Ceramic Membranes Photocatalytically Functionalized on the Permeate Side and Their Application to Water Treatment
Abstract
1. Introduction
2. Background on Photocatalysis and Hybrid Processes Coupling Photocatalysis and Membrane Separation
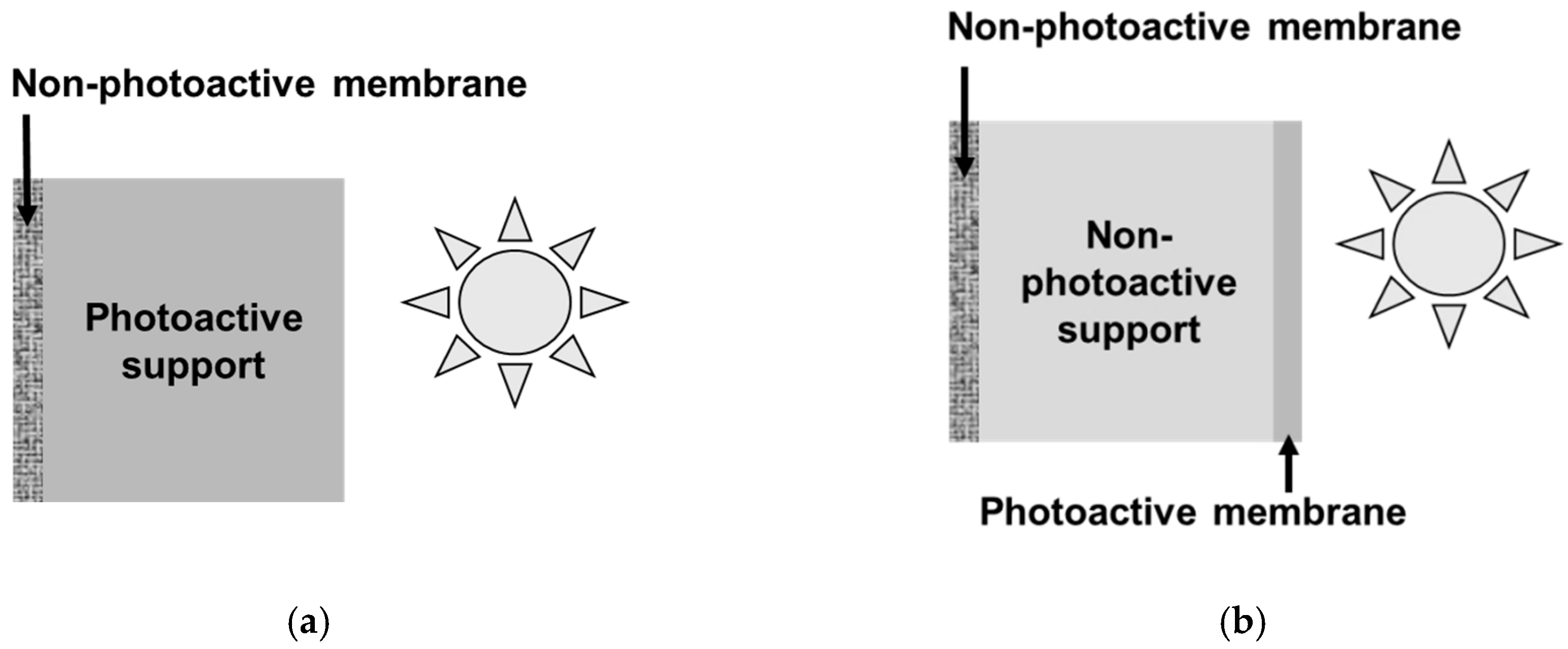
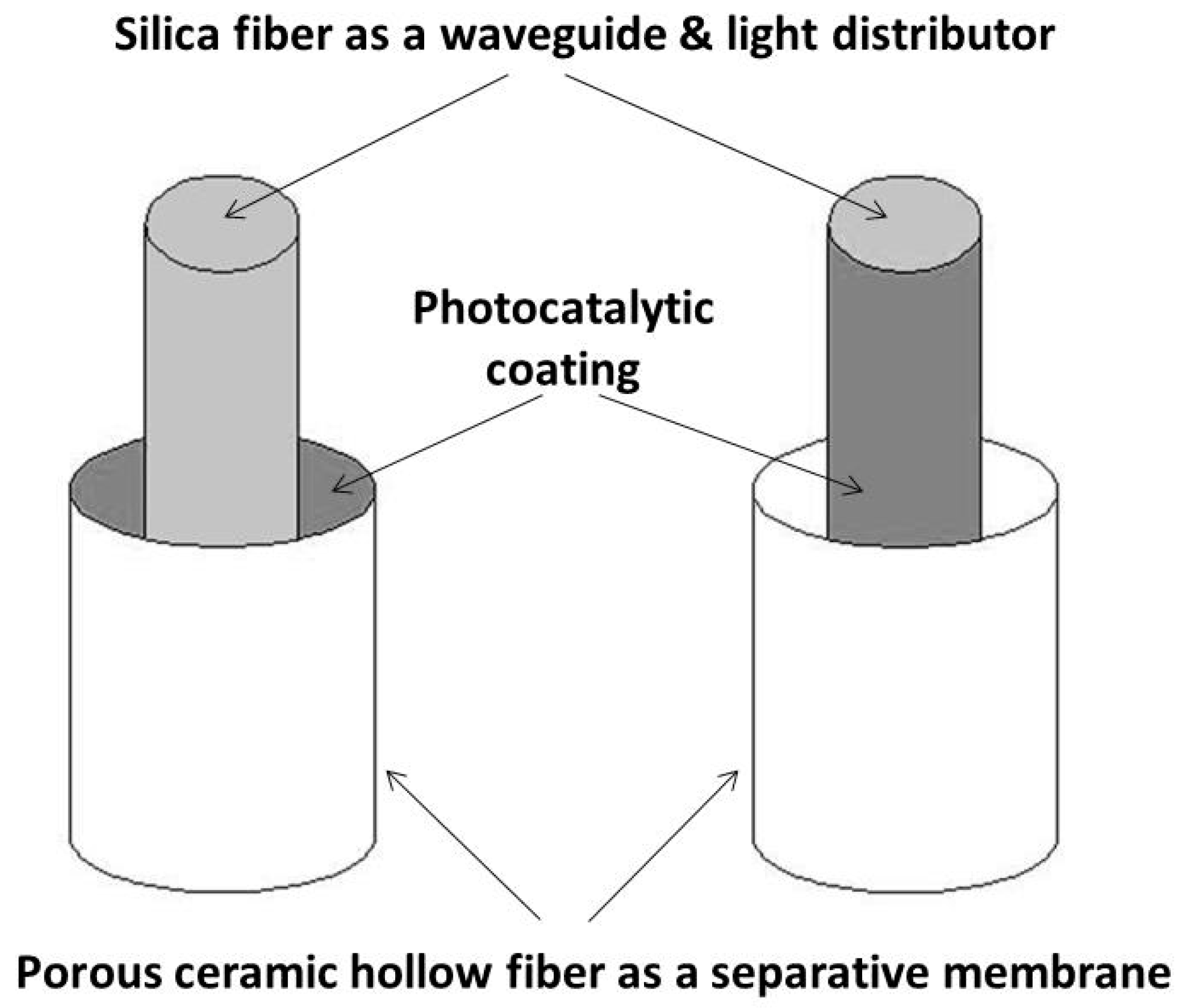
3. Direct Coupling Considering an Unusual Configuration of Photocatalytic Membrane
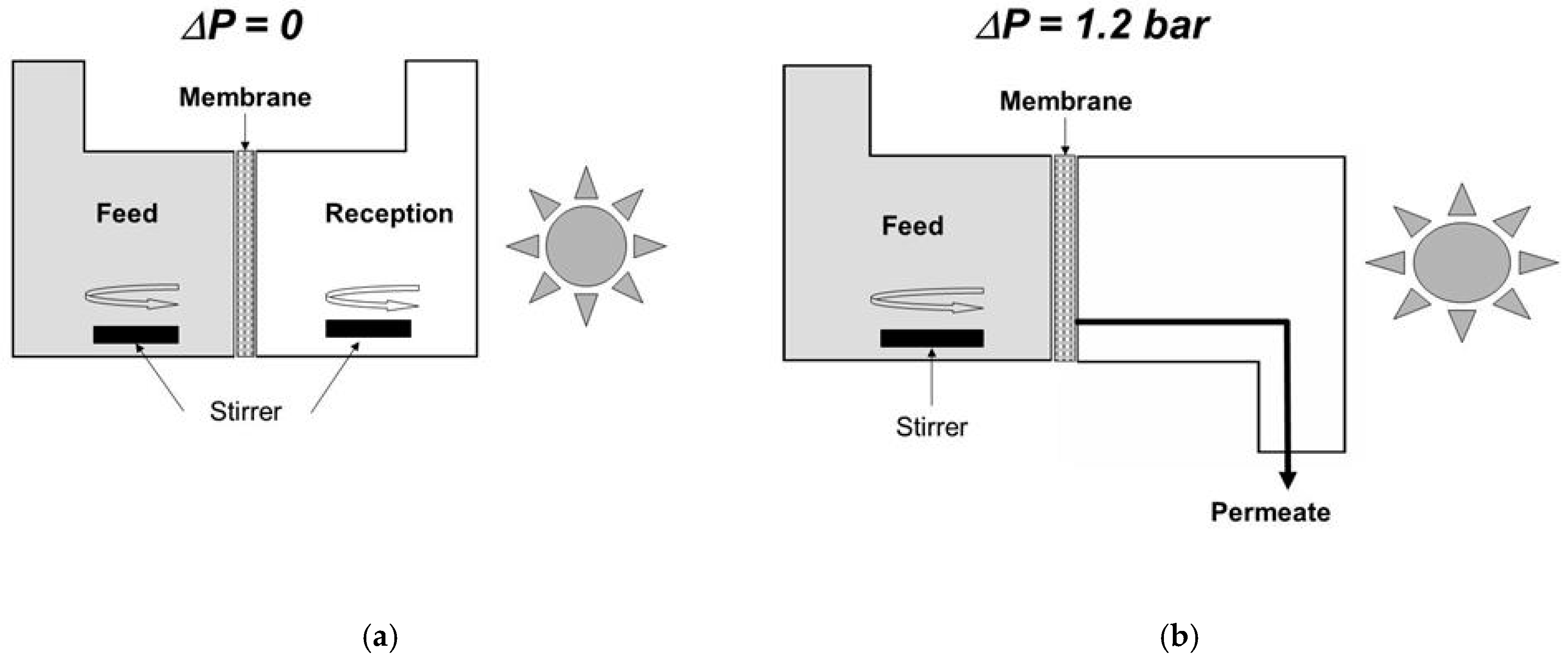
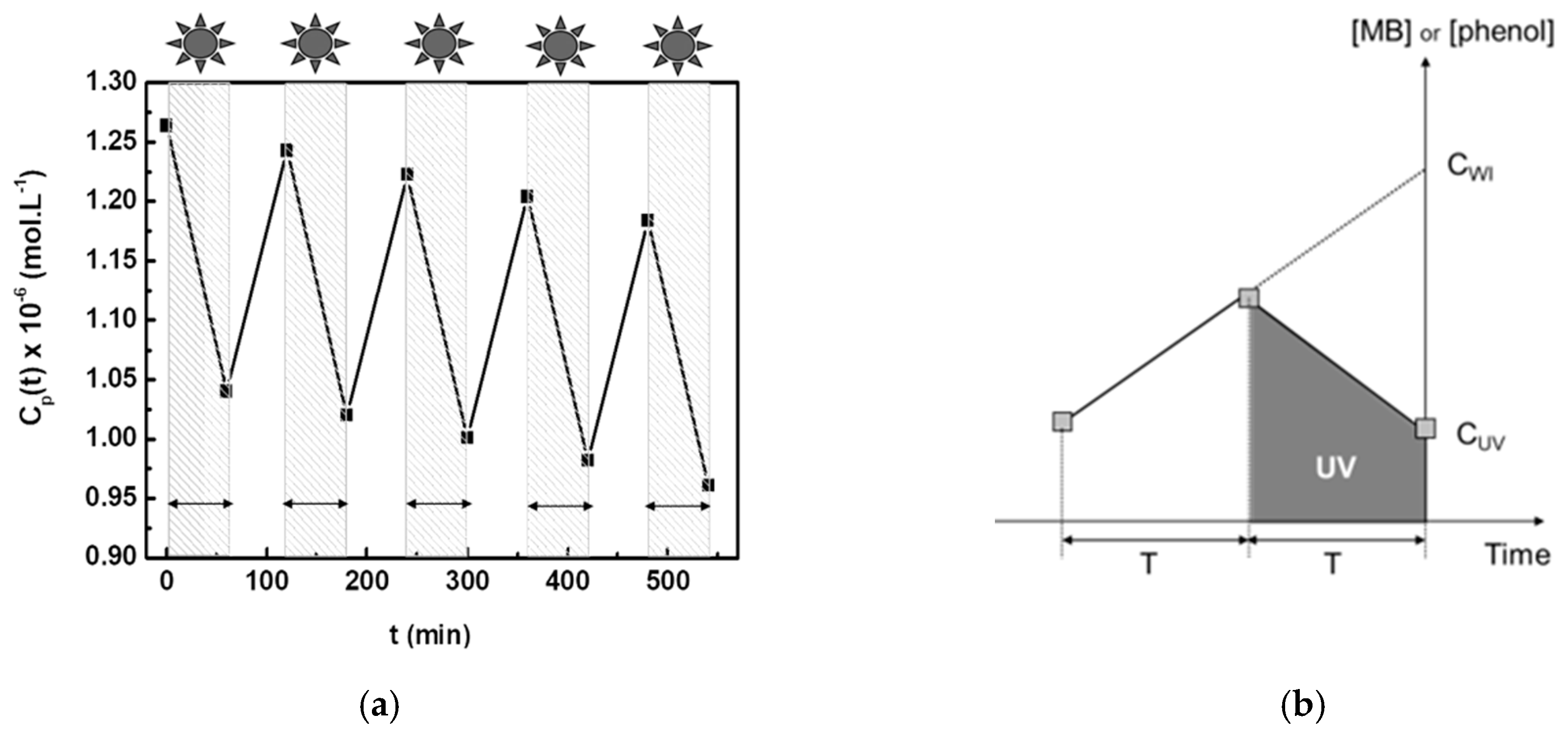
4. A Simple Method for Estimating the Performance of Photocatalytic Membranes
5. Implementation in Representative Conditions and Design of Purification Units
6. Current Technological Challenges and Potential Solutions
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Rivas, L.; Bellobono, I.R.; Ascari, F. Photomineralization of n-alkanoic acids in aqueous solution by photocatalytic membranes. Influence of radiation power. Chemosphere 1998, 37, 1033–1044. [Google Scholar] [CrossRef]
- Bischoff, B.L.; Fain, D.E.; Stockdale, J.A.D. Photocatalytic Reactor. US Patent 5, 862, 449, 1999. [Google Scholar]
- Molinari, R.; Mungari, M.; Drioli, E.; Di Paola, A.; Loddo, V.; Palmisano, L.; Schiavello, M. Study on a photocatalytic membrane reactor for water purification. Catal. Today 2000, 55, 71–78. [Google Scholar] [CrossRef]
- Molinari, R.; Palmisano, L.; Drioli, E.; Schiavello, M. Studies on various reactor configurations for coupling photocatalysis and membrane processes in water purification. J. Membr. Sci. 2002, 206, 399–415. [Google Scholar] [CrossRef]
