Modelling Forward Osmosis Treatment of Automobile Wastewaters
Abstract
1. Introduction
2. Materials and Methods
2.1. Lab-scale Experiments
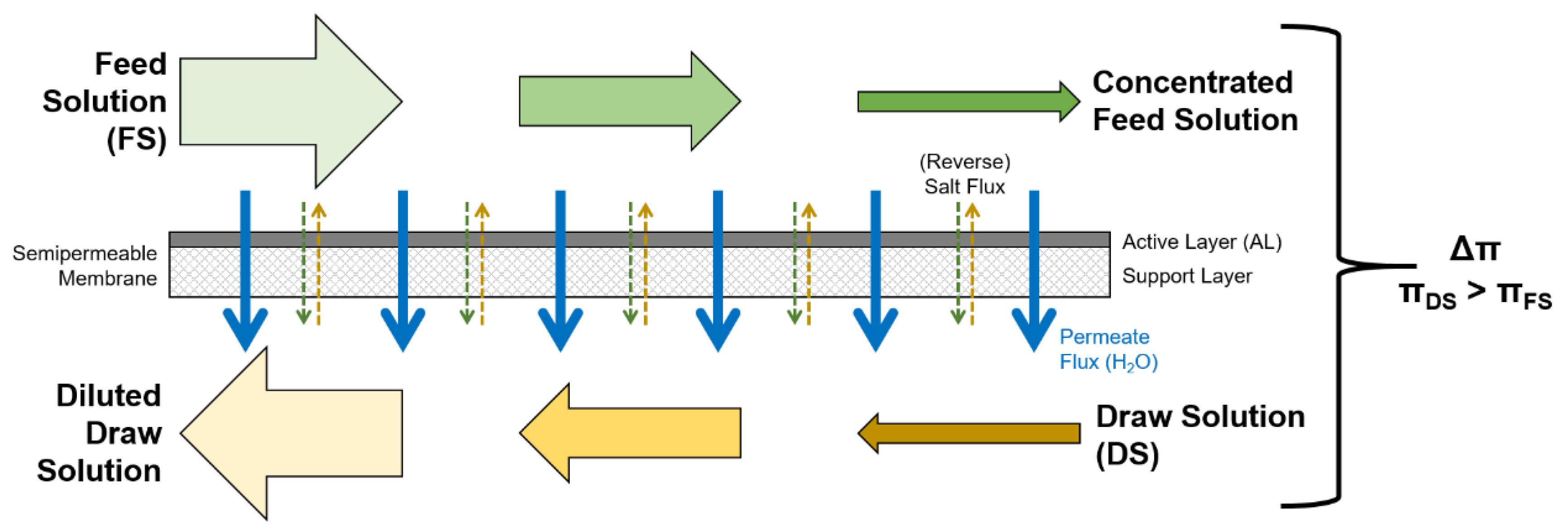
2.2. Model Setup
- FS and DS behave like ideal solutions.
- The temperature is constant during the experiments.
- The permeate flux is directed from FS to DS.
- The reverse solute flux is directed from DS to FS.
- Membrane parameters A, B, and S are the same for all membrane samples.
- Membrane parameters A, B, and S are constant during the experiment.
- The diffusion coefficient D is constant during the experiment.
- Fouling does not occur.
- Chemical reactions do not occur.
- Spacers are not considered although they were used in the experiments.
- The fluxes axial across the membrane are constant; no local dependencies are assumed.
- The system is a steady-state system.
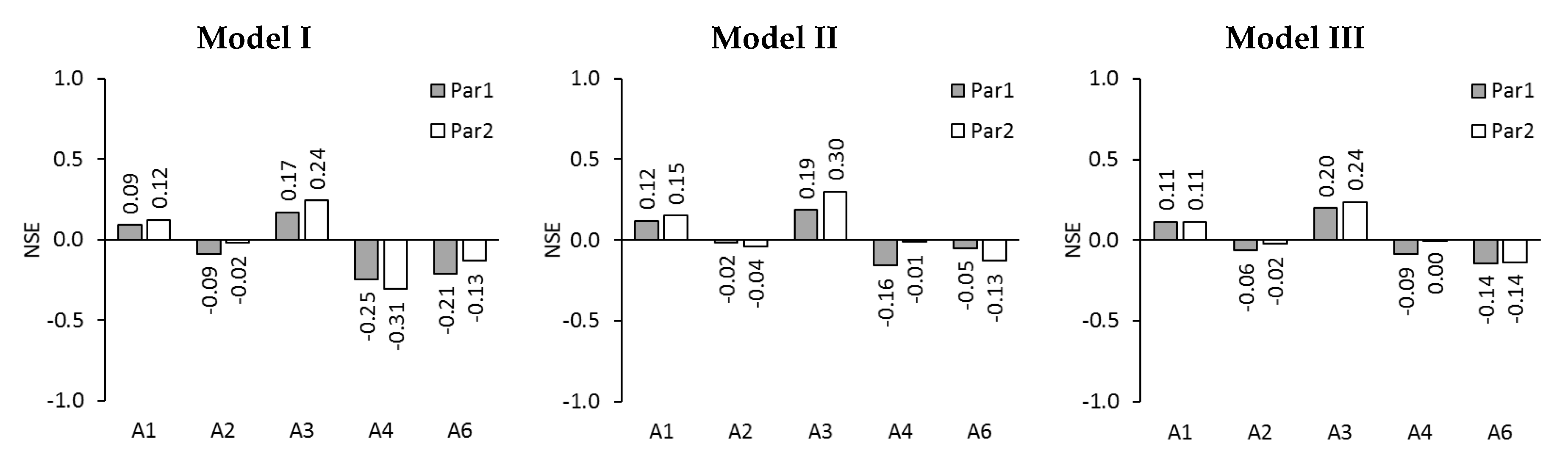
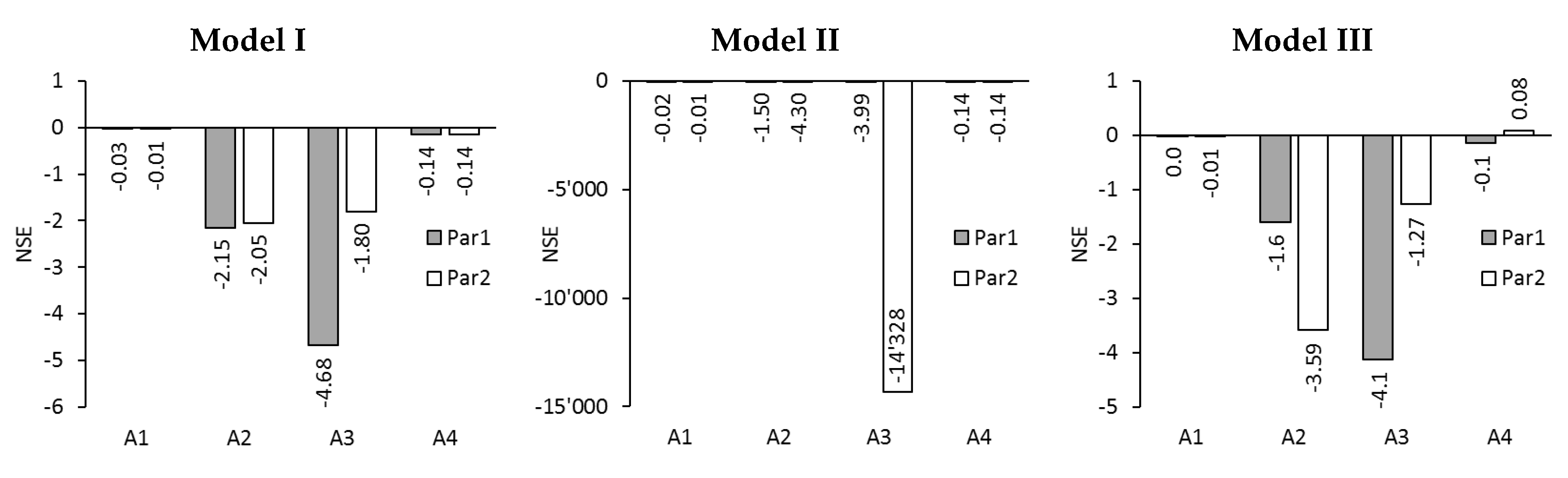
2.3. Model Evaluation
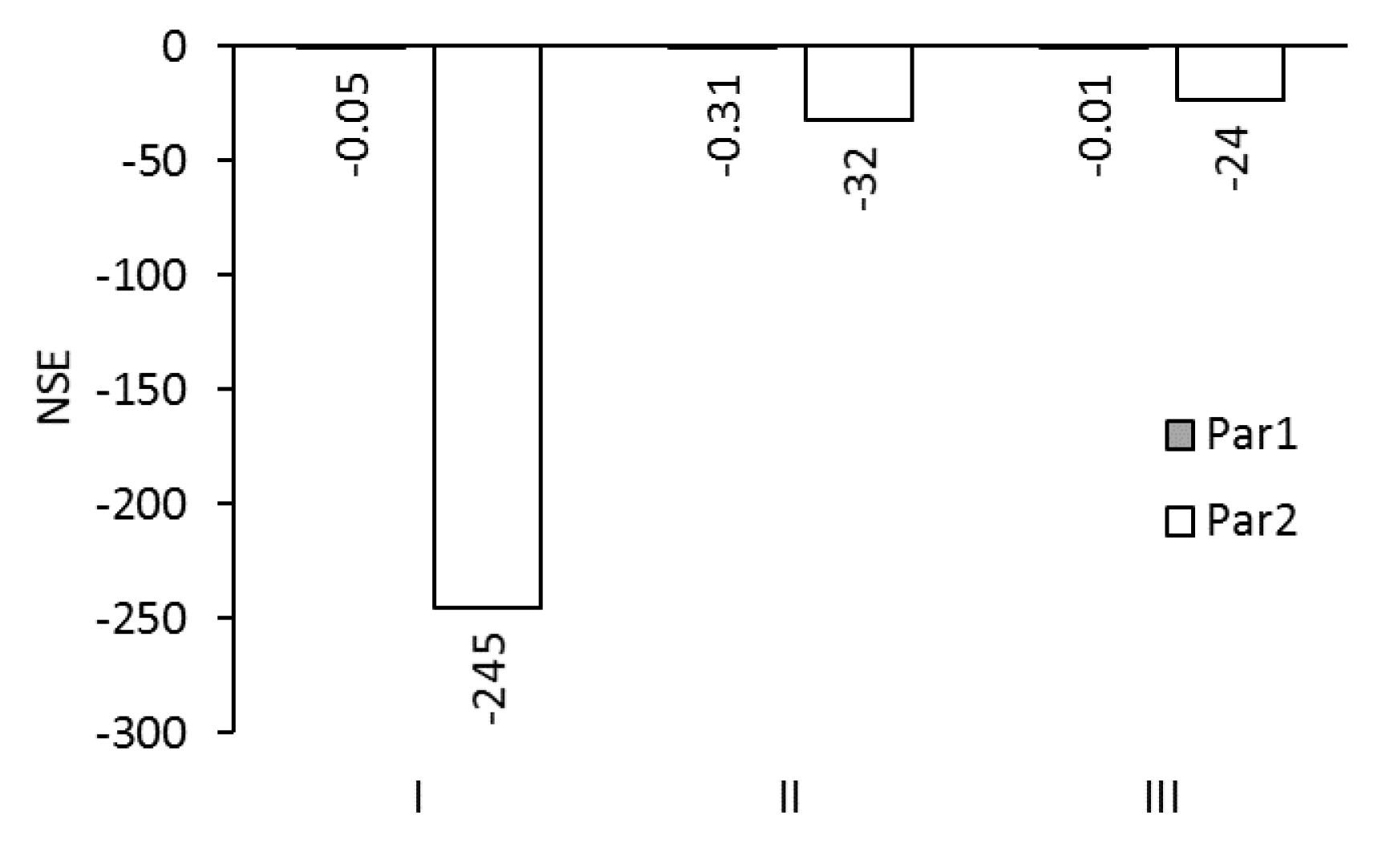
3. Results and Discussion
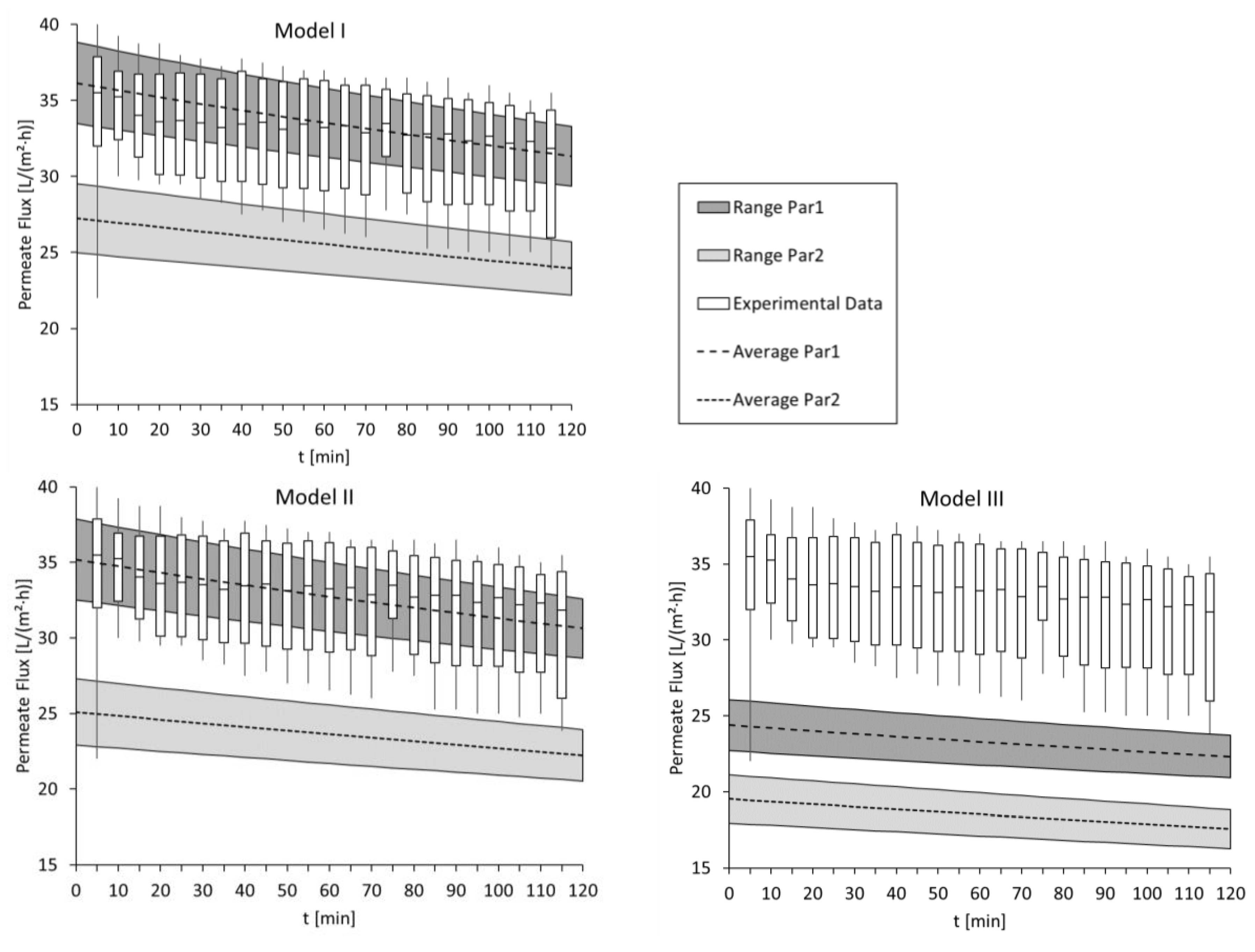
3.1. Modelling the Permeate Flux for Standard Performance Tests with Deionized Water and 1 mol/L NaCl
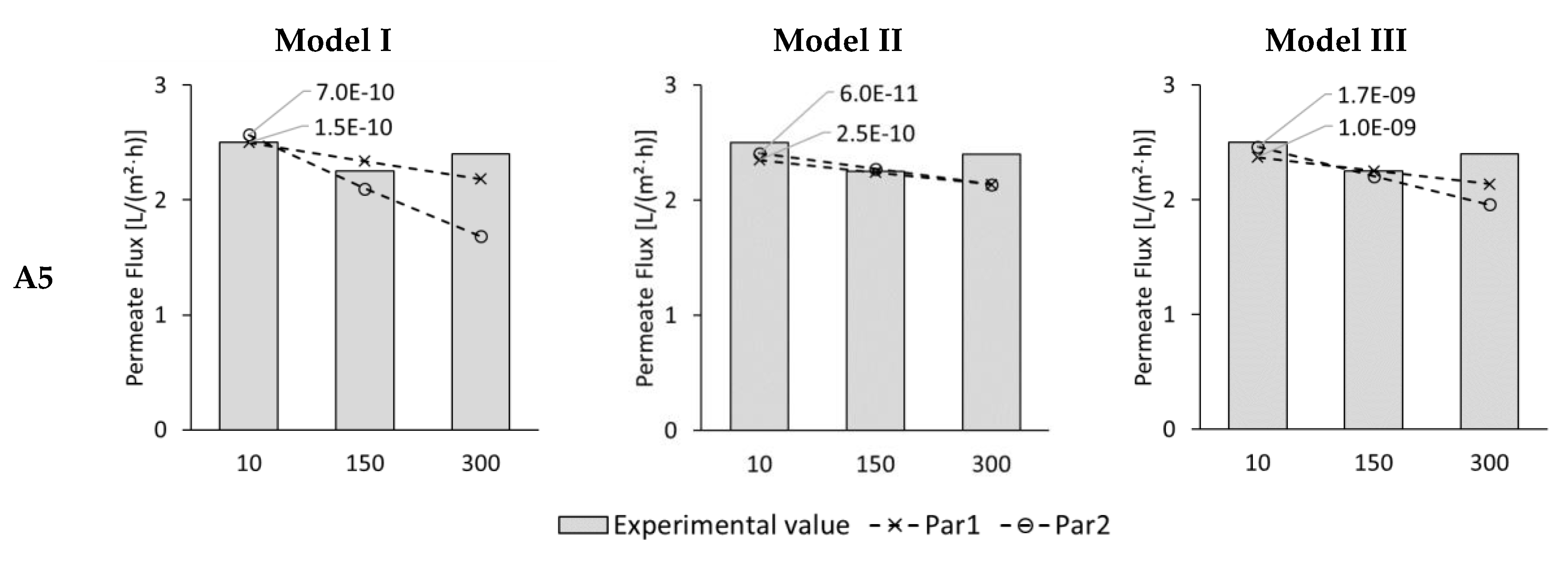
3.2. Modelling of Permeate Flux in ALFS Mode for Wastewater Experiments
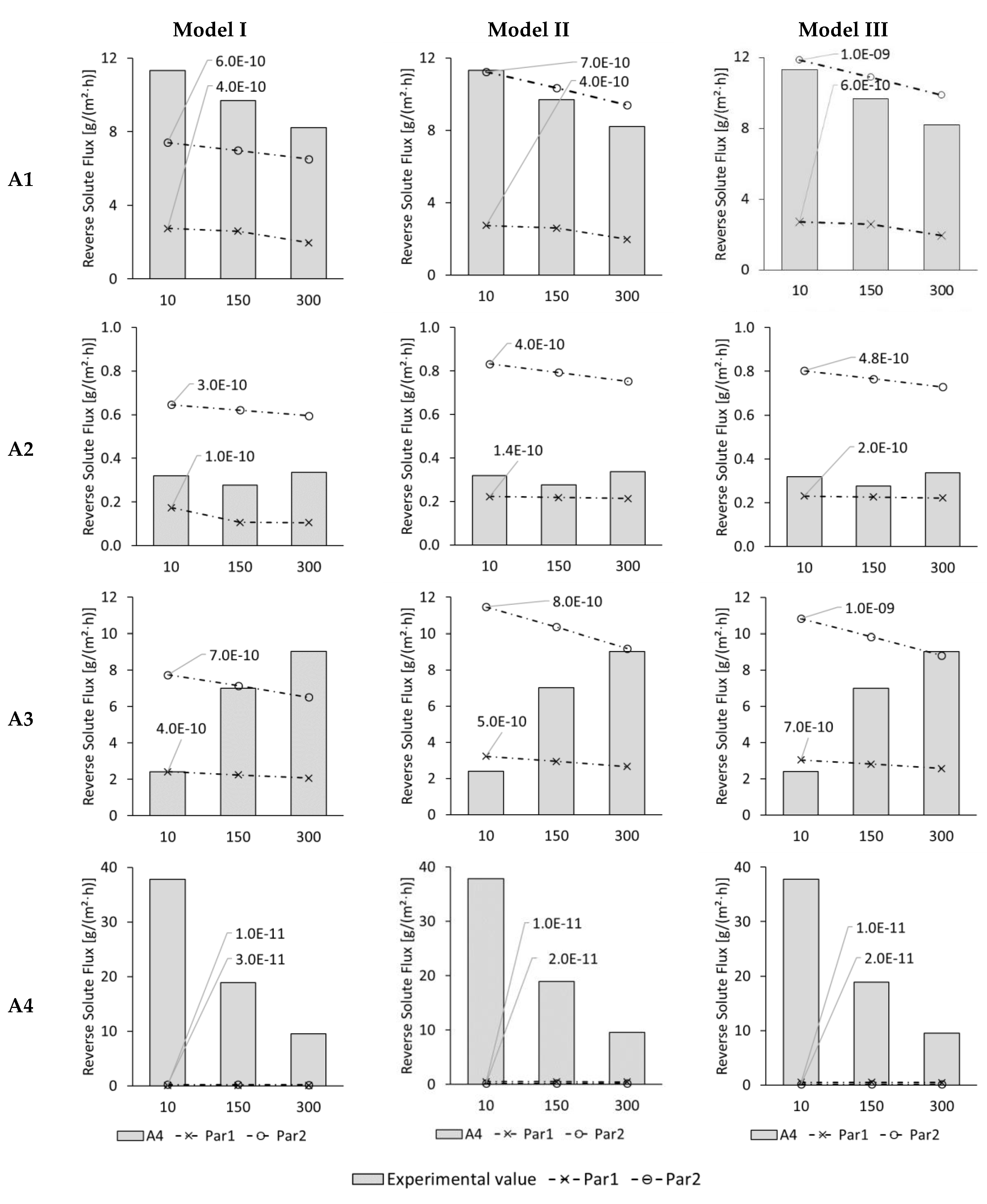
3.3. Modelling of Reverse Solute Flux in ALFS Mode for Wastewater Experiments
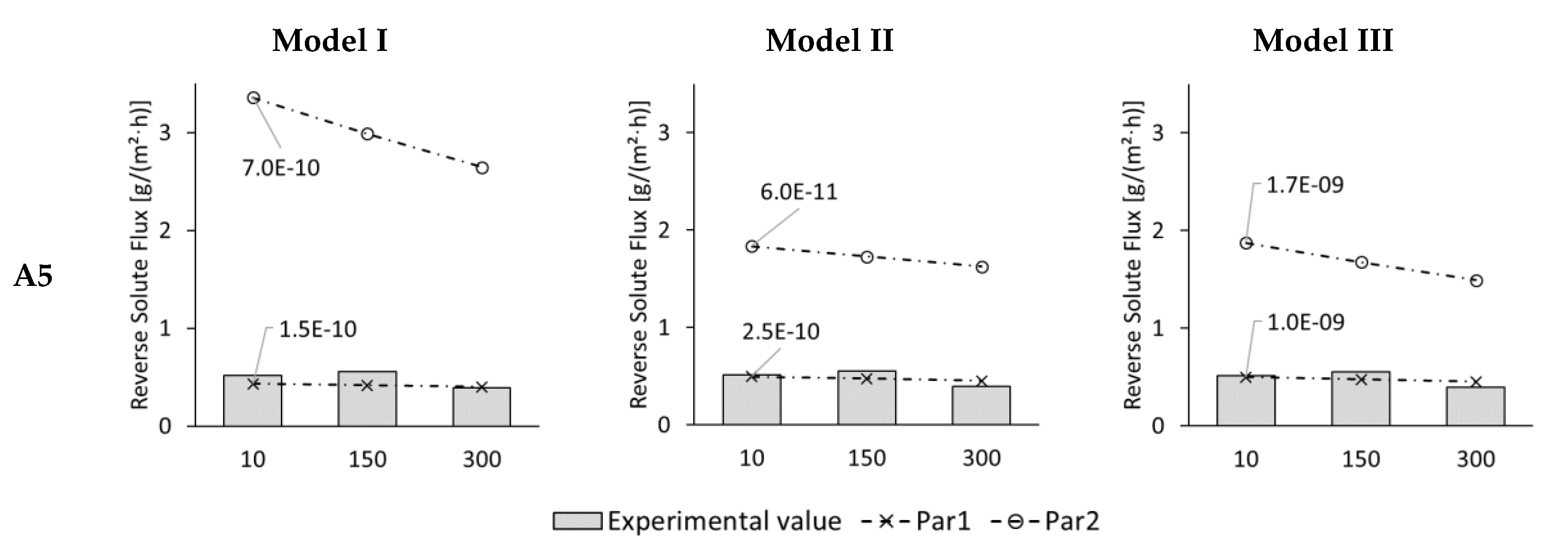
3.4. Modelling of Permeate Flux and Reverse Solute Flux in ALDS Mode for Wastewater Experiments
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| A | Water permeability [L/(m2 h bar)] |
| ACS | Cross-section area [m2] |
| B | Solute permeability [L/(m2 h)] |
| c | Concentration [mol/L] |
| cosm | Osmolality [osmol/kg] |
| dh | Hydraulic diameter [m] |
| D | Diffusion coefficient of solution [m2/s] |
| J | Flux |
| JS | Reverse solute flux [g/(m2·h)] |
| JW | Permeate flux [L/(m2·h)] |
| k | Mass transfer coefficient from bulk solution to membrane interface [m/s] |
| L | Flow channel length [m] |
| lu | Wetted perimeter [m] |
| MSE | Mean square error |
