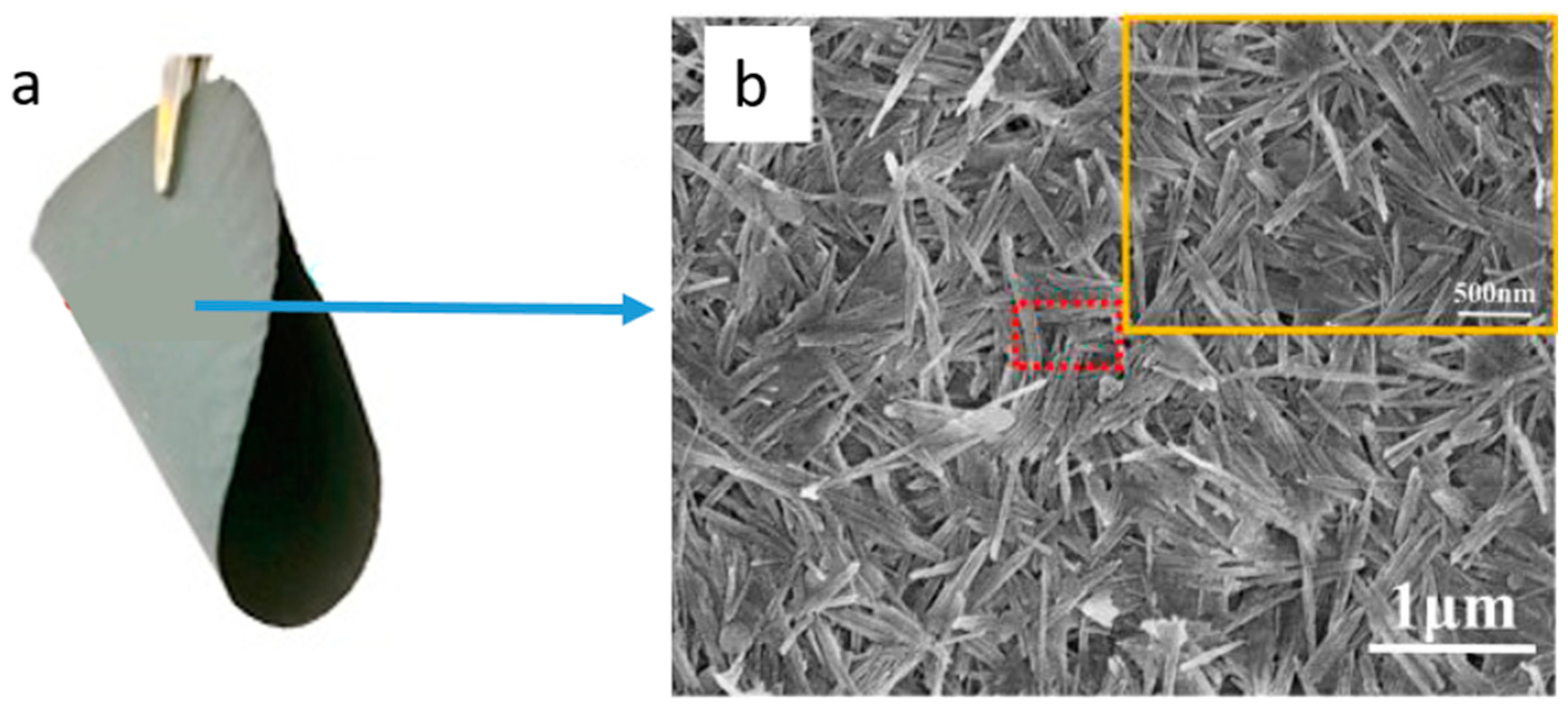

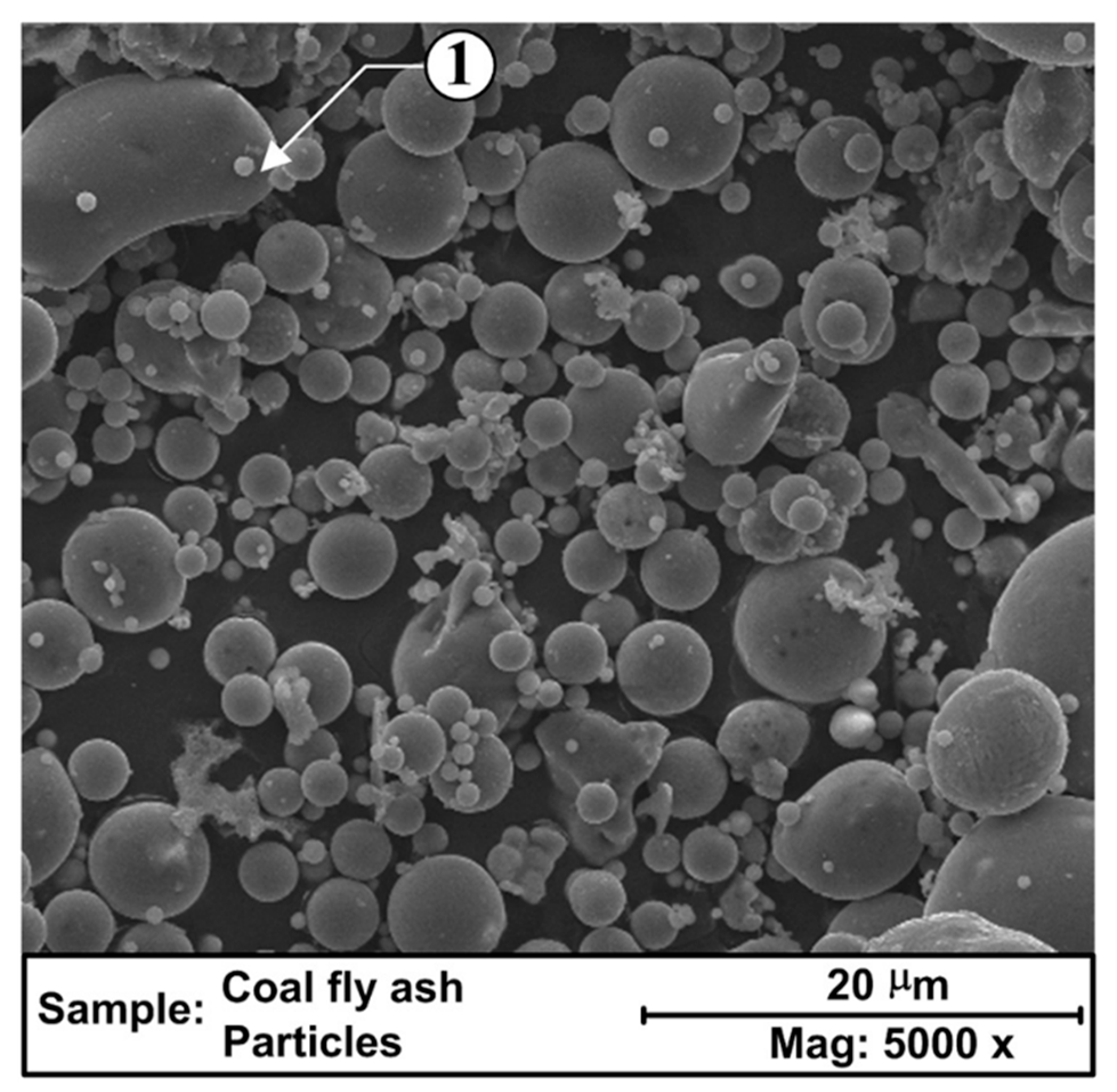
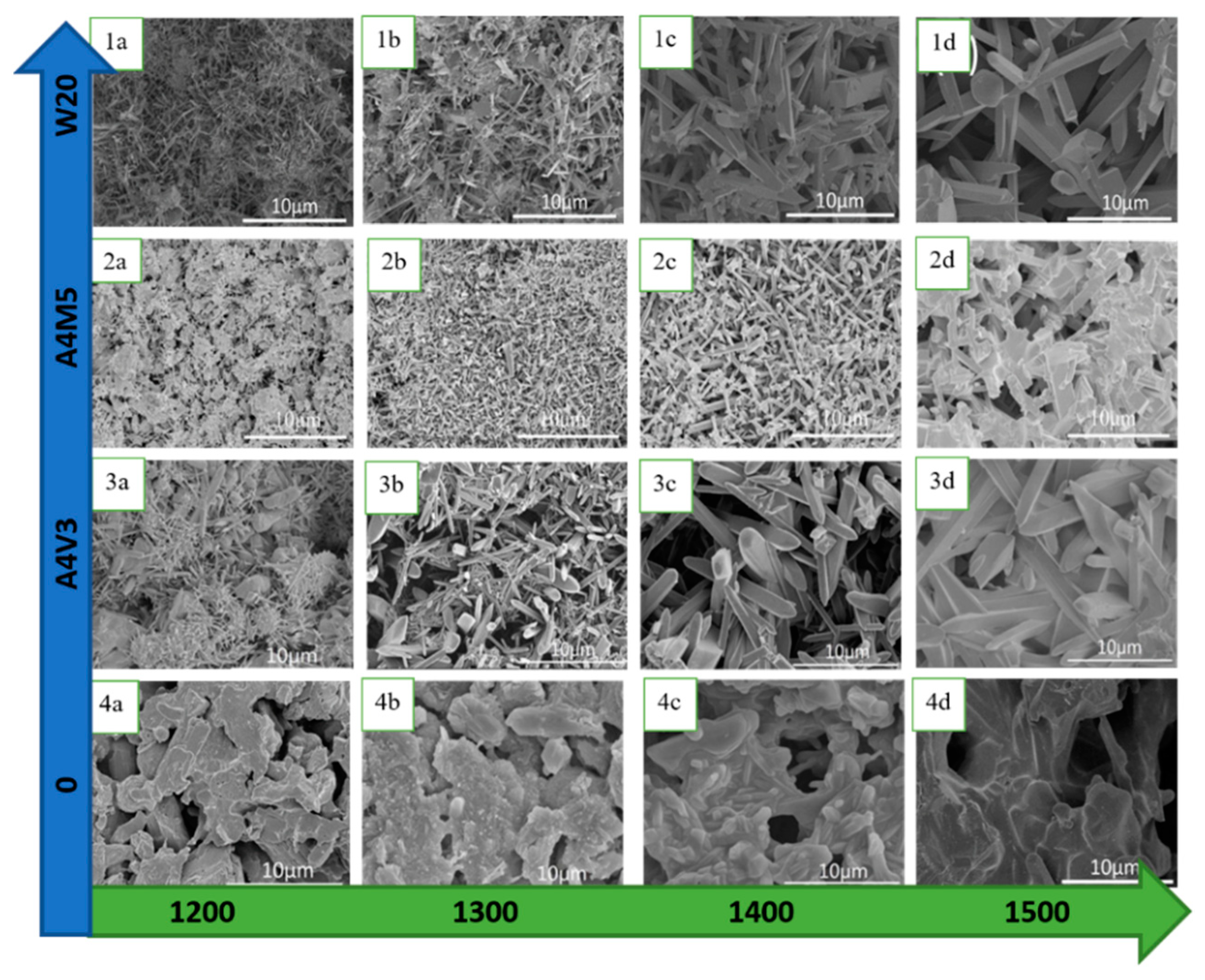
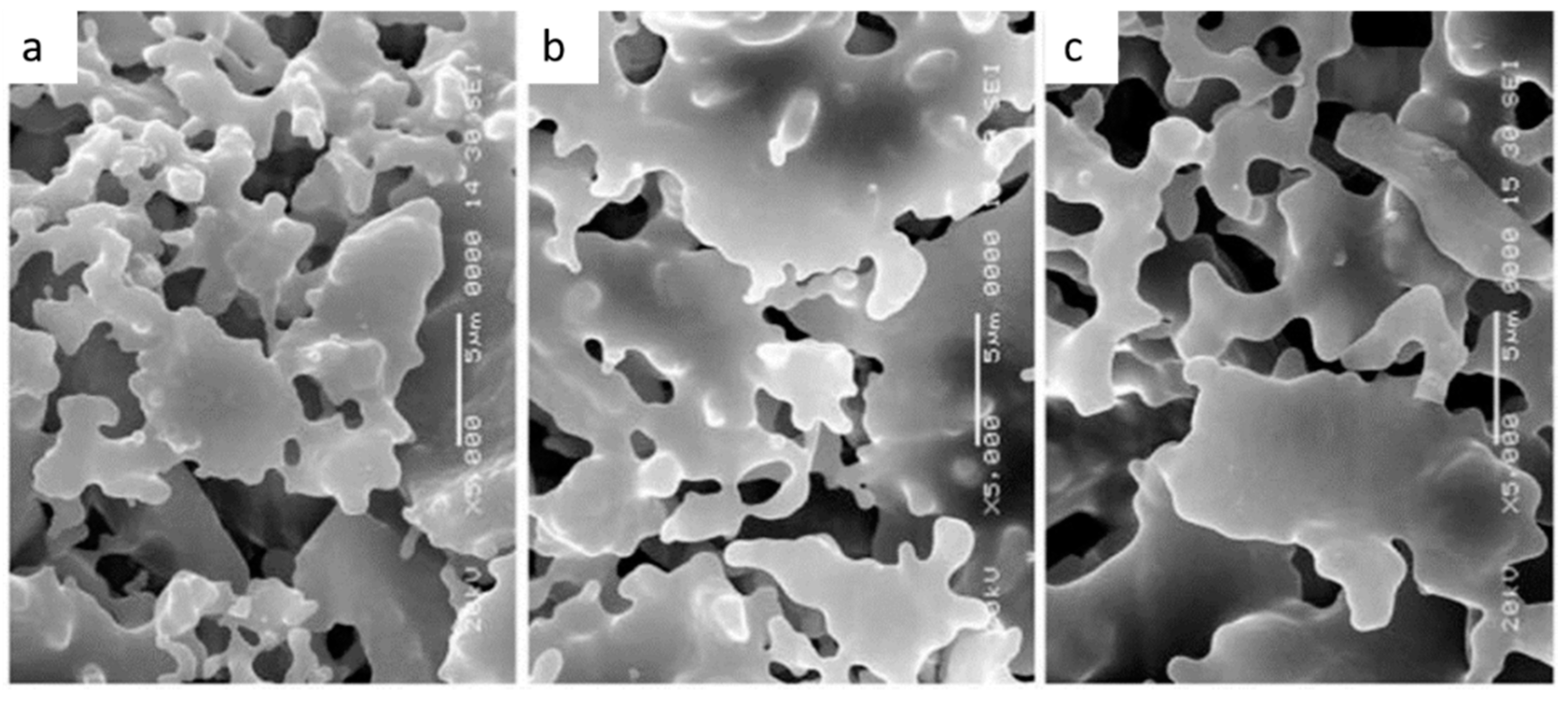
| Materials Mixed with Kaolin | Shaping Technique | Sintering Temperature, °C | Porosity, % | Pore Size, µm | Flexural Strength, MPa | Application |
|---|---|---|---|---|---|---|
| Quartz, sodium carbonate, calcium carbonate, and boric acid | Paste casting | 850–1000 | 33–42 | 0.55–0.81 | 3–8 | MF [20] |
| Quartz, calcium carbonate, sodium carbonate, boric acid, and sodium metasilicate | Pressing | 900 | 35–39 | 0.72–1.69 | 7–11 | MF of mosambi juice [30] |
| Quartz, calcium carbonate, sodium carbonate, boric acid, and sodium metasilicate | Pressing | 900 | 30–37 | 2–3 | - | MF of oil-in-water emulsions [31] |
| Quartz, ball clay, pyrophyllite, and feldspar | Extrusion | 950 | 53 | 0.31 | 12 | MF of oil in water emulsion [37] |
| Quartz and calcium carbonate | Pressing | 900–1000 | 30 | 1.3 | 34 | MF of oil and bacteria [11] |
| Limestone | Extrusion | 800–1100 | 48 | 7 | 30 | Support layer [38] |
| Lime | Extrusion | 800–1100 | 47 | 8 | 30–53 | Support layer [28] |
| Feldspar, sodium metasilicate nanohydrate, and boric acid | Pressing | 850 | 29 | 0.93 | 8.7 | MF [39] |
| Dolomite | Pressing and Extrusion | 1000–1300 | 37–56 | 1.6–48 | 6–15 | Support layer [40] |
| Extrusion | 1100–1300 | 44.6 | 4.7 | 47.6 | Support layer [17] | |