- Molinari, R.; Borgese, M.; Drioli, E.; Palmisano, L.; Schiavello, M. Hybrid processes coupling photocatalysis and membranes for degradation of organic pollutants in water. Catal. Today 2002, 75, 77–85. [Google Scholar] [CrossRef]
- Ollis, D.F. Integrating photocatalysis and membrane technologies for water treatment. Ann. N.Y. Acad. Sci. 2003, 984, 65–84. [Google Scholar] [CrossRef] [PubMed]
- Tsuru, T.; Kan-no, T.; Yoshioka, T.; Asaeda, M. A photocatalytic membrane reactor for gas-phase reactions using porous titanium oxide membranes. Catal. Today 2003, 82, 41–48. [Google Scholar] [CrossRef]
- Villacres, R.; Ikeda, S.; Torimoto, T.; Ohtani, B. Development of a novel photocatalytic reaction system for oxidative decomposition of volatile organic compounds in water with enhanced aeration. J. Photochem. Photobiol. A Chem. 2003, 160, 121–126. [Google Scholar] [CrossRef]
- Fujishima, A.; Hashimoto, K.; Watanabe, T. TiO2 photocatalysis, fundamentals and applications; BKC Inc.: Tokyo, Japan, 2001. [Google Scholar]
- Kaneko, M.; Okura, I. Photocatalysis; Kodansha: Tokyo, Japan, 2002. [Google Scholar]
- Mo, J.; Zhang, Y.; Xu, Q.; Lamson, J.J.; Zhao, R. Photocatalytic purification of volatile organic compounds in indoor air: a literature review. Atmos. Environ. 2009, 43, 2229–2246. [Google Scholar] [CrossRef]
- Herrmann, J.M. Heterogeneous photocatalysis: fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Le Cloirec, P. Les composés organiques volatils dans l’environnement; Lavoisier: Paris, France, 1998. [Google Scholar]
- Rajeshwar, K. Photoelectrochemistry and the environment. J. Appl. Electrochem. 1995, 25, 1067–1082. [Google Scholar] [CrossRef]