| NSE | Nash-Sutcliffe efficiency [-] |
| R | Universal gas constant [J/(K·mol)] |
| Re | Reynolds number [-] |
| S | Structural parameter [m] |
| Sc | Schmidt number [-] |
| Sh | Sherwood number [-] |
| T | Temperature [K] |
| v | Fluid velocity [m/s] |
| Greeks | |
| β | Concentration [g/L] |
| ρ | Density [kg/m3] |
| Δ | Difference |
| κ | Electrical conductivity [µS/cm or mS/cm] |
| η | Dynamic viscosity [Pa·s] |
| π | Osmotic pressure [bar] |
| ϑ | Temperature [°C] |
| Subscripts | |
| DS | Draw solution |
| exp | Experimental value |
| FS | Feed solution |
| mod | Modelled value |
| N | Number of values |
| t | Time |
References
- Sachidananda, M.; Webb, D.P.; Rahimifard, S. A Concept of Water Usage Efficiency to Support Water Reduction in Manufacturing Industry. Sustainavility 2016, 8, 1222. [Google Scholar] [CrossRef]
- Cath, T.; Childress, A.; Elimelech, M. Forward osmosis: Principles, applications, and recent developments. J. Membr. Sci. 2006, 281, 70–87. [Google Scholar] [CrossRef]
- Lutchmiah, K.; Verliefde, A.; Roest, K.; Rietveld, L.; Cornelissen, E. Forward osmosis for application in wastewater treatment: A review. Water Res. 2014, 58, 179–197. [Google Scholar] [CrossRef]
- Shaffer, D.L.; Werber, J.R.; Jaramillo, H.; Lin, S.; Elimelech, M. Forward osmosis: Where are we now? Desalination 2015, 356, 271–284. [Google Scholar] [CrossRef]
- McCutcheon, J.R. Forward osmosis: A technology platform here to stay. Desalination 2017, 421, 1–2. [Google Scholar] [CrossRef]
- Haupt, A.; Lerch, A. Forward Osmosis Application in Manufacturing Industries: A Short Review. Membranes 2018, 8, 47. [Google Scholar] [CrossRef]
- Johnson, D.J.; Suwaileh, W.A.; Mohammed, A.W.; Hilal, N. Osmotic’s potential: An overview of draw solutes for forward osmosis. Desalination 2018, 434, 100–120. [Google Scholar] [CrossRef]
- Awad, A.M.; Jalab, R.; Minier-Matar, J.; Adham, S.; Nasser, M.S.; Judd, S. The status of forward osmosis technology implementation. Desalination 2019, 461, 10–21. [Google Scholar] [CrossRef]
