Recent Progresses in Application of Membrane Bioreactors in Production of Biohydrogen
Abstract
1. Introduction
2. Basic Biohydrogen Production Technologies
2.1. Fermentation
2.1.1. Photo-Fermentation
2.1.2. Dark Fermentation
- Pyruvate: formate lyase (PFL) Pyruvate + CoA → acetyl-CoA + formate
- Pyruvate: ferredoxin oxido reductase (PFOR) Pyruvate + CoA + 2Fd(ox) → acetyl-CoA + CO2 + 2Fd(red).
2.2. Biophotolysis
2.2.1. Direct Biophotolysis
2.2.2. Indirect Biophotolysis
3. General Features of MBR Systems
3.1. Bioreactor Configurations
3.2. Membrane Materials
3.3. Potentials and Limitations of AnMBR Technology
- Ability of retaining anaerobic microbes due to uncoupling of HRT and solid retention time (SRT).
- Providing cost-effective and environmental friendly ambient-temperature anaerobic digestion (AD) by increasing SRT [74].
- Low disposal of biosolids due to low growth yield of anaerobic biomass.
- Improving the stabilization of biosolids by increasing SRT.
- Employing micron pore-sized filtration as appropriate membrane cut-off, which leads to production of high-quality permeate.
- Ability of conversion of biodegradable compounds into gaseous energy carriers like methane and biohydrogen at ambient temperature [30].
- Reducing greenhouse gas emissions by saving energy consumption.
4. Biohydrogen Production in Anaerobic Membrane Bioreactors
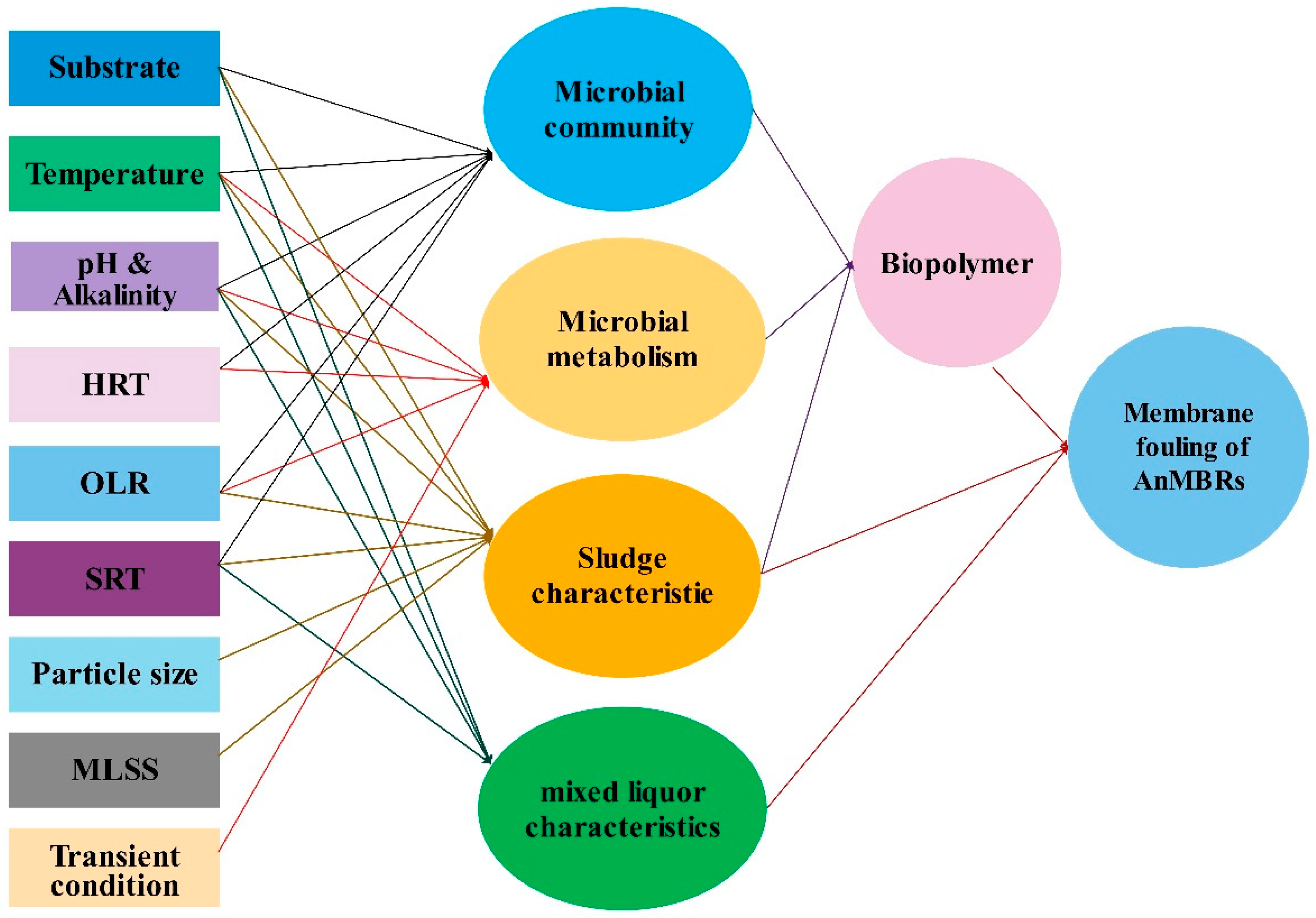
4.1. Factors Affecting Biohydrogen Production in AnMBRs
4.1.1. Substrate Concentration and Nutrients Loading
4.1.2. Hydraulic Retention Time (HRT) and Solid Retention Time (SRT)
4.1.3. Temperature and pH
4.1.4. Hydrogen Partial Pressure
4.1.5. Microbial Culture and Metabolism
5. Membrane Fouling and Fouling Mechanisms
5.1. Biofouling, Organic/Inorganic Fouling
5.1.1. Biofouling
5.1.2. Organic Fouling
5.1.3. Inorganic Fouling
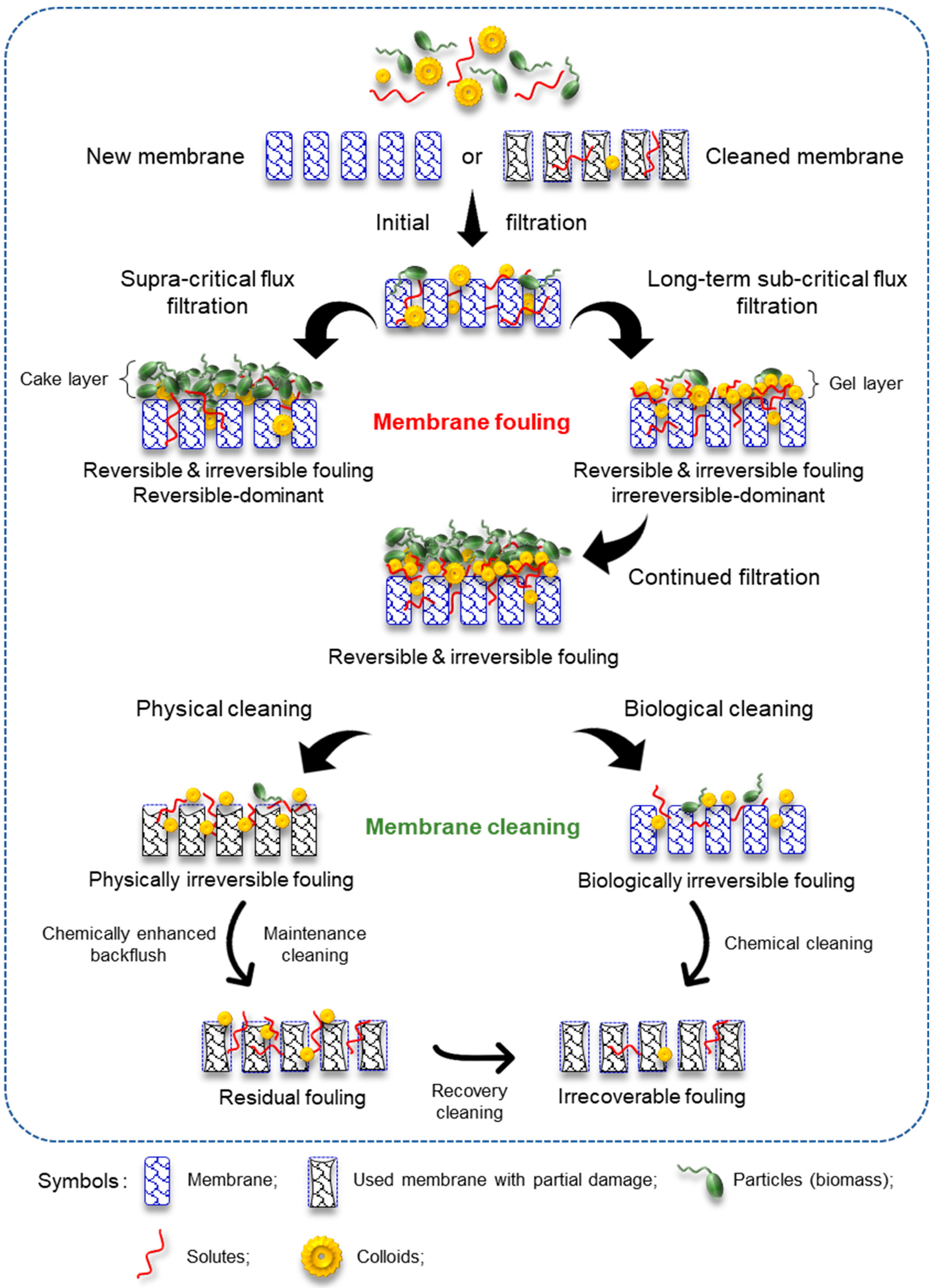
5.2. Reversible, Irreversible, Residual, and Irrecoverable Fouling
5.2.1. Reversible Fouling
5.2.2. Irreversible Fouling
5.2.3. Residual Fouling
5.2.4. Irrecoverable Fouling
5.3. Strategies for Fouling Removal
5.3.1. Physical Cleaning
5.3.2. Chemical Cleaning
5.3.3. Anti-Fouling Membranes
6. Biohydrogen Separation and Purification
H2 Selective Membranes and Operational Conditions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
List of Acronyms
| AD | Aanaerobic digestion |
| AeMBR | Aerobic membrane bioreactor |
| AFBR | Anaerobic fluidized-bed reactor |
| AnMBR | Anaerobic membrane bioreactor |
| ASBR | Anaerobic Sequencing Batch Reactor |
| ATP | Adenosine triphosphate |
| CIGSB | Carrier-induced granular sludge bed reactor |
| CNT | Carbon nanotubes |
| COD | Chemical oxygen demand |
| CSTR | Continuous stirred tank reactor |
| EPS | Extracellular polymeric substances |
| FBBAC | Fixed bed bioreactor with activated carbon |
| FBR | Fixed bed reactor |
| FHL | Formate hydrogenlyase |
| FS | Flat sheet |
| GO | Graphene oxide |
| HF | Hollow fiber |
| HRT | High retention time |
| ICSTR | Intermittent continuous stirred tank reactor |
| LBR | Leaching bed reactor |
| MBR | Membrane bioreactor |
| MF | Microfiltration |
| MFC | Microbial fuel cells |
| MLSS | Mixed liquor suspended solid |
| MT | Multi tubular |
| NADH | Nicotinamide adenine dinucleotide |
| NF | Nanofiltration |
| OLR | Organic loading rate |
| PE | Polyethylene |
| PES | Polyethersulfone |
| PFL | Pyruvate formate lyase |
| PFOR | Pyruvate ferredoxin oxido reductase |
| PNS | Photosynthetic non-sulfur |
| PP | Polypropylene |
| PS | Polysulfone |
| PVDF | Polyvinylidene difluoride |
| SMP | Soluble microbial products |
| SRT | Solid retention time |
| TCA | Tricarboxylic acid cycle |
| ThAeMBR | Thermophilic aerobic membrane bioreactor |
| ThAnMBR | Thermophilic anaerobic membrane bioreactor |
| TMP | Transmembrane pressure |
| UASB | Up-flow Anaerobic Sludge Blanket |
| UF | Ultrafiltration |
| VFA | Volatil fatty acid |
References
- Argun, H.; Kargi, F.; Kapdan, I.K.; Oztekin, R. Biohydrogen production by dark fermentation of wheat powder solution: Effects of C/N and C/P ratio on hydrogen yield and formation rate. Int. J. Hydrogen Energy 2008, 33, 1813–1819. [Google Scholar] [CrossRef]
- Shin, J.-H.; Yoon, J.H.; Lee, S.H.; Park, T.H. Hydrogen production from formic acid in pH-stat fed-batch operation for direct supply to fuel cell. Bioresour. Technol. 2010, 101, S53–S58. [Google Scholar] [CrossRef] [PubMed]
- Manish, S.; Banerjee, R. Comparison of biohydrogen production processes. Int. J. Hydrogen Energy 2008, 33, 279–286. [Google Scholar] [CrossRef]
- Aslam, M.; Ahmad, R.; Yasin, M.; Khan, A.L.; Shahid, M.K.; Hossain, S.; Khan, Z.; Jamil, F.; Rafiq, S.; Bilad, M.R. Anaerobic membrane bioreactors for biohydrogen production: Recent developments, challenges and perspectives. Bioresour. Technol. 2018, 269, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Veziroglu, T.N. Advances in biological hydrogen production processes. Int. J. Hydrogen Energy 2008, 33, 6046–6057. [Google Scholar] [CrossRef]
- Hawkes, F.R.; Hussy, I.; Kyazze, G.; Dinsdale, R.; Hawkes, D.L. Continuous dark fermentative hydrogen production by mesophilic microflora: Principles and progress. Int. J. Hydrogen Energy 2007, 32, 172–184. [Google Scholar] [CrossRef]
- Kraemer, J.T.; Bagley, D.M. Improving the yield from fermentative hydrogen production. Biotechnol. Lett. 2007, 29, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Show, K.; Lee, D.; Tay, J.; Lin, C.; Chang, J.-S. Biohydrogen production: Current perspectives and the way forward. Int. J. Hydrogen Energy 2012, 37, 15616–15631. [Google Scholar] [CrossRef]
- Saifuddin, N.; Priatharsini, P. Developments in bio-hydrogen production from algae: A review. Res. J. Appl. Sci. Eng. Technol. 2016, 12, 968–982. [Google Scholar] [CrossRef]