- Ohno, T.; Sarukawa, K.; Tokieda, K.; Matsumura, M. Morphology of a TiO2 photocatalyst (Degussa, P-25) consisting of anatase and rutile crystalline phases. J. Catal. 2001, 203, 82–86. [Google Scholar] [CrossRef]
- Sun, B.; Smirniotis, P.G. Interaction of anatase and rutile TiO2 particles in aqueous photo-oxidation. Catal. Today 2003, 88, 49–59. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.-C.; Zakaria, R.; Ying, J.Y. Role of particle size in nanocrystalline TiO2-based photocatalysts. J. Phys. Chem. B 1998, 102, 10871–10878. [Google Scholar] [CrossRef]
- Hong, S.S.; Lee, M.S.; Ju Lee, C.S.; Park, S.S.; Lim, K.-T. Photocatalytic decomposition of p-nitrophenol over titanium dioxides prepared in water-in-carbon dioxide microemulsion. Catal. Today 2004, 93–95, 871–876. [Google Scholar] [CrossRef]
- Panagiotopoulou, P.; Kondarides, D.I. Effect of morphological characteristics of TiO2-supported noble metal catalysts on their activity for the water-gas shift reaction. J. Catal. 2004, 225, 327–336. [Google Scholar] [CrossRef]
- Srikant, V.; Clarke, D.R. On the optical band gap of zinc oxide. J. Appl. Phys. 1998, 83, 5447–5451. [Google Scholar] [CrossRef]
- Choi, H.; Stathatos, E.; Dionysiou, D.D. Sol-gel preparation of mesoporous photocatalytic TiO2 films and TiO2/Al2O3 composite membranes for environmental applications. Appl. Catal. B: Environ. 2006, 63, 60–67. [Google Scholar] [CrossRef]
- Choi, H.; Stathatos, E.; Dionysiou, D.D. Photocatalytic TiO2 films and membranes for the development of efficient wastewater treatment and reuse systems. Desalination 2007, 202, 199–206. [Google Scholar] [CrossRef]
