Structures, Properties, and Performances—Relationships of Polymeric Membranes for Pervaporative Desalination
Abstract
:1. Introduction
2. A Brief History of Membranes in Pervaporative Desalination
3. Polymeric Membranes for Pervaporative Desalination
3.1. Synthesis
3.2. Characterization
3.2.1. Morphology
3.2.2. Mechanical Properties
3.2.3. Spectroscopy
3.3. Desalination Performance and Mechanisms
3.3.1. Membrane Structure
3.3.2. Influence of Feed Temperature
3.3.3. Influence of Feed Concentration
4. Polymer Composite/NCP Membranes
4.1. Synthesis
4.1.1. Sol-Gel Method
4.1.2. Solution Mixing and Casting
4.1.3. Vacuum Filtration-Assisted Assembly Method
4.1.4. Direct Spinning and Phase Inversion Method
4.1.5. Electrospraying and Electrospinning
4.2. Characterization
4.2.1. Morphology
4.2.2. FTIR Analyses
4.2.3. Thermal Properties
4.2.4. Mechanical Properties
4.2.5. Swelling
4.2.6. Hydrophilicity
4.3. Desalination Performance and Mechanisms
4.3.1. Membrane Structure
4.3.2. Feed Concentration
4.3.3. Feed Flow Rate
4.3.4. Effect of Permeate Pressure
4.3.5. Temperature
4.3.6. Thickness of Membrane
4.3.7. Electrical Resistance of Membranes
5. Inorganic Membranes for Pervaporative Desalination
5.1. Various Inorganic Membranes Used
5.1.1. Ceramic Membranes
5.1.2. Metal Oxide Membranes
5.1.3. Zeolite Membranes
5.1.4. Silica Membranes
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TDS | Total dissolved solids |
| RO | Reverse osmosis |
| NF | Nanofiltration |
| UF | Ultrafiltration |
| MF | Microfiltration |
| FO | Forward osmosis |
| PRO | Pressure retarded osmosis |
| MD | Membrane distillation |
| PV | Pervaporation |
| VOCs | Volatile organic compounds |
| MSFD | Multistage flash distillation |
| ΔVP | Vapor pressure difference |
| PVA | Polyvinyl alcohol |
| MED | Multi effect distillation |
| TFC | Thin film composite |
| GO | Graphene oxide |
| PAN | Polyacrylonitrile |
| NPs | Nanoparticles |
| PDMS | Polydimethyl siloxane |
| CMS | Carbon molecular sieves |
| MA | Maleic acid |
| TEOS | Tetraethoxysilane |
| FFV | Fractional free volume |
| PET | Polyethylene terephthalate |
| PMDA | Pyromellitic dianhydride |
| SSA | Sulfosuccinic Acid |
| SPTA | 4-Sulfophthalic acid |
| PEBA | Poly(ether block amide) |
| NCP | Nanocomposite |
| GNPs | Graphene nanoplates |
| PI | Polyimide |
| CS | Chitosan |
| MMMs | Mixed matrix membranes |
| PVS | Polyvinyl sulfone |
| MOFs | Metal organic frameworks |
| ZIFs | Zeolitic-imidazolate frameworks |
| PEBAX | Polyether block polyamide |
| PVDF | Polyvinlyidene fluoride |
| MWCNT | Multi-walled carbon nanotube |
| PMMA | Poly(methyl methacrylate) |
| PE | Polyester |
| PSF | Polysulfone |
| NMP | N-Methyl-2-pyrrolidinone |
| DMF | Dimethylformamide |
| GA | Glutaraldehyde |
| Tg | Glass transition temperature |
| TS | Tensile strength |
| EAB | Elongation-at-break |
| CD | Crosslink density |
| SR | Swelling ratio |
| CNTs | Carbon nanotubes |
| GS | Gas separation |
| FAS | Fluoroalkylsilanes |
| CA | Cellulose acetate |
| LTA | Linde Type A |
References
- Singha, N.R.; Karmakar, M.; Mahapatra, M.; Mondal, H.; Dutta, A.; Deb, M.; Mitra, M.; Roy, C.; Chattopadhyay, P.K. An in situ approach for the synthesis of a gum ghatti-g-interpenetrating terpolymer network hydrogel for the high-performance adsorption mechanism evaluation of Cd(II), Pb(II), Bi(III) and Sb(III). J. Mater. Chem. A 2018, 6, 8078–8100. [Google Scholar] [CrossRef]