- Kapdan, I.K.; Kargi, F. Bio-hydrogen production from waste materials. Enzym. Microb. Technol. 2006, 38, 569–582. [Google Scholar] [CrossRef]
- Wang, A.; Sun, D.; Cao, G.; Wang, H.; Ren, N.; Wu, W.-M.; Logan, B.E. Integrated hydrogen production process from cellulose by combining dark fermentation, microbial fuel cells, and a microbial electrolysis cell. Bioresour. Technol. 2011, 102, 4137–4143. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, H.P.; Mortensen, K.K. Advanced genetic strategies for recombinant protein expression in Escherichia coli. J. Biotechnol. 2005, 115, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, K.; Aghaeinejad-Meybodi, A.; Basile, A. Hydrogen production as a green fuel in silica membrane reactor: Experimental analysis and artificial neural network modeling. Fuel 2018, 222, 114–124. [Google Scholar] [CrossRef]
- Chen, W.-H. Biological Hydrogen Production by Anaerobic Fermentation. Ph.D. Thesis, Iowa State University, Ames, ID, USA, 2006. [Google Scholar]
- Turner, J.; Sverdrup, G.; Mann, M.K.; Maness, P.C.; Kroposki, B.; Ghirardi, M.; Evans, R.J.; Blake, D. Renewable hydrogen production. Int. J. Energy Res. 2008, 32, 379–407. [Google Scholar] [CrossRef]
- Robles, Á.; Ruano, M.V.; Charfi, A.; Lesage, G.; Heran, M.; Harmand, J.; Seco, A.; Steyer, J.-P.; Batstone, D.J.; Kim, J. A review on anaerobic membrane bioreactors (AnMBRs) focused on modelling and control aspects. Bioresour. Technol. 2018, 270, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Arya, S.K. Hydrogen from algal biomass: A review of production process. Biotechnol. Rep. 2017, 15, 63–69. [Google Scholar] [CrossRef]
- Akkerman, I.; Janssen, M.; Rocha, J.; Wijffels, R.H. Photobiological hydrogen production: Photochemical efficiency and bioreactor design. Int. J. Hydrogen Energy 2002, 27, 1195–1208. [Google Scholar] [CrossRef]
- Mudhoo, A.; Forster-Carneiro, T.; Sánchez, A. Biohydrogen production and bioprocess enhancement: A review. Crit. Rev. Biotechnol. 2011, 31, 250–263. [Google Scholar] [CrossRef]
- Levin, D.B.; Pitt, L.; Love, M. Biohydrogen production: Prospects and limitations to practical application. Int. J. Hydrogen Energy 2004, 29, 173–185. [Google Scholar] [CrossRef]
- Hallenbeck, P.C.; Ghosh, D. Advances in fermentative biohydrogen production: The way forward? Trends Biotechnol. 2009, 27, 287–297. [Google Scholar] [CrossRef]
- Łukajtis, R.; Hołowacz, I.; Kucharska, K.; Glinka, M.; Rybarczyk, P.; Przyjazny, A.; Kamiński, M. Hydrogen production from biomass using dark fermentation. Renew. Sustain. Energy Rev. 2018, 91, 665–694. [Google Scholar] [CrossRef]
- Logan, B.E.; Call, D.; Cheng, S.; Hamelers, H.V.; Sleutels, T.H.; Jeremiasse, A.W.; Rozendal, R.A. Microbial electrolysis cells for high yield hydrogen gas production from organic matter. Environ. Sci. Technol. 2008, 42, 8630–8640. [Google Scholar] [CrossRef]
- Mohanraj, S.; Pandey, A.; Mohan, S.V.; Anbalagan, K.; Kodhaiyolii, S.; Pugalenthi, V. Metabolic Engineering and Molecular Biotechnology of Biohydrogen Production. In Biohydrogen; Elsevier: Amsterdam, The Netherlands, 2019; pp. 413–434. [Google Scholar]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Yoshida, A.; Nishimura, T.; Kawaguchi, H.; Inui, M.; Yukawa, H. Enhanced hydrogen production from formic acid by formate hydrogen lyase-overexpressing Escherichia coli strains. Appl. Environ. Microbiol. 2005, 71, 6762–6768. [Google Scholar] [CrossRef]
- Oh, Y.-K.; Seol, E.-H.; Lee, E.Y.; Park, S. Fermentative hydrogen production by a new chemoheterotrophic bacterium Rhodopseudomonas palustris P4. Int. J. Hydrogen Energy 2002, 27, 1373–1379. [Google Scholar] [CrossRef]
- Oh, Y.-K.; Seol, E.-H.; Kim, J.R.; Park, S. Fermentative biohydrogen production by a new chemoheterotrophic bacterium Citrobacter sp. Y19. Int. J. Hydrogen Energy 2003, 28, 1353–1359. [Google Scholar] [CrossRef]
- Mandal, B.; Nath, K.; Das, D. Improvement of biohydrogen production under decreased partial pressure of H 2 by Enterobacter cloacae. Biotechnol. Lett. 2006, 28, 831–835. [Google Scholar] [CrossRef]
- Khan, M.; Ngo, H.; Guo, W.; Liu, Y.; Zhou, J.; Zhang, J.; Liang, S.; Ni, B.; Zhang, X.; Wang, J. Comparing the value of bioproducts from different stages of anaerobic membrane bioreactors. Bioresour. Technol. 2016, 214, 816–825. [Google Scholar] [CrossRef]
- Singer, S.; Magnusson, L.; Hou, D.; Lo, J.; Maness, P.-C.; Ren, Z.J. Anaerobic membrane gas extraction facilitates thermophilic hydrogen production from Clostridium thermocellum. Environ. Sci. Water Res. Technol. 2018, 4, 1771–1782. [Google Scholar] [CrossRef]
- Rahman, S.; Masdar, M.; Rosli, M.; Majlan, E.; Husaini, T. Overview of biohydrogen production technologies and application in fuel cell. Am. J. Chem. 2015, 5, 13–23. [Google Scholar]
- Yu, J.; Takahashi, P. Biophotolysis-based hydrogen production by cyanobacteria and green microalgae. Commun. Curr. Res. Educ. Top. Trends Appl. Microbiol. 2007, 1, 79–89. [Google Scholar]
- Türker, L.; Gümüs, S.; Tapan, A. Biohydrogen production: Molecular aspects. J. Sci. Ind. Res. 2008, 67, 994–1016. [Google Scholar]
- Ghirardi, M.L.; Zhang, L.; Lee, J.W.; Flynn, T.; Seibert, M.; Greenbaum, E.; Melis, A. Microalgae: A green source of renewable H2. Trends Biotechnol. 2000, 18, 506–511. [Google Scholar] [CrossRef]
- Larminie, J.; Dicks, A.; McDonald, M.S. Fuel Cell Systems Explained; John Wiley: Chichester, UK, 2003; Volume 2. [Google Scholar]
- Bélafi-Bakó, K.; Búcsú, D.; Pientka, Z.; Bálint, B.; Herbel, Z.; Kovács, K.; Wessling, M. Integration of biohydrogen fermentation and gas separation processes to recover and enrich hydrogen. Int. J. Hydrogen Energy 2006, 31, 1490–1495. [Google Scholar] [CrossRef]