| Calcium carbonate | Extrusion | 1250 | 52 | 4.0 | 23 | Support layer [41] |
| Extrusion | 1150–1300 | 42–50 | 4–8 | 67–77 | Support layer [42] | |
| Calcite | Extrusion | 1150 | 50 | 4 | 28 | Support layer [19] |
| Pressing and extrusion | 1300 and 1100–1250 | 49 | 3 | 87 | Support layer [18] | |
| Bentonite, talc, sodium borate, and carbon black | Pressing | 1000 | 34 | 0.65–1.25 | 58 | MF of oil-in-water emulsion [32] |
| Bauxite | Pressing | 1300–1600 | 31 | 0.15–0.8 | 100 * | MF [26] |
| Ball clay, quartz, alumina, and calcium carbonate | Paste casting | 1100–1400 | 35–46 | 0.1–1 | 20–60 | MF [29] |
| Ball clay, feldspar, calcium carbonate, and pyrophyllite | Pressing | 800–1000 | 44 | 1.01 | 28 | Support layer [12] |
| Alumina and aluminum hydroxide | Pressing | 1300–1550 | 46 | 1.3 | - | Support layer [27] |
| Without reactive additives | Support: extrusion; MF layer: slip casting | Support: 1000–1250; MF layer: 1050 | 46–60 | 0.9–1.4 | 4–24 | MF [43] |
| Extrusion | 1150 | 49 | 1.2 | 5.8 | Solid particle removal from water [36] | |
| Extrusion | 1200–1500 | - | 0.32 | 221 | Arsenic removal and oil removal [35,44] | |
| Extrusion | 1100–1250 | 27 | 0.76 | 28 | MF of cuttlefish effluent [34] | |
| Extrusion | 1200–1500 | 32–57 | 0.53–4.25 | 15–35 | MF of oil-in-water emulsion [25] | |
| Extrusion | 1200–1500 | - | 0.4–0.5 | 70 | MF of wastewater (oil and dye) [22] | |