- Mozia, S. Photocatalytic membrane reactors (PMRs) in water and wastewater treatment. A review. Sep. Purif. Technol. 2010, 73, 71–91. [Google Scholar] [CrossRef]
- Zheng, X.; Shen, Z.-P.; Shi, L.; Cheng, R.; Yuan, D.-H. Photocatalytic membrane reactors (PMRs) in water treatment: configurations and influencing factors. Catalysts 2017, 7, 224. [Google Scholar] [CrossRef]
- Chen, D.; Li, F.; Ray, A.K. Effect of mass transfer and catalyst layer thickness on photocatalytic reaction. AIChE J. 2000, 46, 1034–1045. [Google Scholar] [CrossRef]
- Bosc, F.; Ayral, A.; Guizard, C. Mesoporous anatase coatings for coupling membrane separation and photocatalyzed reactions. J. Membr. Sci. 2005, 265, 13–19. [Google Scholar] [CrossRef]
- Naszalyi, L.; Bosc, F.; El Mansouri, A.; Van Der Lee, A.; Cot, D.; Horvolgyi, Z.; Ayral, A. Sol-gel-derived mesoporous SiO2/ZnO active coating and development of multifunctional ceramic membranes. Sep. Purif. Technol. 2008, 59, 304–309. [Google Scholar] [CrossRef]
- Djafer, L.; Ayral, A.; Ouagued, A. Robust synthesis and performance of a titania-based ultrafiltration membrane with photocatalytic properties. Sep. Purif. Technol. 2010, 75, 198–203. [Google Scholar] [CrossRef]
- Starr, B.J.; Tarabara, V.V.; Zhou, M.; Roualdès, S.; Ayral, A. Coating porous membranes with a photocatalyst: Comparison of LbL self-assembly and plasma-enhanced CVD techniques. J. Membr. Sci. 2016, 514, 340–349. [Google Scholar] [CrossRef]
- Athanasekou, C.P.; Romanos, G.E.; Katsaros, F.K.; Kordatos, K.; Likodimos, V.; Falaras, P. Very efficient composite titania membranes in hybrid ultrafiltration/photocatalysis water treatment processes. J. Membr. Sci. 2012, 392–393, 192–203. [Google Scholar] [CrossRef]