- Jalab, R.; Awad, A.M.; Nasser, M.S.; Minier-Matar, J.; Adham, S.; Judd, S.J. An empirical determination of the whole-life cost of FO-based open-loop wastewater reclamation technologies. Water Res. 2019, 163, 114879. [Google Scholar] [CrossRef]
- Zhao, S.; Zou, L.; Tang, C.Y.; Mulcahy, D. Recent developments in forward osmosis: Opportunities and challenges. J. Membr. Sci. 2012, 396, 1–21. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.P.; Li, H.Q. A review of forward osmosis membrane fouling: Types, research methods and future prospects. Environ. Technol. Rev. 2017, 6, 26–46. [Google Scholar] [CrossRef]
- Qin, D.; Liu, Z.; Liu, Z.; Bai, H.; Sun, D.D. Superior Antifouling Capability of Hydrogel Forward Osmosis Membrane for Treating Wastewaters with High Concentration of Organic Foulants. Environ. Sci. Technol. 2018, 52, 1421–1428. [Google Scholar] [CrossRef]
- Zou, S.; Qin, M.; He, Z. Tackle reverse solute flux in forward osmosis towards sustainable water recovery: Reduction and perspectives. Water Res. 2019, 149, 362–374. [Google Scholar] [CrossRef]
- Qin, J.J.; Lay, W.C.L.; Kekre, K.A. Recent developments and future challenges of forward osmosis for desalination: A review. Desalin. Water Treat. 2012, 39, 123–136. [Google Scholar] [CrossRef]
- Ibrar, I.; Naji, O.; Sharif, A.; Malekizadeh, A.; Alhawari, A.; AlAnezi, A.A.; Altaee, A. A Review of Fouling Mechanisms, Control Strategies and Real-Time Fouling Monitoring Techniques in Forward Osmosis. Water 2019, 11, 695. [Google Scholar] [CrossRef]
- McCutcheon, J.R.; Elimelech, M. Influence of concentrative and dilutive internal concentration polarization on flux behavior in forward osmosis. J. Membr. Sci. 2006, 284, 237–247. [Google Scholar] [CrossRef]
- Kim, B.; Gwak, G.; Hong, S. Review on methodology for determining forward osmosis (FO) membrane characteristics: Water permeability (A), solute permeability (B), and structural parameter (S). Desalination 2017, 422, 5–16. [Google Scholar] [CrossRef]
- Tielen, S. Enhancing reverse osmosis with feed spacer technology. Filtr. Sep. 2016, 53, 24–27. [Google Scholar] [CrossRef]
- Haidari, A.; Heijman, S.; Van Der Meer, W. Optimal design of spacers in reverse osmosis. Sep. Purif. Technol. 2018, 192, 441–456. [Google Scholar] [CrossRef]