- Singha, N.R.; Karmakar, M.; Mahapatra, M.; Mondal, H.; Dutta, A.; Roy, C.; Chattopadhyay, P.K. Systematic synthesis of pectin-g-(sodium acrylate-co-N-isopropylacrylamide) interpenetrating polymer network for superadsorption of dyes/M(II): Determination of physicochemical changes in loaded hydrogels. Polym. Chem. 2017, 8, 3211–3237. [Google Scholar] [CrossRef]
- Singha, N.R.; Mahapatra, M.; Karmakar, M.; Dutta, A.; Mondal, H.; Chattopadhyay, P.K. Synthesis of guar gum-g-(acrylic acid-co-acrylamide-co-3-acrylamido propanoic acid) IPN via in situ attachment of acrylamido propanoic acid for analyzing superadsorption mechanism of Pb(II)/Cd(II)/Cu(II)/MB/MV. Polym. Chem. 2017, 8, 6750–6777. [Google Scholar] [CrossRef]
- Karmakar, M.; Mondal, H.; Mahapatra, M.; Chattopadhyay, P.K.; Chatterjee, S.; Singha, N.R. Pectin-grafted terpolymer superadsorbent via N–H activated strategic protrusion of monomer for removals of Cd(II), Hg(II), and Pb(II). Carbohyd. Polym. 2019, 206, 778–791. [Google Scholar] [CrossRef] [PubMed]
- Mondal, H.; Karmakar, M.; Chattopadhyay, P.K.; Singha, N.R. Starch-g-tetrapolymer hydrogel via in situ attached monomers for removals of Bi(III) and/or Hg(II) and dye(s): RSM-based optimization. Carbohyd. Polym. 2019. [Google Scholar] [CrossRef]
- Singha, N.R.; Mahapatra, M.; Karmakar, M.; Mondal, H.; Dutta, A.; Deb, M.; Mitra, M.; Roy, C.; Chattopadhyay, P.K.; Maiti, D.K. In situ allocation of a monomer in pectin-g-terpolymer hydrogels and effect of comonomer compositions on superadsorption of metal ions/dyes. ACS Omega 2018, 3, 4163–4180. [Google Scholar] [CrossRef]
- Roy, S.; Ragunath, S. Emerging membrane technologies for water and energy sustainability: Future prospects, constraints and challenges. Energies 2018, 11, 2997. [Google Scholar] [CrossRef]
- Roy, S.; Thongsukmak, A.; Tang, J.; Sirkar, K.K. Concentration of aqueous hydrogen peroxide solution by pervaporation. J. Membr. Sci. 2012, 389, 17–24. [Google Scholar] [CrossRef]
- Goh, P.S.; Matsuura, T.; Ismail, A.F.; Ng, B.C. The water-energy Nexus: Solutions towards energy-efficient desalination. Energy Technol. 2017, 5, 1136–1155. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F. Review: Is interplay between nanomaterial and membrane technology the way forward for desalination? J. Chem. Technol. Biotechnol. 2015, 90, 971–980. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications; John Wiley & Sons: New York, NY, USA, 2012. [Google Scholar]
- Ho, W.S.W.; Sirkar, K.K. (Eds.) Membrane Handbook; Van Nostrand Reinhold: New York, NY, USA, 1992. [Google Scholar]
- Bodzek, M.; Bohdziewicz, J.; Konieczny, K. Techniki Membranowe W Ochronie Srodowiska; Wydawnictwo Politechniki Slaskiej: Gliwice, Poland, 1997. [Google Scholar]
- Mulder, M.H.V. Basic Principles of Membrane Technology; Kluwer Academic Publishers: Dordrecht, The Netherlands; Boston, MA, USA; London, UK, 1991. [Google Scholar]
- Feng, X.; Huang, R.Y.M. Liquid separation by membrane pervaporation: A review. Ind. Eng. Chem. Res. 1997, 36, 1048–1066. [Google Scholar] [CrossRef]
