Galactoglucomannan Recovery with Hydrophilic and Hydrophobic Membranes: Process Performance and Cost Estimations
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Equipment and Experimental Procedure
2.2.1. Membranes and Membrane Filtration Set-Up
2.2.2. Influence of Volume-Reduction on Flux and Retention (Concentration Study)
2.2.3. Membrane Cleaning and Fouling Calculations
2.3. Analysis
2.3.1. Lignin Content
2.3.2. Ash and Total Dry Content
2.3.3. Hemicellulose and Acid-Insoluble Solids
2.3.4. Size-Exclusion Chromatography
2.3.5. Two-Dimensional Nuclear Magnetic Resonance Spectroscopy (2D-NMR)
3. Results and Discussion
3.1. SSL Composition
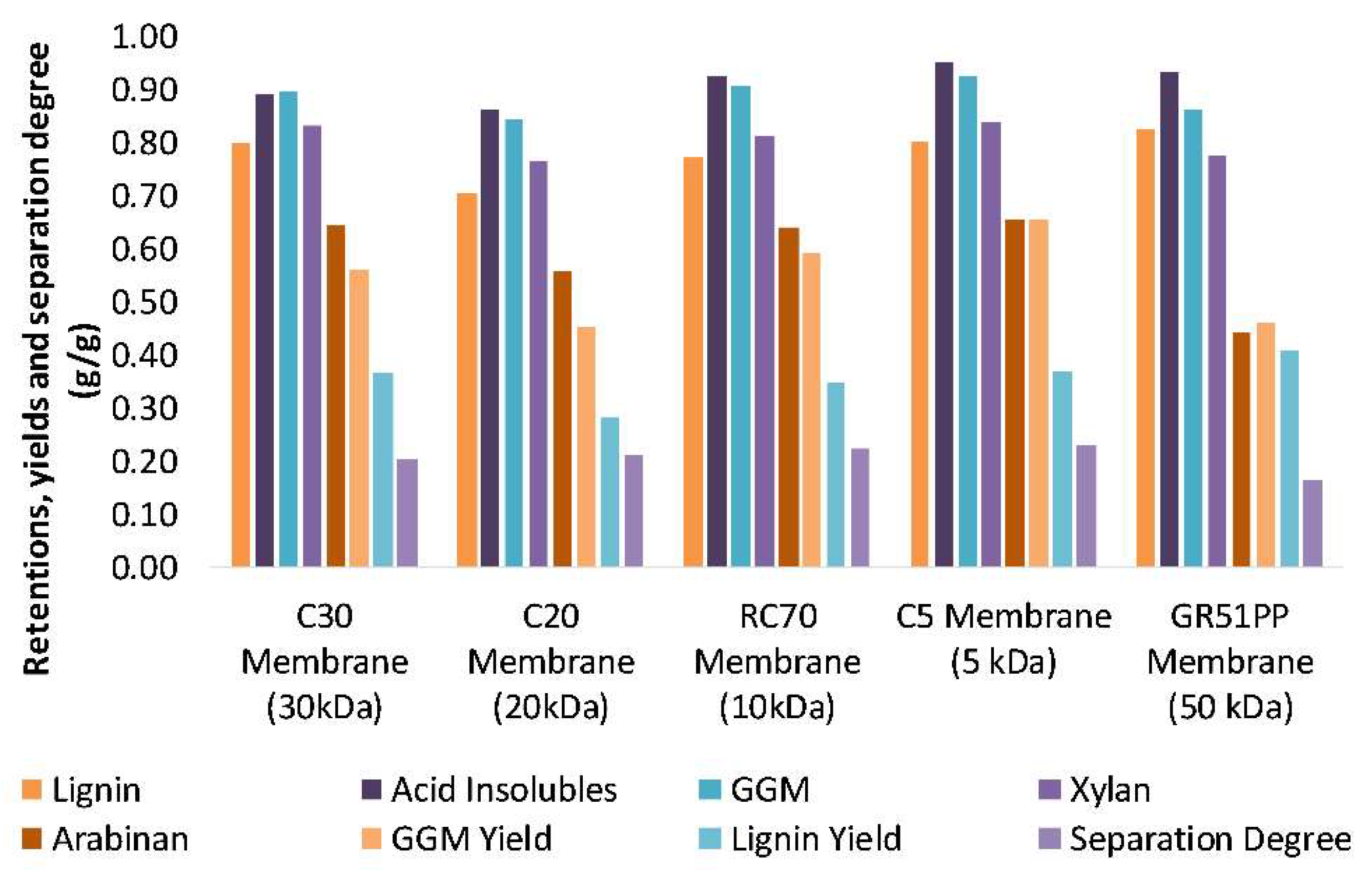