- Kavianipour, O.; Ingram, G.D.; Vuthaluru, H.B. Feed spacers in reverse osmosis. Filtr. Sep. 2015, 52, 20–21. [Google Scholar]
- Koutsou, C.; Yiantsios, S.; Karabelas, A.; Karabelas, A. A numerical and experimental study of mass transfer in spacer-filled channels: Effects of spacer geometrical characteristics and Schmidt number. J. Membr. Sci. 2009, 326, 234–251. [Google Scholar] [CrossRef]
- Karabelas, A.J.; Koutsou, C.P.; Sioutopoulos, D.C. Comprehensive performance assessment of spacers in spiral-wound membrane modules accounting for compressibility effects. J. Membr. Sci. 2018, 549, 602–615. [Google Scholar] [CrossRef]
- Islam, M.S.; Sultana, S.; McCutcheon, J.R.; Rahaman, M.S. Treatment of fracking wastewaters via forward osmosis: Evaluation of suitable organic draw solutions. Desalination 2019, 452, 149–158. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, M.; Deng, Q.; Cai, T. Combination and performance of forward osmosis and membrane distillation (FO-MD) for treatment of high salinity landfill leachate. Desalination 2017, 420, 99–105. [Google Scholar] [CrossRef]
- Qasim, M.; Mohammed, F.; Aidan, A.; Darwish, N.A. Forward osmosis desalination using ferric sulfate draw solute. Desalination 2017, 423, 12–20. [Google Scholar] [CrossRef]
- Wang, X.; Chang, V.W.; Tang, C.Y.; Chang, V.W.C. Osmotic membrane bioreactor (OMBR) technology for wastewater treatment and reclamation: Advances, challenges, and prospects for the future. J. Membr. Sci. 2016, 504, 113–132. [Google Scholar] [CrossRef]
- Chekli, L.; Kim, Y.; Phuntsho, S.; Li, S.; Ghaffour, N.; Leiknes, T.; Shon, H.K. Evaluation of fertilizer-drawn forward osmosis for sustainable agriculture and water reuse in arid regions. J. Environ. Manag. 2017, 187, 137–145. [Google Scholar] [CrossRef]
- Gwak, G.; Kim, D.I.; Hong, S. New industrial application of forward osmosis (FO): Precious metal recovery from printed circuit board (PCB) plant wastewater. J. Membr. Sci. 2018, 552, 234–242. [Google Scholar] [CrossRef]