| Pressing | 1050–1100 | 43 | 0.5 | 20 | MF [33] | |
| Support: pressing; UF: dip coating | Support: 900–1100; UF layer: 850–900 | Support 30–41; UF 27 | Support 1.4–6.3; UF 0.09 | - | UF [23] | |
| Pressing | 950 | 30 | 0.1 | 60 | MF [21] |
| Origin of Clay | Shaping Technique | Sintering Temperature, °C | Porosity, % | Pore Size, µm | Flexural Strength, MPa | Application |
|---|---|---|---|---|---|---|
| Argentina | Paste extrusion; slip casting | 1000; 1200–1400 | 50 | 0.08–0.55 | 16–34 | MF membrane [48] |
| Brazil | Pressing | 1050 | - | 0.1–2 | 4–16 | Water clarification from microalgae [49] |
| China | Dip coating | 600 | - | 3–10 nm | - | Removal of phosphate ions [46] |
| Pressing | 1100–1350 | - | 1.4–1.9 and 10 | 45–69 | Support layer [47] | |
| Paste casting | - | Above 50 | 10 nm | 12.5 | Oil-in-water emulsion filtration [50] | |
| Paste casting | - | Above 50 | 3.6–20 nm | 28 | Oily wastewater and protein separation [51] | |
| Paste casting | 400 | Above 60 | 12 nm | 5–7 | UF of oil-in-water emulsion [52] | |
| India | Paste casting | 800–1000 | 42 | 4.58 | 11.55 | Removal of chromate [20] |
| Paste extrusion | 950 | 53 | 0.309 | 12 | MF of oil-in-water emulsion [37] | |
| Iran | Pressing | 900 | 30 | 0.16–0.3 | - | Removal of cationic dyes [53] |
| Morocco | Pressing | 1000 | 25–40 | 0.01–1 | - | Support layer [54] |
| Paste extrusion | 1250 | 43 | 11 | 10 | Support layer [55] | |