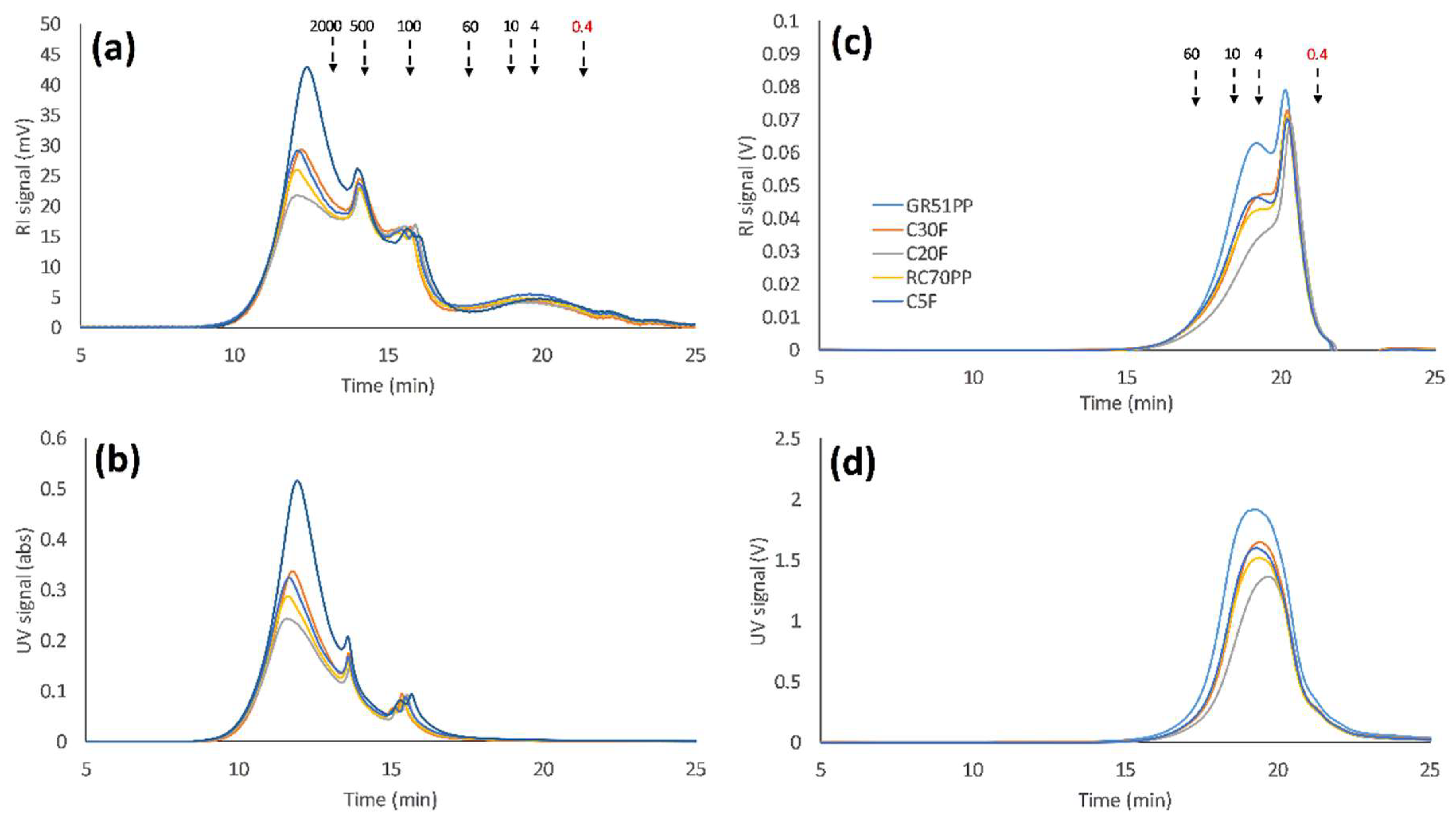
3.2. Influence of Volume Reduction on Flux and Retention
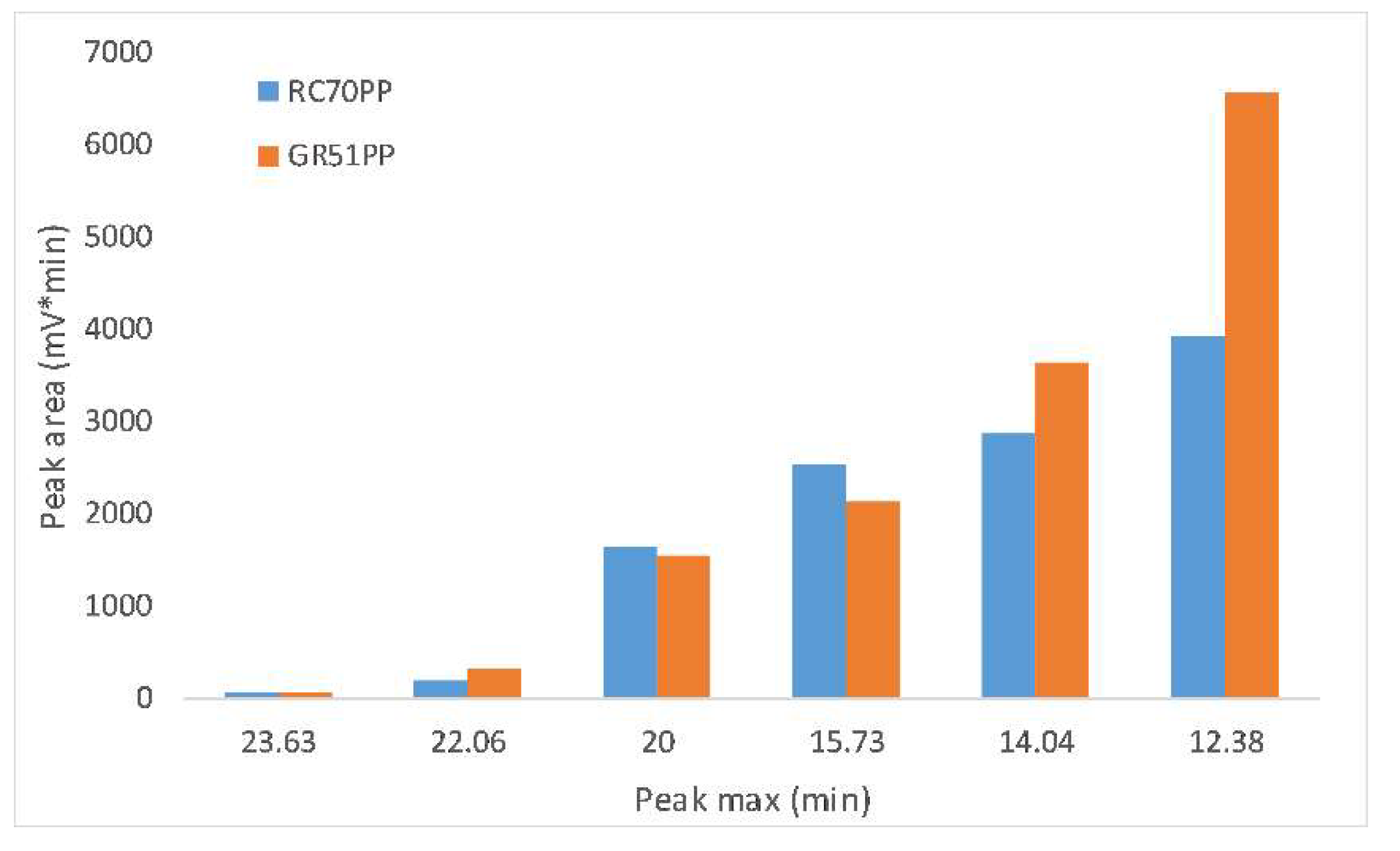
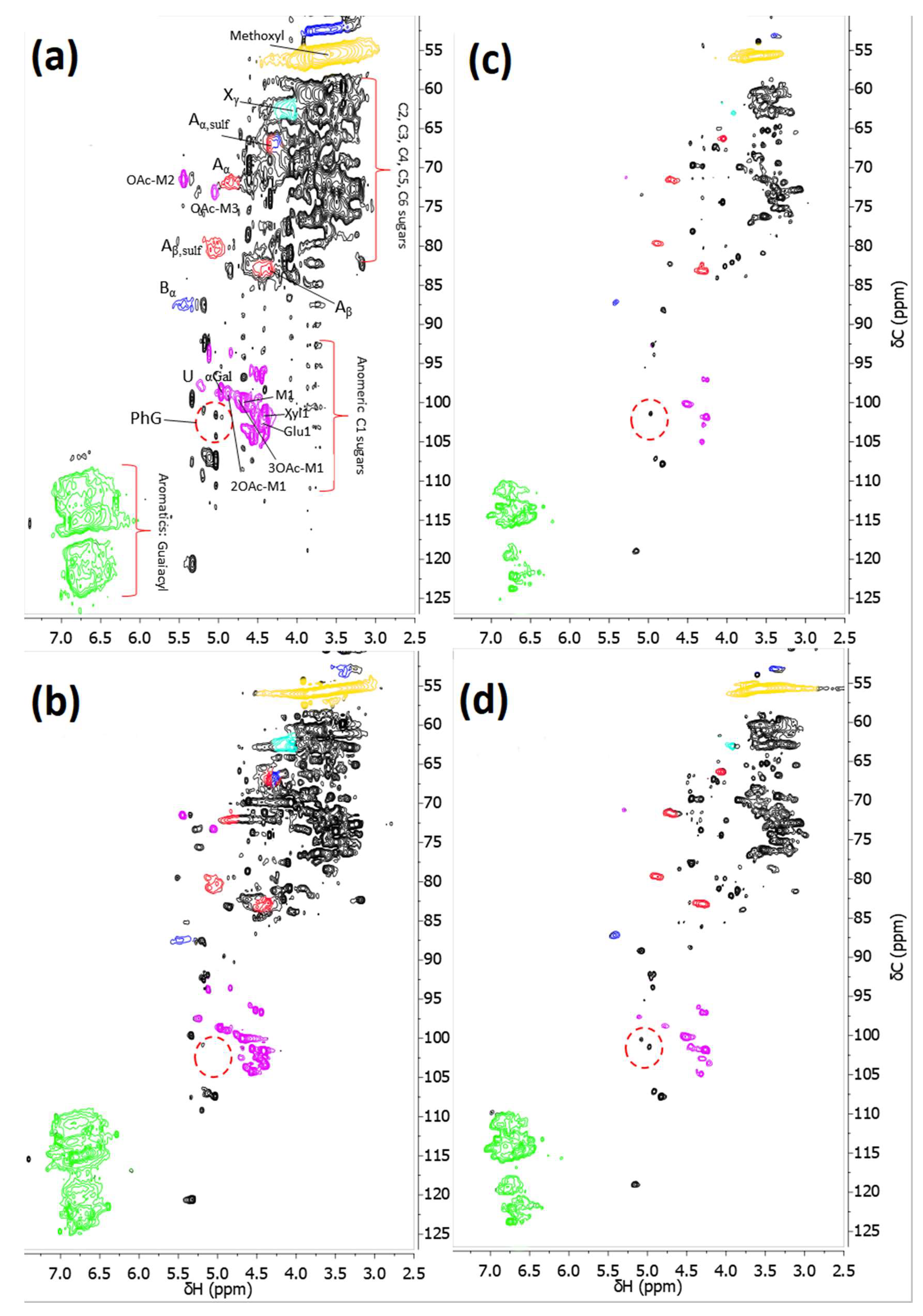
3.3. Differences between the GR51PP and RC70PP Retentates
3.4. Optimal Membrane and Cost Estimates
4. Conclusions
- Initial membrane filtration trials and comparison of the raw material (SSL) and retentates show that most of the solutes are in the same MW range as the cut-off specifications of the membranes. Therefore, the hydrophilic membranes with highest MWCO, experienced the highest degree of fouling.
- The fouling was due to pore blocking as has been seen previously with the hydrophobic membranes.
- The hydrophilic membranes had an overall lower lignin retention and higher GGM retention compared with the hydrophobic, possibly because of the lower lignin affinity and adsorption.
- The hydrophilic membranes were shown to be superior to the hydrophobic membranes when it comes to separation, fouling, and capacity.
- Analyzing the SSL using SEC with water as an eluent has been shown to be a promising method for the separation of lignosulfonates and LCCs from GGM due to the effect of ion exclusion in the SEC column.
- The optimal membrane (RC70PP) was compared with the GR51PP by analyzing the different retentates using SEC and 2D HSQC NMR.