- Law, J.Y.; Mohammad, A.W.; Tee, Z.K.; Zaman, N.K.; Jahim, J.M.; Santanaraj, J.; Sajab, M.S. Recovery of succinic acid from fermentation broth by forward osmosis-assisted crystallization process. J. Membr. Sci. 2019, 583, 139–151. [Google Scholar] [CrossRef]
- Dou, P.; Zhao, S.; Song, J.; He, H.; She, Q.; Li, X.M.; Zhang, Y.; He, T. Forward osmosis concentration of a vanadium leaching solution. J. Membr. Sci. 2019, 582, 164–171. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Porter, C.J.; Cheng, W.; Zhang, X.; Wang, L.; Elimelech, M. Concentration and Recovery of Dyes from Textile Wastewater Using a Self-Standing, Support-Free Forward Osmosis Membrane. Environ. Sci. Technol. 2019, 53, 3078–3086. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Baker, R.; Lonsdale, H. Membranes for power generation by pressure-retarded osmosis. J. Membr. Sci. 1981, 8, 141–171. [Google Scholar] [CrossRef]
- Loeb, S. Effect of porous support fabric on osmosis through a Loeb-Sourirajan type asymmetric membrane. J. Membr. Sci. 1997, 129, 243–249. [Google Scholar] [CrossRef]
- McCutcheon, J.R.; Elimelech, M. Modeling water flux in forward osmosis: Implications for improved membrane design. AIChE J. 2007, 53, 1736–1744. [Google Scholar] [CrossRef]
- Tan, C.H.; Ng, H.Y. Modified models to predict flux behavior in forward osmosis in consideration of external and internal concentration polarizations. J. Membr. Sci. 2008, 324, 209–219. [Google Scholar] [CrossRef]
- Shakaib, M.; Hasani, S.; Mahmood, M. CFD modeling for flow and mass transfer in spacer-obstructed membrane feed channels. J. Membr. Sci. 2009, 326, 270–284. [Google Scholar] [CrossRef]
- Phillip, W.A.; Yong, J.S.; Elimelech, M. Reverse Draw Solute Permeation in Forward Osmosis: Modeling and Experiments. Environ. Sci. Technol. 2010, 44, 5170–5176. [Google Scholar] [CrossRef]
- Yaroshchuk, A. Influence of osmosis on the diffusion from concentrated solutions through composite/asymmetric membranes: Theoretical analysis. J. Membr. Sci. 2010, 355, 98–103. [Google Scholar] [CrossRef]