| Paste extrusion | 1250 | - | - | - | Support layer [56] | |
| Pressing | 700–1100 | - | 0.1–10 | - | Support layer [57] | |
| Pressing | 950–1250 | - | 0.3–1.8 | - | Wastewater treatment [58] | |
| Extrusion | 800 | 41 | 11 | 15 | Support layer [59] | |
| Pressing | 950 | 28–40 | 1.5–2.8 | 14 | Support layer [60] | |
| Pressing | 800–1050 | 32 | 1.2 | 22 | Wastewater filtration [61] | |
| Pressing | 850–1000 | 23–34 | 1.4–1.8 | 14.6 | Support layer [62] | |
| Pressing | 1100 | 28 | 2.5 | 17.5 | MF membrane [63] | |
| Pressing | 1000–1200 | - | 0.08, 0.6 and 3.8 | - | Wastewater treatment [64] | |
| NA | Pressing | 1000–1100 | 36 | 0.29–0.67 | 27–32 | MF of oil-in-water emulsion [65] |
| Nigeria | Pressing | 1300 | - | 5–7 nm | 7–18 | UF of uranium from underground water [66] |
| Spain | Paste extrusion | 850–1050 | 29–38 | 0.3–0.8 | 10–17 | Support layer [67] |
| Pressing or Paste Extrusion | 1160 | 21–51 | 0.9–16 | 11–39 | Support layer [68] | |
| Tunisia | Paste and slip casting | 1080; 900 | 49 | SL: 6.3; MF: 0.18 | - | MF of cuttlefish effluent [69] |
| Slip casted | 800 | - | 15 nm | - | UF of solution purification [70] | |
| Paste extrusion | 900–1100 | 38 | 0.6–1.04 | 19 | Support layer [71] |
| Fabrication Technique | The Particle Size of Fly Ash (Additives) | Sintering Temperature °C | Porosity, % | Pore Size, µm | Flexural Strength, MPa | Application |