- The results showed that the hydrophilic membrane retained a higher amount of polysaccharides compared with the hydrophobic membrane. This resulted in a higher portion of LCCs being retained in the hydrophilic membrane; which occurred because of the lower MWCO of the hydrophilic membrane.
- The 2D HSQC NMR indicated that the retentate from the hydrophilic membrane had a higher amount of LCCs. No other major structural differences were observed between the two retentates.
- The most cost-efficient membrane was chosen based on the flux and product yields. The C20F membrane had the lowest total costs per year; however, the RC70PP led to higher GGM production and yields and thus, the cost per ton product was the lowest for the RC70PP.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wenzl, H. The Chemical Technology of Wood; Elsevier: New York, NY, USA, 2012. [Google Scholar]
- Weissgram, M.; Gstöttner, J.; Lorántfy, B.; Tenhaken, R.; Herwig, C.; Weber, H.K. Generation of PHB from Spent Sulfite Liquor Using Halophilic Microorganisms. Microorganisms 2015, 3, 268–289. [Google Scholar] [CrossRef] [PubMed]
- Lawoko, M. Lignin Polysaccharide Networks in Softwood and Chemical Pulps: Characterisation, Structure and Reactivity. Ph.D. Thesis, Royal Institute of Technology, Stockholm, Sweden, 2005. [Google Scholar]
- Al-Rudainy, B.; Galbe, M.; Schagerlöf, H.; Wallberg, O. Antisolvent precipitation of hemicelluloses, lignosulfonates and their complexes from ultrafiltrated spent sulfite liquor (SSL). Holzforschung 2018, 72, 839–850. [Google Scholar] [CrossRef]
- Giummarella, N.; Lawoko, M.; Zhang, L.; Henriksson, G. Structural features of mildly fractionated lignin carbohydrate complexes (LCC) from spruce. RSC Adv. 2016, 6, 42120–42131. [Google Scholar] [CrossRef]
- Willför, S.; Sjöholm, R.; Laine, C.; Roslund, M.; Hemming, J.; Holmbom, B. Characterisation of water-soluble galactoglucomannans from Norway spruce wood and thermomechanical pulp. Carbohydr. Polym. 2003, 52, 175–187. [Google Scholar] [CrossRef]