- Slater, C.S. A review of: “Pervaporation membrane separation processes”. Sep. Purif. Methods 1991, 20, 109–111. [Google Scholar] [CrossRef]
- Peñate, B.; García-Rodríguez, L. Current trends and future prospects in the design of seawater reverse osmosis desalination technology. Desalination 2012, 284, 1–8. [Google Scholar] [CrossRef]
- Chong, T.H.; Loo, S.; Krantz, W.B. Energy-efficient reverse osmosis desalination process. J. Membr. Sci. 2015, 473, 177–188. [Google Scholar] [CrossRef]
- Stover, R.L. High recovery, low fouling, and low energy reverse osmosis. Desalin. Water Treat. 2016, 55, 26501–26506. [Google Scholar] [CrossRef]
- Ghaffour, N.; Missimer, T.M.; Amy, G.L. Technical review and evaluation of the economics of water desalination: Current and future challenges for better water supply sustainability. Desalination 2013, 309, 197–207. [Google Scholar] [CrossRef]
- Muntha, S.T.; Kausar, A.; Siddiq, M. Advances in polymeric nanofiltration membrane: A review. Polym.-Plast. Technol. Eng. 2017, 56, 841–856. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Singh, D.; Sirkar, K.K. Desalination of brine and produced water by direct contact membrane distillation at high temperatures and pressures. J. Membr. Sci. 2012, 389, 380–388. [Google Scholar] [CrossRef]
- Adham, S.; Hussain, A.; Matar, J.M.; Dores, R.; Janson, A. Application of membrane distillation for desalting brines from thermal desalination plants. Desalination 2013, 314, 101–108. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Produced water treatment: Application of air gap membrane distillation. Desalination 2013, 309, 46–51. [Google Scholar] [CrossRef]
- Mahapatra, M.; Karmakar, M.; Mondal, B.; Singha, N.R. Role of ZDC/S ratio for pervaporative separation of organic liquids through modified EPDM membranes: Rational mechanistic study of vulcanization. RSC Adv. 2016, 6, 69387–69403. [Google Scholar] [CrossRef]
- Karmakar, M.; Mahapatra, M.; Singha, N.R. Separation of tetrahydrofuran using RSM optimized accelerator-sulfur-filler of rubber membranes: Systematic optimization and comprehensive mechanistic study. Korean J. Chem. Eng. 2017, 34, 1416–1434. [Google Scholar] [CrossRef]
- Mahapatra, M.; Karmakar, M.; Dutta, A.; Singha, N.R. Fabrication of composite membranes for pervaporation of tetrahydrofuran-water: Optimization of intrinsic property by response surface methodology and studies on vulcanization mechanism by density functional theory. Korean J. Chem. Eng. 2018, 35, 1889–1910. [Google Scholar] [CrossRef]
- Roy, S.; Singha, N.R. Polymeric Nanocomposite Membranes for Next Generation Pervaporation Process: Strategies, Challenges and Future Prospects. Membranes 2017, 3, 53. [Google Scholar] [CrossRef]
- Singha, N.R.; Parya, T.K.; Ray, S.K. Dehydration of 1, 4-dioxane by pervaporation using filled and crosslinked polyvinyl alcohol membrane. J. Membr. Sci. 2009, 340, 35–44. [Google Scholar] [CrossRef]
- Ray, S.; Singha, N.R.; Ray, S.K. Removal of tetrahydrofuran (THF) from water by pervaporation using homo and blend polymeric membranes. Chem. Eng. J. 2009, 149, 153–161. [Google Scholar] [CrossRef]
- Singha, N.R.; Ray, S.; Ray, S.K.; Konar, B.B. Removal of pyridine from water by pervaporation using filled SBR Membranes. J. Appl. Polym. Sci. 2011, 121, 1330–1334. [Google Scholar] [CrossRef]