- Gruber, M.; Johnson, C.; Tang, C.; Jensen, M.; Yde, L.; Hélix-Nielsen, C. Computational fluid dynamics simulations of flow and concentration polarization in forward osmosis membrane systems. J. Membr. Sci. 2011, 379, 488–495. [Google Scholar] [CrossRef]
- Jung, D.H.; Lee, J.; Kim, D.Y.; Lee, Y.G.; Park, M.; Lee, S.; Yang, D.R.; Kim, J.H. Simulation of forward osmosis membrane process: Effect of membrane orientation and flow direction of feed and draw solutions. Desalination 2011, 277, 83–91. [Google Scholar] [CrossRef]
- Li, W.; Gao, Y.; Tang, C.Y. Network modeling for studying the effect of support structure on internal concentration polarization during forward osmosis: Model development and theoretical analysis with FEM. J. Membr. Sci. 2011, 379, 307–321. [Google Scholar] [CrossRef]
- Park, M.; Lee, J.J.; Lee, S.; Kim, J.H. Determination of a constant membrane structure parameter in forward osmosis processes. J. Membr. Sci. 2011, 375, 241–248. [Google Scholar] [CrossRef]
- Tiraferri, A.; Yip, N.Y.; Phillip, W.A.; Schiffman, J.D.; Elimelech, M. Relating performance of thin-film composite forward osmosis membranes to support layer formation and structure. J. Membr. Sci. 2011, 367, 340–352. [Google Scholar] [CrossRef]
- Yip, N.Y.; Tiraferri, A.; Phillip, W.A.; Schiffman, J.D.; Hoover, L.A.; Kim, Y.C.; Elimelech, M. Thin-Film Composite Pressure Retarded Osmosis Membranes for Sustainable Power Generation from Salinity Gradients. Environ. Sci. Technol. 2011, 45, 4360–4369. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Ng, H.Y. Revised external and internal concentration polarization models to improve flux prediction in forward osmosis process. Desalination 2013, 309, 125–140. [Google Scholar] [CrossRef]
- Tiraferri, A.; Yip, N.Y.; Straub, A.P.; Castrillón, S.R.V.; Elimelech, M. A method for the simultaneous determination of transport and structural parameters of forward osmosis membranes. J. Membr. Sci. 2013, 444, 523–538. [Google Scholar] [CrossRef]
- Bui, N.N.; Arena, J.T.; McCutcheon, J.R. Proper accounting of mass transfer resistances in forward osmosis: Improving the accuracy of model predictions of structural parameter. J. Membr. Sci. 2015, 492, 289–302. [Google Scholar] [CrossRef]