- Du, X.; Pérez-Boada, M.; Fernández, C.; Rencoret, J.; Del Río, J.C.; Jiménez-Barbero, J.; Li, J.; Gutierrez, A.; Martínez, A.T. Analysis of lignin–carbohydrate and lignin–lignin linkages after hydrolase treatment of xylan–lignin, glucomannan–lignin and glucan–lignin complexes from spruce wood. Planta 2014, 239, 1079–1090. [Google Scholar] [CrossRef] [PubMed]
- Al-Rudainy, B.; Galbe, M.; Hernandez, M.A.; Jannasch, P.; Wallberg, O. Impact of Lignin Content on the Properties of Hemicellulose Hydrogels. Polymers (Basel) 2018, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Thuvander, J.; Jönsson, A.-S. Extraction of galactoglucomannan from thermomechanical pulp mill process water by microfiltration and ultrafiltration—Influence of microfiltration membrane pore size on ultrafiltration performance. Chem. Eng. Res. Des. 2016, 105, 171–176. [Google Scholar] [CrossRef]
- Al Manasrah, M.; Kallioinen, M.; Ilvesniemi, H.; Mänttäri, M. Recovery of galactoglucomannan from wood hydrolysate using regenerated cellulose ultrafiltration membranes. Bioresour. Technol. 2012, 114, 375–381. [Google Scholar] [CrossRef]
- Duval, A.; Molina-Boisseau, S.; Chirat, C. Fractionation of lignosulfonates: Comparison of ultrafiltration and ethanol solubility to obtain a set of fractions with distinct properties. Holzforschung 2015, 69, 127–134. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Todi, R.; Tiwari, M.; Bhattacharjee, C.; Bhattacharjee, S.; Datta, S. Studies on ultrafiltration of spent sulfite liquor using various membranes for the recovery of lignosulphonates. Desalination 2005, 174, 287–297. [Google Scholar] [CrossRef]
- Al-Rudainy, B.; Galbe, M.; Wallberg, O. Influence of prefiltration on membrane performance during isolation of lignin-carbohydrate complexes from spent sulfite liquor. Sep. Purif. Technol. 2017, 187, 380–388. [Google Scholar] [CrossRef]
- Song, T.; Pranovich, A.; Holmbom, B. Separation of polymeric galactoglucomannans from hot-water extract of spruce wood. Bioresour. Technol. 2013, 130, 198–203. [Google Scholar] [CrossRef]