- Singha, N.R.; Ray, S.K. Removal of pyridine from water by pervaporation using crosslinked and filled natural rubber membranes. J. Appl. Polym. Sci. 2012, 124, E99–E107. [Google Scholar] [CrossRef]
- Singha, N.R.; Kar, S.; Ray, S.; Ray, S.K. Separation of isopropyl alcohol-water mixtures by pervaporation using crosslink IPN membranes. Chem. Eng. Process. 2009, 48, 1020–1029. [Google Scholar] [CrossRef]
- Kuila, S.B.; Ray, S.K.; Das, P.; Singha, N.R. Synthesis of full interpenetrating network membranes of poly(acrylic acid-co-acrylamide) in the matrix of polyvinyl alcohol for dehydration of ethylene glycol by pervaporation. Chem. Eng. Process. 2011, 50, 391–403. [Google Scholar] [CrossRef]
- Singha, N.R.; Kar, S.; Ray, S.K. Synthesis of chemically modified polyvinyl alcohol membranes for dehydration of dioxane by pervaporation. Sep. Sci. Technol. 2009, 44, 422–446. [Google Scholar] [CrossRef]
- Singha, N.R.; Ray, S.K. Separation of toluene-methanol mixtures by pervaporation using semi-IPN polymer membranes. Sep. Sci. Technol. 2010, 45, 2298–2307. [Google Scholar] [CrossRef]
- Singha, N.R.; Kar, S.; Ray, S.K. Synthesis of novel polymeric membrane for separation of MTBE-methanol by pervaporation. Sep. Sci. Technol. 2009, 44, 1970–1990. [Google Scholar] [CrossRef]
- Ray, S.; Ray, S.K. Effect of copolymer type and composition on separation characteristics of pervaporation membranes-a case study with separation of acetone-water mixtures. J. Membr. Sci. 2006, 270, 73–87. [Google Scholar] [CrossRef]
- Ray, S.; Ray, S.K. Pervaporative dehydration of dimethyl formamide (DMF) by crosslinked copolymer membranes. Ind. Eng. Chem. Res. 2006, 45, 7210–7218. [Google Scholar] [CrossRef]
- Korin, E.; Ladizhensky, I.; Korngold, E. Hydrophilic hollow fibre membranes for water desalination by the pervaporation method. Chem. Eng. Process. 1996, 35, 451–457. [Google Scholar] [CrossRef]
- Korngold, E.; Korin, E.; Ladizhensky, I. Water desalination by pervaporation with hollow fibre membranes. Desalination 1996, 107, 121–129. [Google Scholar] [CrossRef]
- Zwijnenberg, H.J.; Koops, G.H.; Wessling, A. Solar driven membrane pervaporation for desalination processes. J. Membr. Sci. 2005, 250, 235–246. [Google Scholar] [CrossRef]
- García-Payo, M.C.; Izquierdo-Gil, M.A.; Fernández-Pineda, C. Wetting study of hydrophobic membranes via liquidentry pressure measurements with aqueous alcohol solutions. J. Colloid Interface Sci. 2000, 230, 420–431. [Google Scholar] [CrossRef]
- Tijing, L.D.; Woo, Y.C.; Choi, J.; Lee, S.; Kim, S.; Shon, H.K. Fouling and its control in membrane distillation—A review. J. Membr. Sci. 2015, 475, 215–244. [Google Scholar] [CrossRef]
- Xie, Z.; Hoang, M.; Duong, T.; Ng, D.; Dao, B.; Gray, S. Sol-gel derived poly(vinyl alcohol)/maleic acid/silica hybrid membrane for desalination by pervaporation. J. Membr. Sci. 2011, 383, 96–103. [Google Scholar] [CrossRef]
- Lee, S.; Boo, C.; Elimelech, M.; Hong, S. Comparison of fouling behavior in forward osmosis (FO) and reverse osmosis (RO). J. Membr. Sci. 2010, 365, 34–39. [Google Scholar] [CrossRef]
- Pearce, G.K. SWRO pre-treatment: Markets and experience. Filtr. Sep. 2010, 47, 30–33. [Google Scholar] [CrossRef]