- Lei, Z.; Yang, S.; Li, Y.; Wen, W.; Wang, X.C.; Chen, R. Application of anaerobic membrane bioreactors to municipal wastewater treatment at ambient temperature: A review of achievements, challenges, and perspectives. Bioresour. Technol. 2018, 267, 756–768. [Google Scholar] [CrossRef]
- Judd, S.J. The status of industrial and municipal effluent treatment with membrane bioreactor technology. Chem. Eng. J. 2016, 305, 37–45. [Google Scholar] [CrossRef]
- Jalilnejad, E.; Sadeghpour, P.; Ghasemzadeh, K. Advances in MBR Technology. In Current Trends and Future Developments on (Bio-) Membranes, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019; p. 360. [Google Scholar]
- Bakonyi, P.; Nemestóthy, N.; Bélafi-Bakó, K. Biohydrogen purification by membranes: An overview on the operational conditions affecting the performance of non-porous, polymeric and ionic liquid based gas separation membranes. Int. J. Hydrogen Energy 2013, 38, 9673–9687. [Google Scholar] [CrossRef]
- Hallenbeck, P.C. Fermentative hydrogen production: Principles, progress, and prognosis. Int. J. Hydrogen Energy 2009, 34, 7379–7389. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Nguyen, T.M.-L.; Chu, C.-Y.; Leu, H.-J.; Lay, C.-H. Fermentative biohydrogen production and its byproducts: A mini review of current technology developments. Renew. Sustain. Energy Rev. 2018, 82, 4215–4220. [Google Scholar] [CrossRef]
- Nath, K.; Das, D. Improvement of fermentative hydrogen production: Various approaches. Appl. Microbiol. Biotechnol. 2004, 65, 520–529. [Google Scholar] [CrossRef]
- Ramírez-Morales, J.; Tapia-Venegas, E.; Nemestóthy, N.; Bakonyi, P.; Bélafi-Bakó, K.; Ruiz-Filippi, G. Evaluation of two gas membrane modules for fermentative hydrogen separation. Int. J. Hydrogen Energy 2013, 38, 14042–14052. [Google Scholar] [CrossRef]
- Bakonyi, P.; Nemestóthy, N.; Simon, V.; Bélafi-Bakó, K. Fermentative hydrogen production in anaerobic membrane bioreactors: A review. Bioresour. Technol. 2014, 156, 357–363. [Google Scholar] [CrossRef]
- da Silva Veras, T.; Mozer, T.S.; da Silva César, A. Hydrogen: Trends, production and characterization of the main process worldwide. Int. J. Hydrogen Energy 2017, 42, 2018–2033. [Google Scholar] [CrossRef]
- Ren, N.; Chua, H.; Chan, S.; Tsang, Y.; Wang, Y.; Sin, N. Assessing optimal fermentation type for bio-hydrogen production in continuous-flow acidogenic reactors. Bioresour. Technol. 2007, 98, 1774–1780. [Google Scholar] [CrossRef]
- Cheong, D.Y.; Hansen, C.L.; Stevens, D.K. Production of bio-hydrogen by mesophilic anaerobic fermentation in an acid-phase sequencing batch reactor. Biotechnol. Bioeng. 2007, 96, 421–432. [Google Scholar] [CrossRef]
- Chang, J.-S.; Lee, K.-S.; Lin, P.-J. Biohydrogen production with fixed-bed bioreactors. Int. J. Hydrogen Energy 2002, 27, 1167–1174. [Google Scholar] [CrossRef]
- Zhang, Z.-P.; Tay, J.-H.; Show, K.-Y.; Yan, R.; Liang, D.T.; Lee, D.-J.; Jiang, W.-J. Biohydrogen production in a granular activated carbon anaerobic fluidized bed reactor. Int. J. Hydrogen Energy 2007, 32, 185–191. [Google Scholar] [CrossRef]
- Chang, F.-Y.; Lin, C.-Y. Biohydrogen production using an up-flow anaerobic sludge blanket reactor. Int. J. Hydrogen Energy 2004, 29, 33–39. [Google Scholar] [CrossRef]
- Lee, K.-S.; Lo, Y.-C.; Lin, P.-J.; Chang, J.-S. Improving biohydrogen production in a carrier-induced granular sludge bed by altering physical configuration and agitation pattern of the bioreactor. Int. J. Hydrogen Energy 2006, 31, 1648–1657. [Google Scholar] [CrossRef]
- Wu, K.-J.; Chang, C.-F.; Chang, J.-S. Simultaneous production of biohydrogen and bioethanol with fluidized-bed and packed-bed bioreactors containing immobilized anaerobic sludge. Process Biochem. 2007, 42, 1165–1171. [Google Scholar] [CrossRef]
- Zhang, Z.-P.; Show, K.-Y.; Tay, J.-H.; Liang, D.T.; Lee, D.-J. Biohydrogen production with anaerobic fluidized bed reactors—A comparison of biofilm-based and granule-based systems. Int. J. Hydrogen Energy 2008, 33, 1559–1564. [Google Scholar] [CrossRef]
- Oh, S.E.; Iyer, P.; Bruns, M.A.; Logan, B.E. Biological hydrogen production using a membrane bioreactor. Biotechnol. Bioeng. 2004, 87, 119–127. [Google Scholar] [CrossRef]
- Han, S.-K.; Shin, H.-S. Performance of an innovative two-stage process converting food waste to hydrogen and methane. J. Air Waste Manag. Assoc. 2004, 54, 242–249. [Google Scholar] [CrossRef]
- Lee, Z.-K.; Li, S.-L.; Kuo, P.-C.; Chen, I.-C.; Tien, Y.-M.; Huang, Y.-J.; Chuang, C.-P.; Wong, S.-C.; Cheng, S.-S. Thermophilic bio-energy process study on hydrogen fermentation with vegetable kitchen waste. Int. J. Hydrogen Energy 2010, 35, 13458–13466. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pontoni, L.; d’Antonio, G.; Lens, P.N.; Esposito, G.; Pirozzi, F. Dark fermentation of complex waste biomass for biohydrogen production by pretreated thermophilic anaerobic digestate. J. Environ. Manag. 2015, 152, 43–48. [Google Scholar] [CrossRef]
- Cicek, N. A review of membrane bioreactors and their potential application in the treatment of agricultural wastewater. Can. Biosyst. Eng. 2003, 45, 6–37. [Google Scholar]
- Manem, J.; Sanderson, R. Membrane bioreactors. In Water Treatment Membrane Processes; McGraw-Hill: Singapore, 1996; Volume 17, pp. 1–33. [Google Scholar]
- Stephenson, T.; Brindle, K.; Judd, S.; Jefferson, B. Membrane Bioreactors for Wastewater Treatment; IWA Publishing: London, UK, 2000. [Google Scholar]
- Dereli, R.K.; Ersahin, M.E.; Ozgun, H.; Ozturk, I.; Jeison, D.; van der Zee, F.; van Lier, J.B. Potentials of anaerobic membrane bioreactors to overcome treatment limitations induced by industrial wastewaters. Bioresour. Technol. 2012, 122, 160–170. [Google Scholar] [CrossRef]