|---|---|---|---|---|---|---|
| Extrusion | <10 µm | 1100–1130 | 56–48 | 4.0–4.09 | 9.8–22.9 | Support layer [117] |
| - | 1100–1500 | 30 | 0.5–1.0 | 8.5–85.8 | Support layer [122] | |
| Extrusion followed slip casting | 15.41 µm; 5.01 µm; 1.41 µm | 1190; 1150; 1000 | - | 2.13; 1.94 and 0.77 | - | MF membranes [133] |
| Paste casting | - | 900 | 42 | 0.885 | 43.6 | MF of humic acid containing solution [126] |
| - | 800–1000 | 35–40 | 1.2 | 8–20 | MF of oil-in-water emulsions [127] | |
| Pressing | 1.52 µm | 1200–1550 | 35–45 | 0.93–2.2 | 22–65 | Support layer [118] |
| 15.09 µm | 1300–1500 | 39–44 | 6.52–7.28 | 28–36 | Support layer [119] | |
| 3.9 µm (bauxite 7.4 µm) | 1200–1500 | 50 | 0.27–1.18 | 69.8 | Support layer [120] | |
| 11.94 µm (bauxite 5.66 µm) | 1100–1500 | 52 | 0.67–1.78 | 34–87 | MF of oil-in-water emulsion [121] | |
| 2.1 µm (bauxite 1.2 µm) | 1100–1500 | 48 | 0.18–0.26 | 81.2 | Support layer [123] | |
| 2.1 µm (bauxite 1.2 µm) | 1100–1400 | 47.3 | 0.12–0.37 | 60–68 | Support layer [124] | |
| 1.14 µm, (dolomite 4.2 µm) | 1100–1200 | 46 | 0.32 | 73 | Support layer [125] | |
| 2.53 µm (CaCO3 9.15 µm) | 1200–1350 | 49.6 | 0.5–1.2 | 34–90 | Support layer [128] | |
| 1–100 µm | 1100 | 48 | 1.3–2.9 | 13 | MF of oil-in-water emulsions [129] | |
| 1–2.5 µm | 1100 | 30–43 | 1.75–2.0 | 1.68–9.23 | MF of oil-in-water emulsions [130] | |
| 1–20 µm (mullite fiber) | 800–1200 | 34 | 1–2 | 30 | Support layer [134] | |