- Drioli, E.; Stankiewicz, A.I.; Macedonio, F. Membrane engineering in process intensification—An overview. J. Membr. Sci. 2011, 380, 1–8. [Google Scholar] [CrossRef]
- Liang, B.; Zhan, W.; Qi, G.; Lin, S.; Nan, Q.; Liu, Y.; Cao, B.; Pan, K. High performance graphene oxide/polyacrylonitrile composite pervaporation membranes for desalination applications. J. Mater. Chem. A 2015, 3, 5140–5147. [Google Scholar] [CrossRef]
- Wang, H.J.; Ding, S.B.; Zhu, H.L.; Wang, F.; Guo, Y.H.; Zhang, H.P.; Chen, J.Y. Effect of stretching ratio and heating temperature on structure and performance of PTFE hollow fiber membrane in VMD for RO brine. Sep. Purif. Technol. 2014, 126, 82–94. [Google Scholar] [CrossRef]
- Khawaji, A.D.; Kutubkhanah, I.K.; Wie, J. Advances in seawater desalination technologies. Desalination 2008, 221, 47–69. [Google Scholar] [CrossRef]
- Alghoul, M.A.; Poovanaesvaran, P.; Sopian, K.; Sulaiman, M.Y. Review of brackish water reverse osmosis (BWRO) system designs. Renew. Sustain. Energ. Rev. 2009, 13, 2661–2667. [Google Scholar] [CrossRef]
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef]
- Drobek, M.; Yacou, C.; Motuzas, J.; Julbe, A.; Ding, L.; Da Costa, J.C.D. Long term pervaporation desalination of tubular MFI zeolite membranes. J. Membr. Sci. 2012, 415–416, 816–823. [Google Scholar] [CrossRef]
- Lin, C.X.C.; Ding, L.P.; Smart, S.; Da Costa, J.C.D. Cobalt oxide silica membranes for desalination. J. Colloid Interface Sci. 2012, 368, 70–76. [Google Scholar] [CrossRef]
- Smitha, B.; Suhanya, D.; Sridhar, S.; Ramakrishna, M. Separation of organic–organic mixtures by pervaporation—A review. J. Membr. Sci. 2004, 241, 1–21. [Google Scholar] [CrossRef]
- Jiang, L.Y.; Wang, Y.; Chung, T.S.; Qiao, X.Y.; Lai, J.Y. Polyimides membranes for pervaporation and biofuels separation. Prog. Polym. Sci. 2009, 34, 1135–1160. [Google Scholar] [CrossRef]
- Das, P.; Ray, S.K.; Kuila, S.B.; Samanta, H.S.; Singha, N.R. Systematic choice of crosslinker and filler for pervaporation membrane: A case study with dehydration of isopropyl alcohol-water mixtures by polyvinyl alcohol membranes. Sep. Purif. Technol. 2011, 81, 159–173. [Google Scholar] [CrossRef]
- Mujiburohman, M.; Feng, X. Permselectivity, solubility and diffusivity of propyl propionate/water mixtures in poly(ether block amide)membranes. J. Membr. Sci. 2007, 300, 95–103. [Google Scholar] [CrossRef]
- Singha, N.R.; Kuila, S.B.; Das, P.; Ray, S.K. Separation of toluene–methanol mixtures by pervaporation using crosslink IPN membranes. Chem. Eng. Process. Process Intensif. 2009, 48, 1560–1565. [Google Scholar] [CrossRef]
- Singha, N.R.; Das, P.; Ray, S.K. Recovery of pyridine from water by pervaporation using filled and crosslinked EPDM membranes. J. Ind. Eng. Chem. 2013, 19, 2034–2045. [Google Scholar] [CrossRef]
- Pereira, C.C.; Claudio, P.R.; Ronaldo, N.; Cristiano, P.B. Pervaporative recovery of volatile aroma compounds from fruit juices. J. Membr. Sci. 2006, 274, 1–23. [Google Scholar] [CrossRef]
- Samanta, H.S.; Ray, S.K.; Das, P.; Singha, N.R. Separation of acid–water mixtures by pervaporation using nanoparticle filled mixed matrix copolymer membranes. J. Chem. Technol. Biotechnol. 2012, 87, 608–622. [Google Scholar] [CrossRef]