- Haupt, A.; Lerch, A. Forward osmosis treatment of effluents from dairy and automobile industry—Results from short-term experiments to show general applicability. Water Sci. Technol. 2018, 78, 467–475. [Google Scholar] [CrossRef]
- Chun, Y.; Qing, L.; Sun, G.; Bilad, M.R.; Fane, A.G.; Chong, T.H. Prototype aquaporin-based forward osmosis membrane: Filtration properties and fouling resistance. Desalination 2018, 445, 75–84. [Google Scholar] [CrossRef]
- Kim, J.; Blandin, G.; Phuntsho, S.; Verliefde, A.; Le-Clech, P.; Shon, H. Practical considerations for operability of an 8″ spiral wound forward osmosis module: Hydrodynamics, fouling behaviour and cleaning strategy. Desalination 2017, 404, 249–258. [Google Scholar] [CrossRef]
- Grattoni, A.; Canavese, G.; Montevecchi, F.M.; Ferrari, M. Fast Membrane Osmometer as Alternative to Freezing Point and Vapor Pressure Osmometry. Anal. Chem. 2008, 80, 2617–2622. [Google Scholar] [CrossRef] [PubMed]
- Kune, J. Dimensionless Physical Quantities in Science and Engineering; Elsevier Insights; Elsevier: Amsterdam, The Netherlands; Heidelberg, Germany, 2012; ISBN 978-0-12-416013-2. [Google Scholar]
- Bollrich, G. Technische Hydromechanik 1; Verlag Für Bauwesen: Berlin, Germany, 1996; ISBN 978-3-345-00608-1. [Google Scholar]
- Verein Deutscher Ingenieure. VDI-Wärmeatlas: Berechnungsblätter für den Wärmeübergang. In Gesellschaft Verfahrenstechnik und Chemieingenieurwesen, 5th ed.; VDI-Verlag: Düsseldorf, Germany, 1988; ISBN 978-3-18-400850-5. [Google Scholar]
- Perry, R.H.; Green, D.W.; Maloney, J.O. Perry’s Chemical Engineers’ Handbook, 6th ed.; McGraw-Hill Companies, Inc.: New York, NY, USA, 1984; ISBN 0-07-049479-7. [Google Scholar]
- Gupta, H.V.; Kling, H. On typical range, sensitivity, and normalization of Mean Squared Error and Nash-Sutcliffe Efficiency type metrics. Water Resour. Res. 2011, 47, 47. [Google Scholar] [CrossRef]
- Moriasi, D.N.; Arnold, J.G.; Van Liew, M.W.; Bingner, R.L.; Harmel, R.D.; Veith, T.L. Model Evaluation Guidelines for Systematic Quantification of Accuracy in Watershed Simulations. Trans. ASABE 2007, 50, 885–900. [Google Scholar] [CrossRef]