| Slip casting | 1 µm | 800 | 51 | 0.25 | - | MF of textile industry effluent [116] |
| Main Materials | Pore Size, µm | Oil Droplet Size, µm | Feed Concentration, mg/L | Removal of Oil, % |
|---|---|---|---|---|
| Clay | 0.5 | 6.9 | 200 | 96 [179] |
| 0.65 | 2.84 | 100 | 96.7 [32] | |
| 0.012 | 0.050–0.150 | 1350 | 97.4 [52] | |
| Fly ash and bauxite | 0.48 | 2 | 250 | 99 [121] |
| Fly ash and titania | 0.11 | 1.1 | 200 | 97 [122] |
| Fly ash, quartz, and calcium carbonate | 1.36 | 6.9 | 200 | 97 [130] |
| Fly ash, quartz, titania | 1.32 | 6.9 | 200 | 99.2 [129] |
| Kaolin | 1.42–0.35 | 12 | - | 90–100 [25] |
| Kaolin, ball clay | 0.31 | 1.21 | 200 | 99.98 [37] |
| Kaolin, bentonite | <0.4 | 2.2 | 600 | 92.9 [65] |
| Kaolin, quartz | 2.2 | - | 400 | 98.5 [31] |
| Kaolin, quartz, calcium carbonate | 1.3 | 0.92 | 250 | 85 [11] |
| Sugarcane bagasse ash | 1.8 | - | - | 99.9 [146] |
| Mullite-carbon nanotube composite | 0.038 | 1.09 | 200 | 99.99 [180] |
| α-Alumina | 0.05 | - | 500 | 97.7 [181] |
| Zirconia/α-alumina | 0.2 | 1.79 | 1000 | >97.8 [182] |
| Titania composite | 0.9 | - | 200 | 99.56 [183] |
| The Material Used for the Preparation of Membrane | Cost of Raw Material (USD) |
|---|---|
| Clay, sodium metasilicate, sodium carbonate, and boric acid | 19 [20] |
| Fly ash quartz and calcium carbonate | 5 [130] |
| Fly ash, calcium carbonate, sodium carbonate, and boric acid | 17 [127] |