- Abeynayaka, A.; Visvanathan, C. Performance comparison of mesophilic and thermophilic aerobic sidestream membrane bioreactors treating high strength wastewater. Bioresour. Technol. 2011, 102, 5345–5352. [Google Scholar] [CrossRef]
- Qu, X.; Gao, W.; Han, M.; Chen, A.; Liao, B. Integrated thermophilic submerged aerobic membrane bioreactor and electrochemical oxidation for pulp and paper effluent treatment–towards system closure. Bioresour. Technol. 2012, 116, 1–8. [Google Scholar] [CrossRef]
- Qu, X.; Gao, W.; Han, M.; Chen, A.; Liao, B. Effect of hydraulic retention time on sludge properties, cake layer structure, and membrane fouling in a thermophilic submerged aerobic membrane bioreactor. Sep. Sci. Technol. 2013, 48, 1529–1536. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Castagnola, F.; Sordi, M.; Bertanza, G. Sewage sludge treatment in a thermophilic membrane reactor (TMR): Factors affecting foam formation. Environ. Sci. Pollut. Res. 2017, 24, 2316–2325. [Google Scholar] [CrossRef]
- Wijekoon, K.C.; Hai, F.I.; Kang, J.; Price, W.E.; Guo, W.; Ngo, H.H.; Cath, T.Y.; Nghiem, L.D. A novel membrane distillation–thermophilic bioreactor system: Biological stability and trace organic compound removal. Bioresour. Technol. 2014, 159, 334–341. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Bertanza, G. Why use a thermophilic aerobic membrane reactor for the treatment of industrial wastewater/liquid waste? Environ. Technol. 2015, 36, 2115–2124. [Google Scholar] [CrossRef]
- Meabe, E.; Déléris, S.; Soroa, S.; Sancho, L. Performance of anaerobic membrane bioreactor for sewage sludge treatment: Mesophilic and thermophilic processes. J. Membr. Sci. 2013, 446, 26–33. [Google Scholar] [CrossRef]
- Pehlivaner, G.; Buyukkamaci, N. Effect of different temperatures on the performance of AnMBR systems. J. Selcuk Univ. Nat. Appl. Sci. 2014, 217–224. [Google Scholar]
- Kale, M.M.; Singh, K.S. Comparison of a mesophilic and thermophilic novel sludge-bed anaerobic membrane bioreactor treating prehydrolysis liquor from a dissolving pulp mill. J. Environ. Eng. 2016, 142, 04016030. [Google Scholar] [CrossRef]
- Fan, R.; Ebrahimi, M.; Quitmann, H.; Czermak, P. Lactic acid production in a membrane bioreactor system with thermophilic Bacillus coagulans: Online monitoring and process control using an optical sensor. Sep. Sci. Technol. 2017, 52, 352–363. [Google Scholar] [CrossRef]
- Pileggi, V.; Parker, W.J. AnMBR digestion of mixed WRRF sludges: Impact of digester loading and temperature. J. Water Process Eng. 2017, 19, 74–80. [Google Scholar] [CrossRef]
- Crone, B.C.; Garland, J.L.; Sorial, G.A.; Vane, L.M. Significance of dissolved methane in effluents of anaerobically treated low strength wastewater and potential for recovery as an energy product: A review. Water Res. 2016, 104, 520–531. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, J.; Tang, C.Y.; Kimura, K.; Wang, Q.; Han, X. Membrane cleaning in membrane bioreactors: A review. J. Membr. Sci. 2014, 468, 276–307. [Google Scholar] [CrossRef]
- Batstone, D.; Hülsen, T.; Mehta, C.; Keller, J. Platforms for energy and nutrient recovery from domestic wastewater: A review. Chemosphere 2015, 140, 2–11. [Google Scholar] [CrossRef]
- Yue, X.; Koh, Y.K.K.; Ng, H.Y. Effects of dissolved organic matters (DOMs) on membrane fouling in anaerobic ceramic membrane bioreactors (AnCMBRs) treating domestic wastewater. Water Res. 2015, 86, 96–107. [Google Scholar] [CrossRef]
- Trad, Z.; Vial, C.; Fontaine, J.-P.; Larroche, C. Modeling of hydrodynamics and mixing in a submerged membrane bioreactor. Chem. Eng. J. 2015, 282, 77–90. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Li, Y.-Y.; Noike, T.; Cha, G.-C. Behavior of extracellular polymers and bio-fouling during hydrogen fermentation with a membrane bioreactor. J. Membr. Sci. 2008, 322, 13–18. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Li, Y.-Y.; Oh, Y.-K.; Kim, M.-S.; Noike, T. Effect of iron concentration on continuous H2 production using membrane bioreactor. Int. J. Hydrogen Energy 2009, 34, 1244–1252. [Google Scholar] [CrossRef]
- Shen, L.; Zhou, Y.; Mahendran, B.; Bagley, D.M.; Liss, S.N. Membrane fouling in a fermentative hydrogen producing membrane bioreactor at different organic loading rates. J. Membr. Sci. 2010, 360, 226–233. [Google Scholar] [CrossRef]
- Van Ginkel, S.; Logan, B.E. Inhibition of biohydrogen production by undissociated acetic and butyric acids. Environ. Sci. Technol. 2005, 39, 9351–9356. [Google Scholar] [CrossRef]
- Noblecourt, A.; Christophe, G.; Larroche, C.; Santa-Catalina, G.; Trably, E.; Fontanille, P. High hydrogen production rate in a submerged membrane anaerobic bioreactor. Int. J. Hydrogen Energy 2017, 42, 24656–24666. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Li, Y.-Y.; Noike, T. Continuous H2 production by anaerobic mixed microflora in membrane bioreactor. Bioresour. Technol. 2009, 100, 690–695. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Xu, K.-Q.; Kobayashi, T.; Li, Y.-Y.; Inamori, Y. Effect of organic loading rate on continuous hydrogen production from food waste in submerged anaerobic membrane bioreactor. Int. J. Hydrogen Energy 2014, 39, 16863–16871. [Google Scholar] [CrossRef]
- Mohan, S.V. Harnessing of biohydrogen from wastewater treatment using mixed fermentative consortia: Process evaluation towards optimization. Int. J. Hydrogen Energy 2009, 34, 7460–7474. [Google Scholar] [CrossRef]
- Hafez, H.; Baghchehsaraee, B.; Nakhla, G.; Karamanev, D.; Margaritis, A.; El Naggar, H. Comparative assessment of decoupling of biomass and hydraulic retention times in hydrogen production bioreactors. Int. J. Hydrogen Energy 2009, 34, 7603–7611. [Google Scholar] [CrossRef]