- Lobo, V.M.M. Mutual diffusion coefficients in aqueous electrolyte solutions (Technical Report). Pure Appl. Chem. 1993, 65, 2613–2640. [Google Scholar] [CrossRef]
- D’Haese, A.K.; Motsa, M.M.; Van Der Meeren, P.; Verliefde, A.R. A refined draw solute flux model in forward osmosis: Theoretical considerations and experimental validation. J. Membr. Sci. 2017, 522, 316–331. [Google Scholar] [CrossRef]
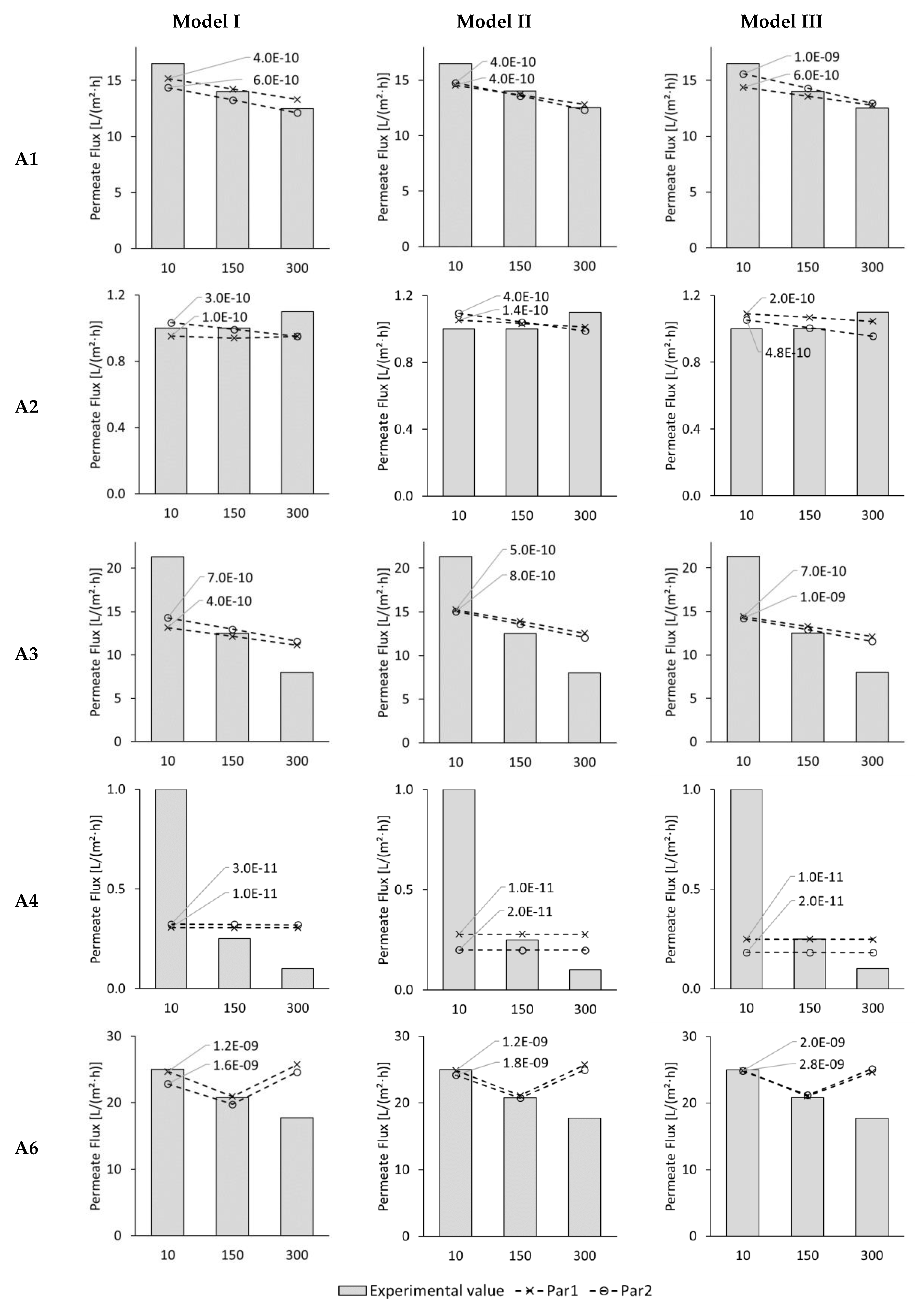

| Test Series | Feed Solution (FS) | Draw Solution (DS) | ∆π [bar] | Membrane Orientation | |
|---|---|---|---|---|---|
| ALFS | ALDS | ||||
| A1 | Cathodic dip painting rinsing water | 1 mol/L NaCl | 44.8 | ✓ | - |
| A2 | Deionized Water (DI) | Cooling tower circulation water | 1.1 | ✓ | - |
| A3 | Paint shop pre-treatment wastewater | 1 mol/L NaCl | 44.5 | ✓ | - |
| A4 | Deionized Water (DI) | Cathodic dip painting wastewater | 2.1 | ✓ | - |
| A5 | Deionized Water (DI) | Cooling tower circulation water | 1.1 | - | ✓ |
| A6 | Cathodic dip painting wastewater | 1 mol/L NaCl | 43.5 | ✓ | - |
| P | Deionized Water (DI) | 1 mol/L NaCl | 44.5 | ✓ | - |
| Model | Permeate Flux | Reverse Solute Flux | Ref. |
|---|---|---|---|
| I | [16] mod., [46] | ||
| IIALFS | |||
| III | [47] |
| Model | Permeate Flux | Reverse Solute Flux | Ref. |
|---|---|---|---|
| I | [16] mod., [44] | ||
| IIALDS | |||
| III | [47] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haupt, A.; Marx, C.; Lerch, A. Modelling Forward Osmosis Treatment of Automobile Wastewaters. Membranes 2019, 9, 106. https://doi.org/10.3390/membranes9090106
Haupt A, Marx C, Lerch A. Modelling Forward Osmosis Treatment of Automobile Wastewaters. Membranes. 2019; 9(9):106. https://doi.org/10.3390/membranes9090106
Chicago/Turabian StyleHaupt, Anita, Christian Marx, and André Lerch. 2019. "Modelling Forward Osmosis Treatment of Automobile Wastewaters" Membranes 9, no. 9: 106. https://doi.org/10.3390/membranes9090106
APA StyleHaupt, A., Marx, C., & Lerch, A. (2019). Modelling Forward Osmosis Treatment of Automobile Wastewaters. Membranes, 9(9), 106. https://doi.org/10.3390/membranes9090106