- Zasadowski, D.; Yang, J.; Edlund, H.; Norgren, M. Antisolvent precipitation of water-soluble hemicelluloses from TMP process water. Carbohydr. Polym. 2014, 113, 411–419. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, X.; Liu, Y.; Zhang, L. Enhancing the Compatibility, Hydrophilicity and Mechanical Properties of Polysulfone Ultrafiltration Membranes with Lignocellulose Nanofibrils. Polymers (Basel) 2016, 8, 349. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; O’Hagan, T.; MacLeod, J. Using ultrafiltration to analyze the molar mass distribution of kraft lignin at pH 13. Can. J. Chem. Eng. 1996, 74, 110–117. [Google Scholar] [CrossRef]
- Puro, L.; Tanninen, J.; Nyström, M. Analyses of organic foulants in membranes fouled by pulp and paper mill effluent using solid-liquid extraction. Desalination 2002, 143, 1–9. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples (NREL/TP-510-42623); NREL: Golden, CO, USA, 2008. [Google Scholar]
- Steinmetz, V.; Villain-Gambier, M.; Klem, A.; Gambier, F.; Dumarcay, S.; Trebouet, D. Unveiling TMP Process Water Potential as an Industrial Sourcing of Valuable Lignin–Carbohydrate Complexes toward Zero-Waste Biorefineries. ACS Sustain. Chem. Eng. 2019, 7, 6390–6400. [Google Scholar] [CrossRef]
- Hannuksela, T.; Herve, C. NMR structural determination of dissolved O-acetylated galactoglucomanna isolated from spruce thermomechanical pulp. Carbohydr. Res. 2004, 339, 301–312. [Google Scholar] [CrossRef]
- Lutnaes, B.F.; Myrvold, B.O.; Lauten, R.A.; Endeshaw, M.M. 1H and13C NMR data of benzylsulfonic acids—Model compounds for lignosulfonate. Magn. Reson. Chem. 2008, 46, 299–305. [Google Scholar] [CrossRef]
- Marques, A.P.; Evtuguin, D.V.; Magina, S.; Amado, F.M.L.; Prates, A.; Evtuguin, D.; Amado, F. Structure of Lignosulphonates from Acidic Magnesium-Based Sulphite Pulping ofEucalyptus globulus. J. Wood Chem. Technol. 2009, 29, 337–357. [Google Scholar] [CrossRef]
- Myrvold, B.O. Salting-out and salting-in experiments with lignosulfonates (LSs). Holzforschung 2013, 67, 549–557. [Google Scholar] [CrossRef]
- Lawoko, M. Unveiling the structure and ultrastructure of lignin carbohydrate complexes in softwoods. Int. J. Biol. Macromol. 2013, 62, 705–713. [Google Scholar] [CrossRef]
- Forss, K.; Stenlund, B. The Influence of Charged Groups in Gel Permeation Chromatography of Polyelec-Trolytes. J. Polym. Sci. Polym. Symp. 1973, 42, 951–963. [Google Scholar] [CrossRef]