- Lee, K.-S.; Lin, P.-J.; Fangchiang, K.; Chang, J.-S. Continuous hydrogen production by anaerobic mixed microflora using a hollow-fiber microfiltration membrane bioreactor. Int. J. Hydrogen Energy 2007, 32, 950–957. [Google Scholar] [CrossRef]
- Shen, L.; Bagley, D.M.; Liss, S.N. Effect of organic loading rate on fermentative hydrogen production from continuous stirred tank and membrane bioreactors. Int. J. Hydrogen Energy 2009, 34, 3689–3696. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Li, Y.-Y.; Noike, T. Influence of solids retention time on continuous H2 production using membrane bioreactor. Int. J. Hydrogen Energy 2010, 35, 52–60. [Google Scholar] [CrossRef]
- Kim, M.-S.; Lee, D.-Y.; Kim, D.-H. Continuous hydrogen production from tofu processing waste using anaerobic mixed microflora under thermophilic conditions. Int. J. Hydrogen Energy 2011, 36, 8712–8718. [Google Scholar] [CrossRef]
- Han, W.; Huang, J.; Zhao, H.; Li, Y. Continuous biohydrogen production from waste bread by anaerobic sludge. Bioresour. Technol. 2016, 212, 1–5. [Google Scholar] [CrossRef]
- Buitrón, G.; Muñoz-Páez, K.M.; Hernández-Mendoza, C.E. Biohydrogen production using a granular sludge membrane bioreactor. Fuel 2019, 241, 954–961. [Google Scholar] [CrossRef]
- Hernandez-Mendoza, C.E.; Moreno-Andrade, I.; Buitron, G. Comparison of hydrogen-producing bacterial communities adapted in continuous and discontinuous reactors. Int. J. Hydrogen Energy 2014, 39, 14234–14239. [Google Scholar] [CrossRef]
- Sivagurunathan, P.; Kumar, G.; Bakonyi, P.; Kim, S.-H.; Kobayashi, T.; Xu, K.Q.; Lakner, G.; Tóth, G.; Nemestóthy, N.; Bélafi-Bakó, K. A critical review on issues and overcoming strategies for the enhancement of dark fermentative hydrogen production in continuous systems. Int. J. Hydrogen Energy 2016, 41, 3820–3836. [Google Scholar] [CrossRef]
- Méndez-Contreras, J.M.; López-Escobar, L.A.; Martínez-Hernández, S.; Cantú-Lozano, D.; Ortiz-Ceballos, A.I. Rheological behavior of physicochemical sludges during methanogenesis suppression and hydrogen production at different organic loading rates. J. Environ. Sci. Health Part A 2016, 51, 515–522. [Google Scholar] [CrossRef]
- Ghasemzadeh, K.; Jalilnejad, E.; Tilebon, S.M.S. Hydrogen Production Technologies From Ethanol. In Ethanol; Elsevier: Amsterdam, The Netherlands, 2019; pp. 307–340. [Google Scholar]
- Van Ginkel, S.W.; Oh, S.-E.; Logan, B.E. Biohydrogen gas production from food processing and domestic wastewaters. Int. J. Hydrogen Energy 2005, 30, 1535–1542. [Google Scholar] [CrossRef]
- Kim, M.-S.; Oh, Y.-K.; Yun, Y.-S.; Lee, D.-Y. Fermentative hydrogen production from anaerobic bacteria using a membrane bioreactor. In Proceedings of the 16th world hydrogen energy conference, Lyon, France, 13–16 June 2006. [Google Scholar]
- Li, C.; Fang, H.H. Fermentative hydrogen production from wastewater and solid wastes by mixed cultures. Crit. Rev. Environ. Sci. Technol. 2007, 37, 1–39. [Google Scholar] [CrossRef]
- Hung, C.-H.; Chang, Y.-T.; Chang, Y.-J. Roles of microorganisms other than Clostridium and Enterobacter in anaerobic fermentative biohydrogen production systems—A review. Bioresour. Technol. 2011, 102, 8437–8444. [Google Scholar] [CrossRef]
- Krzeminski, P.; Leverette, L.; Malamis, S.; Katsou, E. Membrane bioreactors–a review on recent developments in energy reduction, fouling control, novel configurations, LCA and market prospects. J. Membr. Sci. 2017, 527, 207–227. [Google Scholar] [CrossRef]
- Harada, H.; Momonoi, K.; Yamazaki, S.; Takizawa, S. Application of anaerobic-UF membrane reactor for treatment of a wastewater containing high strength particulate organics. Water Sci. Technol. 1994, 30, 307. [Google Scholar] [CrossRef]
- Altmann, J.; Ripperger, S. Particle deposition and layer formation at the crossflow microfiltration. J. Membr. Sci. 1997, 124, 119–128. [Google Scholar] [CrossRef]
- Trzcinski, A.P.; Stuckey, D.C. Treatment of municipal solid waste leachate using a submerged anaerobic membrane bioreactor at mesophilic and psychrophilic temperatures: Analysis of recalcitrants in the permeate using GC-MS. Water Res. 2010, 44, 671–680. [Google Scholar] [CrossRef]
- Visvanathan, C.; Choudhary, M.; Montalbo, M.; Jegatheesan, V. Landfill leachate treatment using thermophilic membrane bioreactor. Desalination 2007, 204, 8–16. [Google Scholar] [CrossRef]
- Abeynayaka, A.; Visvanathan, C. Mesophilic and thermophilic aerobic batch biodegradation, utilization of carbon and nitrogen sources in high-strength wastewater. Bioresour. Technol. 2011, 102, 2358–2366. [Google Scholar] [CrossRef]
- Shahata, A.; Urase, T. Treatment of Saline wastewater by thermophilic membrane bioreactor. J. Water Environ. Technol. 2016, 14, 76–81. [Google Scholar] [CrossRef][Green Version]
- Duncan, J.; Bokhary, A.; Fatehi, P.; Kong, F.; Lin, H.; Liao, B. Thermophilic membrane bioreactors: A review. Bioresour. Technol. 2017, 243, 1180–1193. [Google Scholar] [CrossRef]
- Nie, Y.; Chen, R.; Tian, X.; Li, Y.-Y. Impact of water characteristics on the bioenergy recovery from sewage treatment by anaerobic membrane bioreactor via a comprehensive study on the response of microbial community and methanogenic activity. Energy 2017, 139, 459–467. [Google Scholar] [CrossRef]
- Dagnew, M.; Parker, W.; Seto, P.; Waldner, K.; Hong, Y.; Bayly, R.; Cumin, J. Pilot testing of an AnMBR for municipal wastewater treatment. Proc. Water Environ. Fed. 2011, 2011, 4931–4941. [Google Scholar] [CrossRef]
- Sweity, A.; Ying, W.; Belfer, S.; Oron, G.; Herzberg, M. pH effects on the adherence and fouling propensity of extracellular polymeric substances in a membrane bioreactor. J. Membr. Sci. 2011, 378, 186–193. [Google Scholar] [CrossRef]