- Romanos, G.E.; Athanasekou, C.P.; Katsarosa, F.K.; Kanellopoulos, N.K.; Dionysiou, D.D.; Likodimos, V.; Falaras, P. Double-side active TiO2-modified nanofiltration membranes in continuous flow photocatalytic reactors for effective water purification. J. Hazard. Mater. 2012, 211–212, 304–316. [Google Scholar] [CrossRef]
- Romanos, G.E.; Athanasekou, C.P.; Likodimos, V.; Aloupogiannis, P.; Falaras, P. Hybrid ultrafiltration/photocatalytic membranes for efficient water treatment. Ind. Eng. Chem. Res. 2013, 52, 13938–13947. [Google Scholar] [CrossRef]
- Athanasekou, C.P.; Morales-Torres, S.; Likodimos, V.; Romanos, G.E.; Pastrana-Martinez, L.M.; Falaras, P.; Dionysiou, D.D.; Faria, J.L.; Figueiredo, J.L.; Silva, A.M.T. Prototype composite membranes of partially reduced graphene oxide/TiO2 for photocatalytic ultrafiltration water treatment under visible light. Appl. Catal. B: Environ. 2014, 158–159, 361–372. [Google Scholar] [CrossRef]
- Athanasekou, C.P.; Moustakas, N.G.; Morales-Torres, S.; Pastrana-Martínez, L.M.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M.T.; Dona-Rodriguez, J.M.; Romanos, G.E.; Falaras, P. Ceramic photocatalytic membranes for water filtration under UV and visible light. Appl. Catal. B: Environ. 2015, 178, 12–19. [Google Scholar] [CrossRef]
- Guo, B.; Pasco, E.V.; Xagoraraki, I.; Tarabara, V.V. Virus removal and inactivation in a hybrid microfiltration-UV process with a photocatalytic membrane. Separ. Purif. Technol. 2015, 149, 245–254. [Google Scholar] [CrossRef]