- Tolbert, A.; Akinosho, H.; Khunsupat, R.; Naskar, A.K.; Ragauskas, A.J. Characterization and analysis of the molecular weight of lignin for biorefining studies. Biofuels Bioprod. Biorefining 2014, 8, 836–856. [Google Scholar] [CrossRef]
- Brião, V.B.; Seguenka, B.; Zanon, C.D.; Milani, A. Cake formation and the decreased performance of whey ultrafiltration. Acta Sci. Technol. 2017, 39, 517–524. [Google Scholar] [CrossRef][Green Version]
- Hwang, K.-J.; Liao, C.-Y.; Tung, K.-L. Effect of membrane pore size on the particle fouling in membrane filtration. Desalination 2008, 234, 16–23. [Google Scholar] [CrossRef]
- Du, X.; Gellerstedt, G.; Li, J. Universal fractionation of lignin–carbohydrate complexes (LCCs) from lignocellulosic biomass: An example using spruce wood. Plant. J. 2013, 74, 328–338. [Google Scholar] [CrossRef]
- Jönsson, A.-S.; Nordin, A.-K.; Wallberg, O. Concentration and purification of lignin in hardwood kraft pulping liquor by ultrafiltration and nanofiltration. Chem. Eng. Res. Des. 2008, 86, 1271–1280. [Google Scholar] [CrossRef]
- Jönsson, A.-S.; Wallberg, O. Cost estimates of kraft lignin recovery by ultrafiltration. Desalination 2009, 237, 254–267. [Google Scholar] [CrossRef]
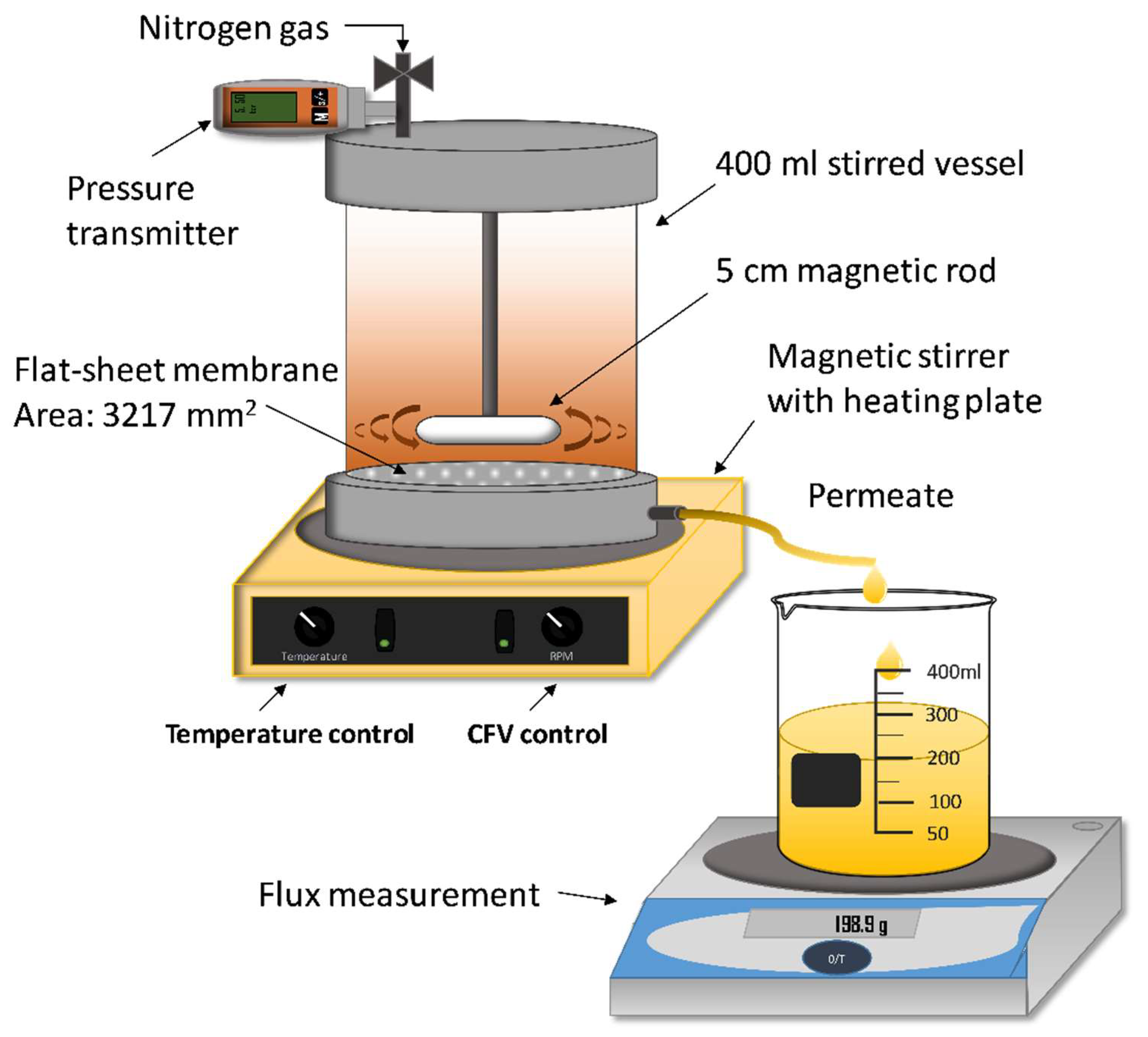
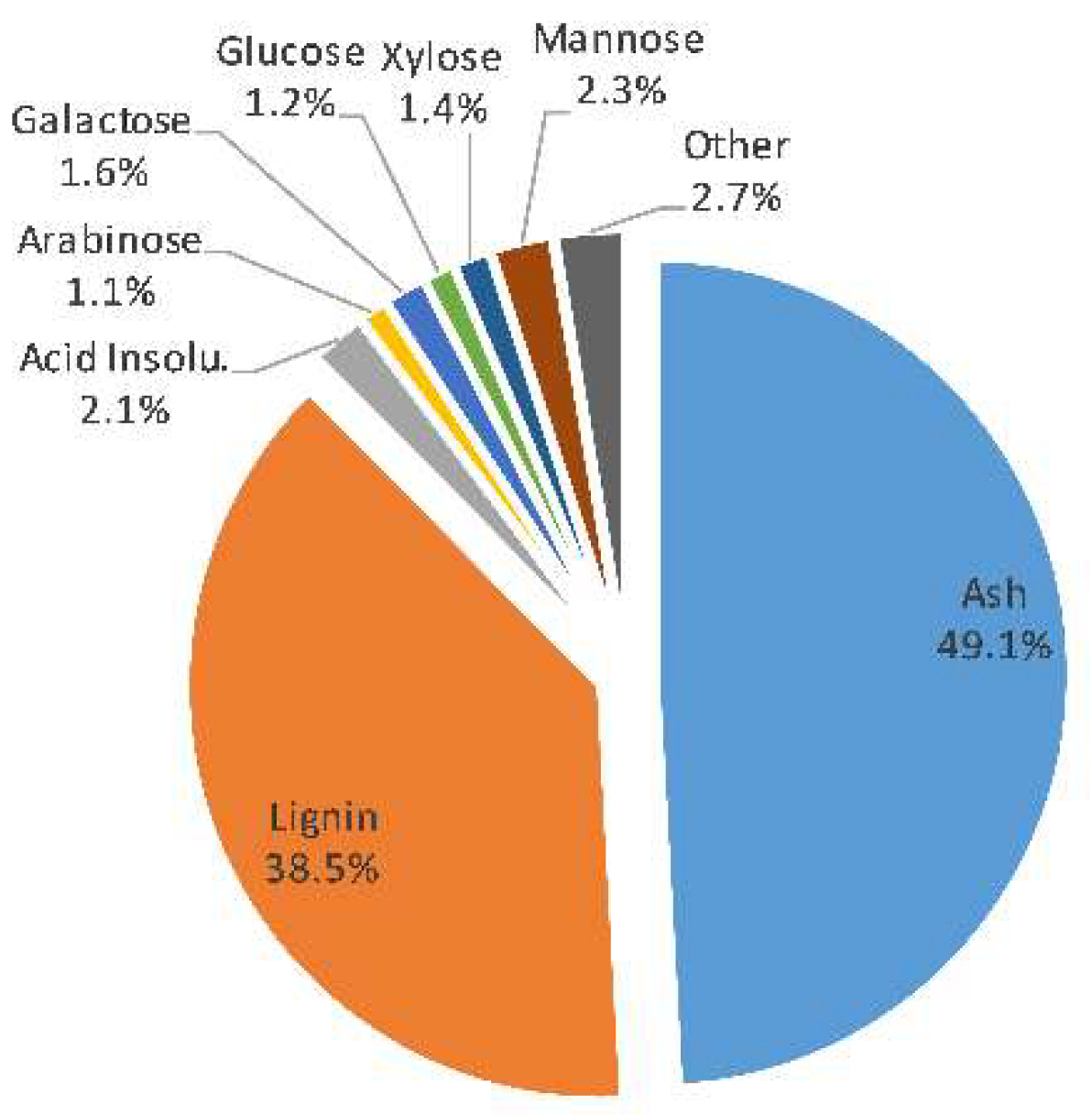

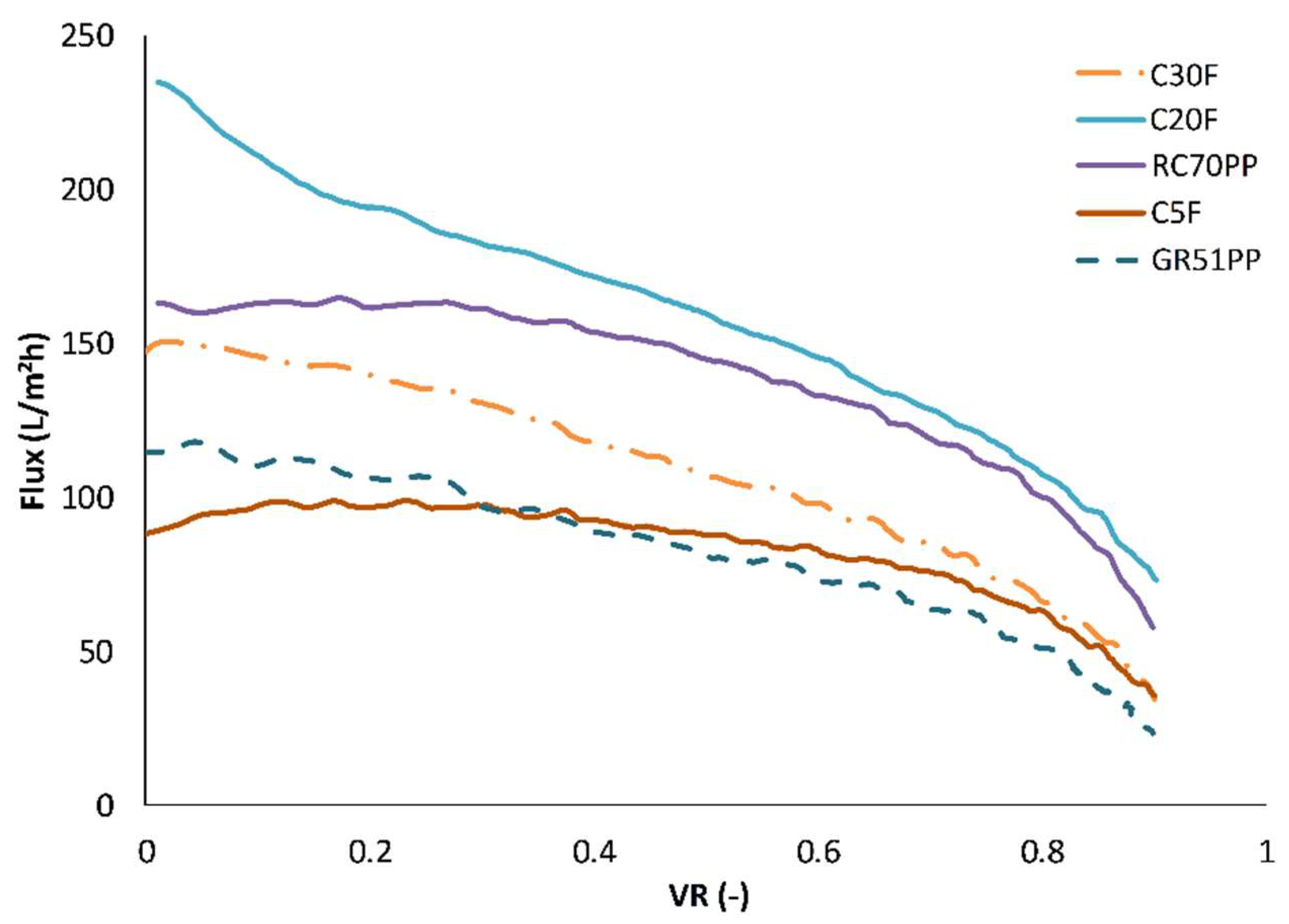
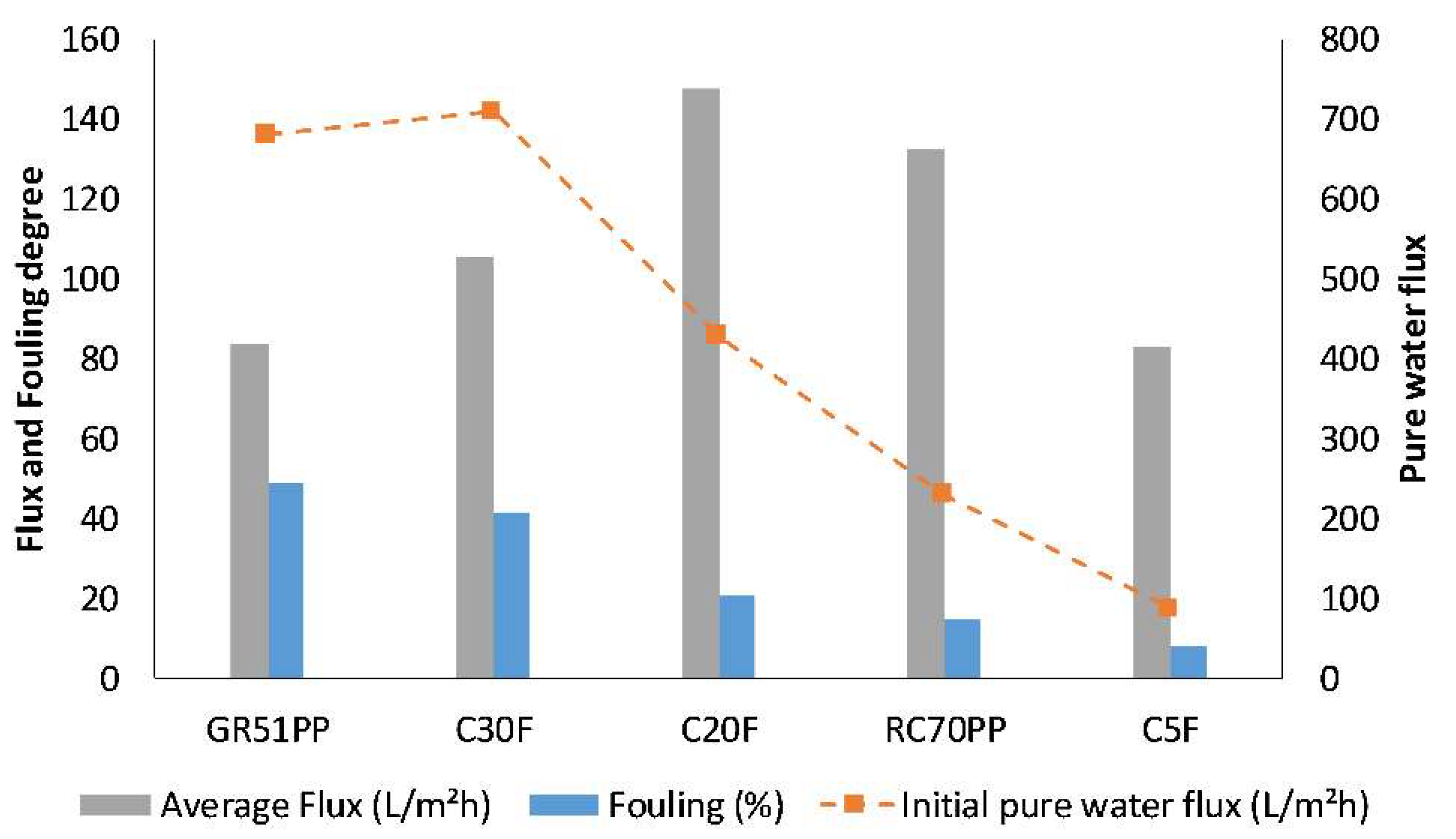
| Designation | Manufacturer | MWCO (kDa) | Membrane Material | Hydrophilicity |
|---|---|---|---|---|
| GR51PP | Alfa Laval Nordic A/S | 50 | Polysulfone | Hydrophobic |
| C30F | Microdyn-Nadir GmbH | 30 | Regenerated cellulose | Hydrophilic |
| C20F | Microdyn-Nadir GmbH | 20 | Regenerated cellulose | Hydrophilic |
| RC70PP | Alfa Laval Nordic A/S | 10 | Regenerated cellulose | Hydrophilic |
| C5F | Microdyn-Nadir GmbH | 5 | Regenerated cellulose | Hydrophilic |
| Investment cost (€/m2) | 500 |
| Annuity factor (/year) | 0.1 |
| Membrane cost (less housing) (€/m2) | 50 |
| Membrane life-time (years) | 1.5 |
| Electricity price (€/MWh) | 38 |
| Cleaning Cost (€/m2/cycle) | 0.13 |
| Cleaning time (h/day) | 1 |
| Extra membrane area for cleaning (%) | 20 |
| Maintenance and labor costs (% of capital cost) | 5 |
| Operation Time (h/year) | 8000 |
| Pump efficiency (-) | 0.8 |