- Dohare, D.; Trivedi, R. A review on membrane bioreactors: An emerging technology for industrial wastewater treatment. Int. J. Emerg. Technol. Adv. Eng. 2014, 4, 226–236. [Google Scholar]
- Le-Clech, P.; Chen, V.; Fane, T.A. Fouling in membrane bioreactors used in wastewater treatment. J. Membr. Sci. 2006, 284, 17–53. [Google Scholar] [CrossRef]
- Park, H.-D.; Chang, I.-S.; Lee, K.-J. Principles of Membrane Bioreactors for Wastewater Treatment; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Charfi, A.; Thongmak, N.; Benyahia, B.; Aslam, M.; Harmand, J.; Amar, N.B.; Lesage, G.; Sridang, P.; Kim, J.; Heran, M. A modelling approach to study the fouling of an anaerobic membrane bioreactor for industrial wastewater treatment. Bioresour. Technol. 2017, 245, 207–215. [Google Scholar] [CrossRef]
- Gkotsis, P.; Banti, D.; Peleka, E.; Zouboulis, A.; Samaras, P. Fouling issues in membrane bioreactors (MBRs) for wastewater treatment: Major mechanisms, prevention and control strategies. Processes 2014, 2, 795–866. [Google Scholar] [CrossRef]
- Aslam, M.; Charfi, A.; Lesage, G.; Heran, M.; Kim, J. Membrane bioreactors for wastewater treatment: A review of mechanical cleaning by scouring agents to control membrane fouling. Chem. Eng. J. 2017, 307, 897–913. [Google Scholar] [CrossRef]
- Burman, I.; Sinha, A. A Review on Membrane Fouling in Membrane Bioreactors: Control and Mitigation. In Environmental Contaminants; Springer: Berlin/Heidelberg, Germany, 2018; pp. 281–315. [Google Scholar]
- Pang, C.M.; Hong, P.; Guo, H.; Liu, W.-T. Biofilm formation characteristics of bacterial isolates retrieved from a reverse osmosis membrane. Environ. Sci. Technol. 2005, 39, 7541–7550. [Google Scholar] [CrossRef]
- Wang, S.; Guillen, G.; Hoek, E.M. Direct observation of microbial adhesion to membranes. Environ. Sci. Technol. 2005, 39, 6461–6469. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Schaule, G.; Griebe, T.; Schmitt, J.; Tamachkiarowa, A. Biofouling—The Achilles heel of membrane processes. Desalination 1997, 113, 215–225. [Google Scholar] [CrossRef]
- Ramesh, A.; Lee, D.; Lai, J. Membrane biofouling by extracellular polymeric substances or soluble microbial products from membrane bioreactor sludge. Appl. Microbiol. Biotechnol. 2007, 74, 699–707. [Google Scholar] [CrossRef]
- Jinhua, P.; Fukushi, K.; Yamamoto, K. Bacterial community structure on membrane surface and characteristics of strains isolated from membrane surface in submerged membrane bioreactor. Sep. Sci. Technol. 2006, 41, 1527–1549. [Google Scholar] [CrossRef]
- Meng, F.; Chae, S.-R.; Drews, A.; Kraume, M.; Shin, H.-S.; Yang, F. Recent advances in membrane bioreactors (MBRs): Membrane fouling and membrane material. Water Res. 2009, 43, 1489–1512. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Z.; Wu, Z.; Ma, J.; Jiang, Z. Insights into membrane fouling of submerged membrane bioreactors by characterizing different fouling layers formed on membrane surfaces. Chem. Eng. J. 2012, 179, 169–177. [Google Scholar] [CrossRef]
- Metzger, U.; Le-Clech, P.; Stuetz, R.M.; Frimmel, F.H.; Chen, V. Characterisation of polymeric fouling in membrane bioreactors and the effect of different filtration modes. J. Membr. Sci. 2007, 301, 180–189. [Google Scholar] [CrossRef]
- Mutamim, N.S.A.; Noor, Z.Z.; Hassan, M.A.A.; Yuniarto, A.; Olsson, G. Membrane bioreactor: Applications and limitations in treating high strength industrial wastewater. Chem. Eng. J. 2013, 225, 109–119. [Google Scholar] [CrossRef]
- Kang, I.-J.; Yoon, S.-H.; Lee, C.-H. Comparison of the filtration characteristics of organic and inorganic membranes in a membrane-coupled anaerobic bioreactor. Water Res. 2002, 36, 1803–1813. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Z.; Yin, X.; Tian, L. Membrane fouling in a submerged membrane bioreactor (MBR) under sub-critical flux operation: Membrane foulant and gel layer characterization. J. Membr. Sci. 2008, 325, 238–244. [Google Scholar] [CrossRef]
- You, H.; Tseng, C.; Peng, M.; Chang, S.; Chen, Y.; Peng, S. A novel application of an anaerobic membrane process in wastewater treatment. Water Sci. Technol. 2005, 51, 45–50. [Google Scholar] [CrossRef]
- Kraume, M.; Wedi, D.; Schaller, J.; Iversen, V.; Drews, A. Fouling in MBR: What use are lab investigations for full scale operation? Desalination 2009, 236, 94–103. [Google Scholar] [CrossRef]
- Judd, S. The MBR Book: Principles and Applications of Membrane Bioreactors for Water and Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Gan, Q.; Howell, J.; Field, R.; England, R.; Bird, M.; McKechinie, M. Synergetic cleaning procedure for a ceramic membrane fouled by beer microfiltration. J. Membr. Sci. 1999, 155, 277–289. [Google Scholar] [CrossRef]
- Resosudarmo, A.; Ye, Y.; Le-Clech, P.; Chen, V. Analysis of UF membrane fouling mechanisms caused by organic interactions in seawater. Water Res. 2013, 47, 911–921. [Google Scholar] [CrossRef]
- Ayala, D.; Ferre, V.; Judd, S.J. Membrane life estimation in full-scale immersed membrane bioreactors. J. Membr. Sci. 2011, 378, 95–100. [Google Scholar] [CrossRef]
- Bouhabila, E.H.; Aïm, R.B.; Buisson, H. Fouling characterisation in membrane bioreactors. Sep. Purif. Technol. 2001, 22, 123–132. [Google Scholar] [CrossRef]
- Psoch, C.; Schiewer, S. Resistance analysis for enhanced wastewater membrane filtration. J. Membr. Sci. 2006, 280, 284–297. [Google Scholar] [CrossRef]
- Jiang, T.; Kennedy, M.D.; Guinzbourg, B.; Vanrolleghem, P.A.; Schippers, J. Optimising the operation of a MBR pilot plant by quantitative analysis of the membrane fouling mechanism. Water Sci. Technol. 2005, 51, 19–25. [Google Scholar] [CrossRef]