- Horovitz, I.; Avisar, D.; Baker, M.A.; Grilli, R.; Lozzi, L.; Di Camillo, D.; Mamane, H. Carbamazepine degradation using a N-doped TiO2 coated photocatalytic membrane reactor: Influence of physical parameters. J. Hazard. Mater. 2016, 310, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Luster, E.; Avisar, D.; Horovitz, I.; Lozzi, L.; Baker, M.A.; Grilli, R.; Mamane, H. N-doped TiO2-coated ceramic membrane for carbamazepine degradation in different water qualities. Nanomaterials 2017, 7, 206. [Google Scholar] [CrossRef]
- Bosc, F.; Ayral, A.; Albouy, P.A.; Guizard, C. A simple route for low-temperature synthesis of mesoporous and nanocrystalline anatase thin films. Chem. Mater. 2003, 15, 2463–2468. [Google Scholar] [CrossRef]
- Bosc, F.; Ayral, A.; Albouy, P.A.; Datas, L.; Guizard, C. Mesostructure of anatase thin films prepared by mesophase templating. Chem. Mater. 2004, 16, 2208–2214. [Google Scholar] [CrossRef]
- Zhou, M.; Roualdes, S.; Zhao, J.; Autès, V.; Ayral, A. Nanocrystalline TiO2 thin film prepared by low-temperature plasma-enhanced chemical vapor deposition for photocatalytic applications. Thin Solid Films 2015, 589, 770–777. [Google Scholar] [CrossRef]
- Zhou, M.; Roualdes, S.; Ayral, A. New photocatalytic contactors obtained by PECVD deposition of TiO2 thin layers on the surface of macroporous supports. Eur. Phys. J. Spec. Top. 2015, 224, 1871–1882. [Google Scholar] [CrossRef]
- Djafer, L.; Ayral, A.; Ouagued, A. Multifunctional membrane coupling separation and photocatalysis for wastewater treatment. In Récents Progrès en Génie des Procédés n° 101; Société Française de Génie des Procédés: Paris, France, 2011. [Google Scholar]