| Permeate density (kg/m3) | 1100 |
| Maximum pressure drop (at 1.5 m3/h) (bar) | 0.6 |
| Membrane area per module (m2) | 1.2 |
| Plant feed flow (m3/h) | 1 |
| Plant volume reduction (%) | 90 |
| Plant transmembrane pressure (bar) | 5.5 |
| Parameters | C30F | C20F | RC70PP | C5F | GR51PP |
|---|---|---|---|---|---|
| Average flux (L/m2 h) | 105.0 | 147.4 | 132.2 | 82.8 | 83.9 |
| GGM yield (%) | 56.2 | 45.0 | 59.3 | 65.6 | 45.9 |
| GGM produced (kg/h) | 2.61 | 2.09 | 2.75 | 3.04 | 2.51 |
| Membrane Area (m2) | 7.79 | 5.55 | 6.19 | 9.89 | 9.75 |
| Feed pump energy demand (kW) | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 |
| Recirculation pump energy demand (kW) | 0.20 | 0.14 | 0.16 | 0.26 | 0.25 |
| Capital cost (€/year) | 468 | 333 | 371 | 593 | 585 |
| Electricity cost (€/year) | 114 | 97 | 102 | 131 | 130 |
| Membrane replacement cost (€/year) | 312 | 222 | 247 | 395 | 390 |
| Cleaning cost (€/year) | 405 | 288 | 321 | 514 | 507 |
| Maintenance and labor costs (€/year) | 23 | 17 | 19 | 30 | 29 |
| Total cost (€/year) | 1322 | 957 | 1060 | 1663 | 1641 |
| Cost per ton product (€/ton GGM) | 63.36 | 57.26 | 48.23 | 68.31 | 81.86 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Rudainy, B.; Galbe, M.; Lipnizki, F.; Wallberg, O. Galactoglucomannan Recovery with Hydrophilic and Hydrophobic Membranes: Process Performance and Cost Estimations. Membranes 2019, 9, 99. https://doi.org/10.3390/membranes9080099
Al-Rudainy B, Galbe M, Lipnizki F, Wallberg O. Galactoglucomannan Recovery with Hydrophilic and Hydrophobic Membranes: Process Performance and Cost Estimations. Membranes. 2019; 9(8):99. https://doi.org/10.3390/membranes9080099
Chicago/Turabian StyleAl-Rudainy, Basel, Mats Galbe, Frank Lipnizki, and Ola Wallberg. 2019. "Galactoglucomannan Recovery with Hydrophilic and Hydrophobic Membranes: Process Performance and Cost Estimations" Membranes 9, no. 8: 99. https://doi.org/10.3390/membranes9080099
APA StyleAl-Rudainy, B., Galbe, M., Lipnizki, F., & Wallberg, O. (2019). Galactoglucomannan Recovery with Hydrophilic and Hydrophobic Membranes: Process Performance and Cost Estimations. Membranes, 9(8), 99. https://doi.org/10.3390/membranes9080099