- Visvanathan, C.; Yang, B.-S.; Muttamara, S.; Maythanukhraw, R. Application of air backflushing technique in membrane bioreactor. Water Sci. Technol. 1997, 36, 259–266. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, S.; Oh, Y.; Zhou, Z.; Shin, H.-S.; Chae, S.-R. Fouling in membrane bioreactors: An updated review. Water Res. 2017, 114, 151–180. [Google Scholar] [CrossRef]
- Shahid, M.K.; Choi, Y.-G. The comparative study for scale inhibition on surface of RO membranes in wastewater reclamation: CO2 purging versus three different antiscalants. J. Membr. Sci. 2018, 546, 61–69. [Google Scholar] [CrossRef]
- Al-Amoudi, A.; Lovitt, R.W. Fouling strategies and the cleaning system of NF membranes and factors affecting cleaning efficiency. J. Membr. Sci. 2007, 303, 4–28. [Google Scholar] [CrossRef]
- Rezaei, M.; Mehrnia, M. The influence of zeolite (clinoptilolite) on the performance of a hybrid membrane bioreactor. Bioresour. Technol. 2014, 158, 25–31. [Google Scholar] [CrossRef]
- Li, H.; Yang, M.; Zhang, Y.; Liu, X.; Gao, M.; Kamagata, Y. Comparison of nitrification performance and microbial community between submerged membrane bioreactor and conventional activated sludge system. Water Sci. Technol. 2005, 51, 193–200. [Google Scholar] [CrossRef]
- Mauter, M.S.; Wang, Y.; Okemgbo, K.C.; Osuji, C.O.; Giannelis, E.P.; Elimelech, M. Antifouling ultrafiltration membranes via post-fabrication grafting of biocidal nanomaterials. ACS Appl. Mater. Interfaces 2011, 3, 2861–2868. [Google Scholar] [CrossRef]
- Zeynali, R.; Ghasemzadeh, K.; Iulianelli, A.; Basile, A. Experimental evaluation of graphene oxide/TiO2-alumina nanocomposite membranes performance for hydrogen separation. Int. J. Hydrogen Energy 2019, in press. [Google Scholar] [CrossRef]
- Bae, T.-H.; Tak, T.-M. Effect of TiO2 nanoparticles on fouling mitigation of ultrafiltration membranes for activated sludge filtration. J. Membr. Sci. 2005, 249, 1–8. [Google Scholar] [CrossRef]
- Moghadam, M.T.; Lesage, G.; Mohammadi, T.; Mericq, J.P.; Mendret, J.; Heran, M.; Faur, C.; Brosillon, S.; Hemmati, M.; Naeimpoor, F. Improved antifouling properties of TiO2/PVDF nanocomposite membranes in UV-coupled ultrafiltration. J. Appl. Polym. Sci. 2015, 132, 41731. [Google Scholar] [CrossRef]
- You, S.-J.; Semblante, G.U.; Lu, S.-C.; Damodar, R.A.; Wei, T.-C. Evaluation of the antifouling and photocatalytic properties of poly (vinylidene fluoride) plasma-grafted poly (acrylic acid) membrane with self-assembled TiO2. J. Hazard. Mater. 2012, 237, 10–19. [Google Scholar] [CrossRef]
- Hu, W.; Yin, J.; Deng, B.; Hu, Z. Application of nano TiO2 modified hollow fiber membranes in algal membrane bioreactors for high-density algae cultivation and wastewater polishing. Bioresour. Technol. 2015, 193, 135–141. [Google Scholar] [CrossRef]
- Qu, X.; Alvarez, P.J.; Li, Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013, 47, 3931–3946. [Google Scholar] [CrossRef]
- Rahimi, Z.; Zinatizadeh, A.; Zinadini, S. Preparation of high antibiofouling amino functionalized MWCNTs/PES nanocomposite ultrafiltration membrane for application in membrane bioreactor. J. Ind. Eng. Chem. 2015, 29, 366–374. [Google Scholar] [CrossRef]
- Lee, J.; Chae, H.-R.; Won, Y.J.; Lee, K.; Lee, C.-H.; Lee, H.H.; Kim, I.-C.; Lee, J.-m. Graphene oxide nanoplatelets composite membrane with hydrophilic and antifouling properties for wastewater treatment. J. Membr. Sci. 2013, 448, 223–230. [Google Scholar] [CrossRef]
- Bagheri, M.; Mirbagheri, S.A. Critical review of fouling mitigation strategies in membrane bioreactors treating water and wastewater. Bioresour. Technol. 2018, 258, 318–334. [Google Scholar] [CrossRef]
- Sołowski, G.; Shalaby, M.S.; Abdallah, H.; Shaban, A.M.; Cenian, A. Production of hydrogen from biomass and its separation using membrane technology. Renew. Sustain. Energy Rev. 2018, 82, 3152–3167. [Google Scholar] [CrossRef]
- Zornoza, B.; Casado, C.; Navajas, A. Chapter 11: Advances in hydrogen separation and purification with membrane technology. in book. Renew. Hydrog. Technol. 2013, 245–268. [Google Scholar]
- Murmura, M.; Sheintuch, M. Permeance inhibition of Pd-based membranes by competitive adsorption of CO: Membrane size effects and first principles predictions. Chem. Eng. J. 2018, 347, 301–312. [Google Scholar] [CrossRef]
- Sinha, P.; Pandey, A. An evaluative report and challenges for fermentative biohydrogen production. Int. J. Hydrogen Energy 2011, 36, 7460–7478. [Google Scholar] [CrossRef]
- Zeynali, R.; Ghasemzadeh, K.; Sarand, A.B.; Kheiri, F.; Basile, A. Performance evaluation of graphene oxide (GO) nanocomposite membrane for hydrogen separation: Effect of dip coating sol concentration. Sep. Purif. Technol. 2018, 200, 169–176. [Google Scholar] [CrossRef]
- George, S.C.; Thomas, S. Transport phenomena through polymeric systems. Prog. Polym. Sci. 2001, 26, 985–1017. [Google Scholar] [CrossRef]
- Car, A.; Stropnik, C.; Yave, W.; Peinemann, K.-V. Pebax®/polyethylene glycol blend thin film composite membranes for CO2 separation: Performance with mixed gases. Sep. Purif. Technol. 2008, 62, 110–117. [Google Scholar] [CrossRef]
- Reijerkerk, S.R.; Knoef, M.H.; Nijmeijer, K.; Wessling, M. Poly (ethylene glycol) and poly (dimethyl siloxane): Combining their advantages into efficient CO2 gas separation membranes. J. Membr. Sci. 2010, 352, 126–135. [Google Scholar] [CrossRef]
- Yave, W.; Car, A.; Peinemann, K.-V.; Shaikh, M.Q.; Rätzke, K.; Faupel, F. Gas permeability and free volume in poly (amide-b-ethylene oxide)/polyethylene glycol blend membranes. J. Membr. Sci. 2009, 339, 177–183. [Google Scholar] [CrossRef]
- David, O.C.; Gorri, D.; Urtiaga, A.; Ortiz, I. Mixed gas separation study for the hydrogen recovery from H2/CO/N2/CO2 post combustion mixtures using a Matrimid membrane. J. Membr. Sci. 2011, 378, 359–368. [Google Scholar] [CrossRef]