- Web page presenting the Lightex® panels in the web site of the French company Brochier Technologies. Available online: http://www.brochiertechnologies.com/en/products/ (accessed on 26 April 2019).
- Ayral, A.; Julbe, A.; Guizard, C. Ceramic membrane processing: new approaches in design and applications. In Chemical Processing of Ceramics, 2nd ed.; Lee, B., Komarneni, S., Eds.; CRC Press: New York, NY, USA, 2005; pp. 629–666. [Google Scholar]
- Dannion, A.; Disdier, J.; Guillard, C.; Abdelmalek, F.; Jaffrezic-Renault, N. Characterization and study of a single-TiO2-coated optical fiber reactor. Appl. Catal. B: Environ. 2004, 52, 213–223. [Google Scholar] [CrossRef]
- Danion, A.; Bordes, C.; Disdier, J.; Gauvrit, J.Y.; Guillard, C.; Lanteri, P.; Jaffrezic-Renault, N. Optimization of a single TiO2-coated optical fiber reactor using experimental design. J. Photochem. Photobiol. A-Chem. 2004, 168, 161–167. [Google Scholar] [CrossRef]
- Youssef, L.; Kinfack Leoga, A.J.; Roualdes, S.; Bassil, J.; Zakhour, M.; Rouessac, V.; Ayral, A.; Nakhl, M. Optimization of N-doped TiO2 multifunctional thin layers by low frequency PECVD process. J. Eur. Ceram. Soc. 2017, 37, 5289–5303. [Google Scholar] [CrossRef]
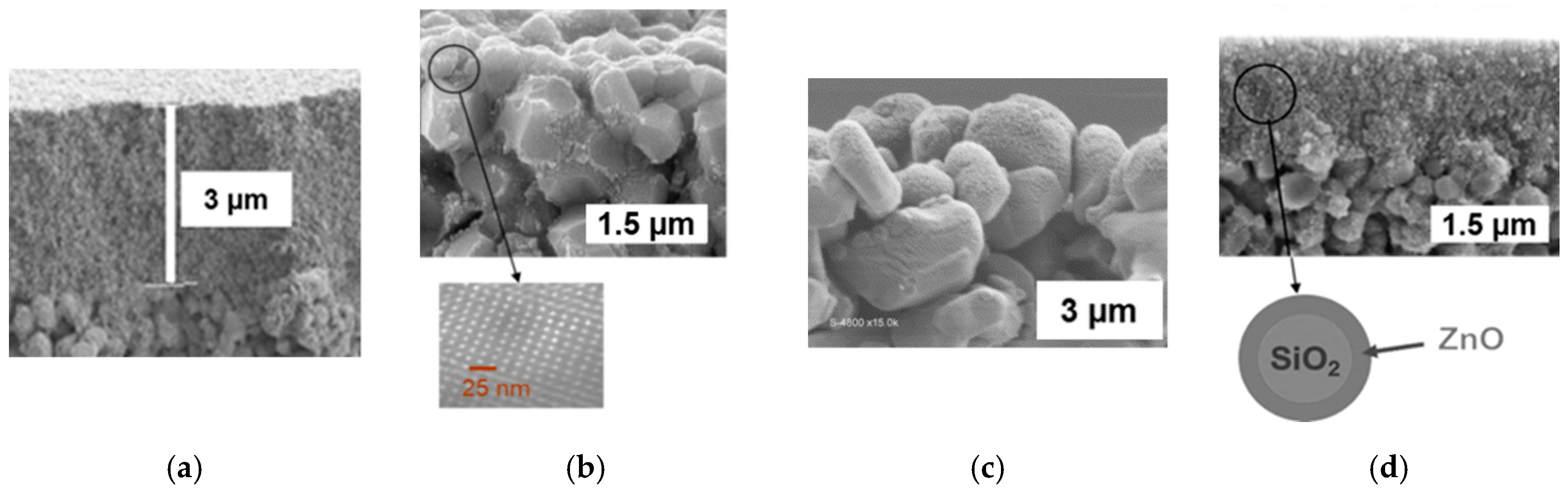
| Tested Membrane | TiO2 film from Commercial Hydrosol (Figure 2a) | Mesostructured TiO2 Coating on Substrate Grains (Figure 2b) | PE-CVD TiO2 Coating on Substrate Grains (Figure 2c) | Film of SiO2/ZnO Particles (Figure 2d) |
|---|---|---|---|---|
| δ for MB (mol·s−1·m−2) | (2.0 ± 0.5) × 10−8 | (1.0 ± 0.2) × 10−7 | (1.5 ± 0.5) × 10−8 | (1.5 ± 0.5) × 10−9 |