| Fly ash, quartz, calcium carbonate, and titania | 25 [129] |
| Fly ash and titania | 2 [122] |
| Kaolin, ball clay, feldspar, calcium carbonate, and pyrophyllite | 10 [12] |
| Kaolin, quartz, ball clay, pyrophyllite, and feldspar | 4 [37] |
| Kaolin, quartz, calcium carbonate | 61 [11] |
| Kaolin, quartz, calcium carbonate, sodium carbonate, boric acid, sodium metasilicate, and polyvinyl alcohol | 78 [30] |
| kaolin, quartz, calcium carbonate, sodium carbonate, boric acid, and sodium metasilicate | 130 [10] |
| 135 [191] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullayev, A.; Bekheet, M.F.; Hanaor, D.A.H.; Gurlo, A. Materials and Applications for Low-Cost Ceramic Membranes. Membranes 2019, 9, 105. https://doi.org/10.3390/membranes9090105
Abdullayev A, Bekheet MF, Hanaor DAH, Gurlo A. Materials and Applications for Low-Cost Ceramic Membranes. Membranes. 2019; 9(9):105. https://doi.org/10.3390/membranes9090105
Chicago/Turabian StyleAbdullayev, Amanmyrat, Maged F. Bekheet, Dorian A.H. Hanaor, and Aleksander Gurlo. 2019. "Materials and Applications for Low-Cost Ceramic Membranes" Membranes 9, no. 9: 105. https://doi.org/10.3390/membranes9090105
APA StyleAbdullayev, A., Bekheet, M. F., Hanaor, D. A. H., & Gurlo, A. (2019). Materials and Applications for Low-Cost Ceramic Membranes. Membranes, 9(9), 105. https://doi.org/10.3390/membranes9090105