- Ghasemzadeh, K.; Aghaeinejad-Meybodi, A.; Iulianelli, A.; Basile, A. Theoretical Performance Evaluation of Inorganic (Non Pd-Based) Membranes for Hydrogen Separation. J. Membr. Sci. Res. 2018, 4, 198–203. [Google Scholar]
- Zeynali, R.; Ghasemzadeh, K.; Sarand, A.B.; Kheiri, F.; Basile, A. Experimental study on graphene-based nanocomposite membrane for hydrogen purification: Effect of temperature and pressure. Catal. Today 2019, 330, 16–23. [Google Scholar] [CrossRef]
| Microorganisms | Substrate | Type of Reactor | H2 Rate (l H2/l/h) | Reference |
|---|---|---|---|---|
| Sludge (wastewater treatment plant) | Molasses | CSTR | 0.20 | [48] |
| Sludge (wastewater treatment plant) | Glucose | ASBR | 0.23 | [49] |
| Sludge (wastewater treatment plant) | Sucrose | FBBAC | 1.2 | [50] |
| Activated sludge and digested sludge | Glucose | AFBR | 2.4 | [51] |
| Sludge (wastewater treatment plant) | Sucrose | UASB | 0.27 | [52] |
| Sludge (wastewater treatment plant) | Sucrose | CIGSB | 9.3 | [53] |
| Sludge (wastewater treatment plant) | Sucrose | FBR | 1.4 | [54] |
| Sludge (wastewater treatment plant) | Glucose | AFBR | 7.6 biofilm; 6.6 granules | [55] |
| Heat-treated soil | Glucose | MBR | 0.38 | [56] |
| Heat shock treated anaerobic sludge | Food waste | LBR | 0.15 | [57] |
| Sludge (wastewater treatment plant) | Vegetable kitchen waste | ICSTR | 0.04 | [58] |
| Adapted anaerobic sludge | Cheese whey | Batch | 0.003 | [59] |
| Type of Wastewater | Type of MBR | Membrane Characteristics | Configuration | Flux (LMH) | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| Membrane Process | Module | Pore Size (μm) | Area (m2) | Material | |||||
| Molasses-based synthetic wastewater | ThAeMBRs | MF/UF | Submerged | 0.45 μm/ 150 kDa | 0.0125 | Filtanium Ceramic | Hollow fiber | 48/72 | [64] |
| TMP pressate | ThAeMBRs | MF | Submerged | 0.3 μm | 0.03 | PVDF | Flat sheet | 6.8–11.8 | [65] |
| Paper drinking wastewater | ThAeMBRs | UF | Submerged | 0.04 μm | 0.34 | PVDF | Flat sheet | 6–25 | [66] |
| Pharmaceutical wastewater | ThAeMBRs | UF | External | 300 kDa | N.S. | Ceramic | Tubular | N.S. | [67] |
| Sewage sludge | ThAeMBRs | UF | External | 10 nm | N.S. | Ceramic | N.S. | N.S. | [68] |
| Industrial liquid wastes | ThAeMBRs | UF | External | 300 kDa | N.S. | Ceramic | Tubular | N.S. | [69] |
| Sewage sludge | ThAnMBRs | UF | External | 300 kDa | 0.0226 | TiO2/ ZrO2 Ceramic | Tubular | 7 | [70] |
| Synthetic molasses | ThAnMBRs | UF | Submerged | 10 kDa | 0.1 | polysulphone | Tubular | 6 | [71] |
| Prehydrolysis Liquor | ThAnMBRs | MF | Submerged | 0.4 μm | 0.11 | Chlorinated polyethylene | Flat sheet | 4 | [72] |
| Glucose model solution | ThAnMBRs | N.S. | External | 40 nm | ~0.033 | Ceramic (α-Al2O3) | Hollow fiber | 40.3–72.2 | [73] |
| Inoculum | Substrate | Retention Time | H2 Generation Performance | Ref. | ||
|---|---|---|---|---|---|---|
| Hydraulic | Solid/Biomass | Yield | Productivity | |||
| Heat-treated soil inocula | Glucose | 3.3–5 | 3.3–48 h | N.S. | 9.2 L H2/L-d | [56] |
| Acid-treated, acclimated sludge | 3 Hexoses | 1–4 h | N.S. | 39 L H2/mol glucose | 66 L H2/L-d* | [89] |
| Anaerobic sludge | Glucose | 4 h | N.S. | 38.1 L H2/mol glucose | 25 L H2/L-d | [89] |
| Heat-treated sludge | Glucose | 9 h | 450 d | N.S. | 2.5 L H2/L-d | [80] |
| Screened anaerobic digester sludge | Glucose | 8 h | 24 h | 40.2 L H2/mol glucose | 4.5 L H2/L-d | [90] |
| Heat-treated sludge | Glucose | 9 h | 12.5 h | 35.4 L H2/mol glucose | 5.9 L H2/L-d | [81] |
| Heat-treated, acclimated sludge | Glucose | N.S. | 90 d | 19.5 L H2/mol glucose | 2.5 L H2/L-d | [85] |
| Screened anaerobic digester sludge | Glucose | 8 h | 24 h | 40.3 L H2/mol glucose | 4.5 L H2/L-d | [90] |
| Sludge | Glucose | 9 h | 90 d | 19.2 L H2/mol glucose | 2.56 L H2/L-d | [85] |
| Heat-treated, acclimated sludge | Glucose | 9 h | 2–90 d | 27 L H2/mol glucose | 5.8 L H2/L-d | [91] |
| Acclimated sludge | Glucose | 8 h | 24 h | N.S. | 4.4 L H2/L-d | [82] |
| Heat-treated sludge | TPW | 2–8 h | N.S. | 42.4 L H2/mol hexose** | 19.8 L H2/L-d | [92] |
| Sludge | Waste bread | 6 h | N.S. | 0.109 L H2/mol waste bread | 7.4 L H2/L-d | [93] |
| Anaerobic granular sludge | Glucose | 4 h | N.S. | 44.8 L H2/mol glucose | 11.4 L H2/L-d | [94] |
| Definition | Fouling Rate (mbar/min) | Time Interval | Cleaning Method Applied |
|---|---|---|---|
| Reversible/temporary fouling | 0.1–1 | 10 min | Physical cleaning |
| Residual fouling | 0.01–0.1 | 1–2 week | Maintenance cleaning (e.g., chemically enhanced backflush) |
| Irreversible/permanent fouling | 0.001–0.01 | 6–12 months | Chemical cleaning |
| Irrecoverable fouling | 0.0001–0.001 | Several years | Cannot be removed |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabbari, B.; Jalilnejad, E.; Ghasemzadeh, K.; Iulianelli, A. Recent Progresses in Application of Membrane Bioreactors in Production of Biohydrogen. Membranes 2019, 9, 100. https://doi.org/10.3390/membranes9080100
Jabbari B, Jalilnejad E, Ghasemzadeh K, Iulianelli A. Recent Progresses in Application of Membrane Bioreactors in Production of Biohydrogen. Membranes. 2019; 9(8):100. https://doi.org/10.3390/membranes9080100
Chicago/Turabian StyleJabbari, Bahman, Elham Jalilnejad, Kamran Ghasemzadeh, and Adolfo Iulianelli. 2019. "Recent Progresses in Application of Membrane Bioreactors in Production of Biohydrogen" Membranes 9, no. 8: 100. https://doi.org/10.3390/membranes9080100
APA StyleJabbari, B., Jalilnejad, E., Ghasemzadeh, K., & Iulianelli, A. (2019). Recent Progresses in Application of Membrane Bioreactors in Production of Biohydrogen. Membranes, 9(8), 100. https://doi.org/10.3390/membranes9080100