| δ for phenol (mol·s−1·m−2) | (3 ± 1) × 10−8 | - | - | - |
| Mean pore size of the photoactive layer (nm) | 11* | 200 | 800 | 6* |
| Thickness of photoactive layer (µm) | ~3 | >>10 infiltration in the substrate | <5 | ~1.5 |
| Crystallite size of the photocatalytic phase (nm) | 5–10 | 8 | 20 | 10 |
| Specific surface area of the photocatalytic phase (m2·g−1) | 143* | 190* | - | 36* |
| Mean pore size of the photocatalytic phase (nm) | 11* | 4.2* | - | 6* |
| Porosity of the photocatalytic phase (%) | 65* | 40* | 33 | 15* |
| Concentration (10−6 mol·L−1) | C0 | CPV | CUV | CPV − CUV | CPI | CSI | CPI − CSI |
| 9.8 ± 0.2 | 7.4 ± 0.2 | 5.7 ± 0.2 | 1.7 ± 0.4 | 8.2 ± 0.4 | 6.2 ± 0.2 | 2.0 ± 0.6 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayral, A. Ceramic Membranes Photocatalytically Functionalized on the Permeate Side and Their Application to Water Treatment. Membranes 2019, 9, 64. https://doi.org/10.3390/membranes9050064
Ayral A. Ceramic Membranes Photocatalytically Functionalized on the Permeate Side and Their Application to Water Treatment. Membranes. 2019; 9(5):64. https://doi.org/10.3390/membranes9050064
Chicago/Turabian StyleAyral, André. 2019. "Ceramic Membranes Photocatalytically Functionalized on the Permeate Side and Their Application to Water Treatment" Membranes 9, no. 5: 64. https://doi.org/10.3390/membranes9050064
APA StyleAyral, A. (2019). Ceramic Membranes Photocatalytically Functionalized on the Permeate Side and Their Application to Water Treatment. Membranes, 9(5), 64. https://doi.org/10.3390/membranes9050064