- Kahlenberg, L. On the nature of the process of osmosis and osmotic pressure with observation concerning dialysis. J. Phys. Chem. 1906, 10, 141–209. [Google Scholar] [CrossRef]
- Shao, P.; Huang, R.Y.M. Polymeric membrane pervaporation. J. Membr. Sci. 2007, 287, 162–179. [Google Scholar] [CrossRef]
- Koschikowski, J.; Wieghaus, M.; Rommel, M. Solar thermal-driven desalination plants based on membrane distillation. Desalination 2003, 156, 295–304. [Google Scholar] [CrossRef]
- Heintz, A.; Stephan, W. A generalized solution-diffusion model of the pervaporation process through composite membranes. Part I. Prediction of mixture solubilities in the dense active layer using the UNIQUAC model. J. Membr. Sci. 1994, 89, 143–151. [Google Scholar] [CrossRef]
- Heintz, A.; Stephan, W. A generalized solution-diffusion model of the pervaporation process through composite membranes. Part II. Concentration polarization, coupled diffusion and the influence of the porous support layer. J. Membr. Sci. 1994, 89, 153–169. [Google Scholar] [CrossRef]
- Lipnizki, F.; Trägårdh, G. Modeling of pervaporation: Models to analyze and predict the mass transport in pervaporation. Sep. Purif. Rev. 2001, 30, 49–125. [Google Scholar] [CrossRef]
- Marriott, J.; Sørensen, E. A general approach to modelling membrane modules. Chem. Eng. Sci. 2003, 58, 4975–4990. [Google Scholar] [CrossRef]
- Schaetzel, P.; Vauclair, C.; Nguyen, Q.T.; Bouzerar, R. A simplified solution-diffusion theory in pervaporation: The total solvent volume fraction model. J. Membr. Sci. 2004, 244, 117–127. [Google Scholar] [CrossRef]
- Sukitpaneenit, P.; Chung, T.-S.; Jiang, L.Y. Modified pore-flow model for pervaporation mass transport in PVDF hollow fiber membranes for ethanol-water separation. J. Membr. Sci. 2010, 362, 393–406. [Google Scholar] [CrossRef]
- Rautenbach, R.; Herion, C.; Meyer-Blumentoth, U. Pervaporation Membrane Separation Processes; Elsevier: Amsterdam, The Netherlands; Oxford, UK, 1990. [Google Scholar]
- Mizsey, P.; Koczka, K.; Deák, A.; Fonyó, Z. Simulation of pervaporation using the “solution–diffusion” model. Hung. Chem. J. 2005, 7, 239–242. [Google Scholar]
- Lovasz, A.; Mizsey, P.; Fonyo, Z. Methodology for parameter estimation of modelling of pervaporation in flowsheeting environment. Chem. Eng. J. 2007, 133, 219–227. [Google Scholar] [CrossRef]
- Cséfalvay, E.; Szitkai, Z.; Mizsey, P.; Fonyó, Z. Experimental data based modelling and simulation of isopropanol dehydration by pervaporation. Desalination 2008, 229, 94–108. [Google Scholar] [CrossRef]
- Valentínyi, N.; Cséfalvaya, E.; Mizsey, P. Modelling of pervaporation: Parameter estimation and model development. Chem. Eng. Res. Des. 2013, 91, 174–183. [Google Scholar] [CrossRef]
- Khajavi, S.; Jansen, J.C.; Kapteijn, F. Production of ultra-pure water by desalination of seawater using a hydroxy sodalite membrane. J. Membr. Sci. 2010, 356, 52–57. [Google Scholar] [CrossRef]
- Liang, B.; Pan, K.; Li, L.; Giannelis, C. High performance hydrophilic pervaporation composite membranes for water desalination. Desalination 2014, 347, 199–206. [Google Scholar] [CrossRef]
- Cho, C.H.; Oh, K.Y.; Kim, S.K.; Yeo, J.G.; Sharma, P. Pervaporative seawater desalination using NaA zeolite membrane: Mechanisms of high water flux and high salt rejection. J. Membr. Sci. 2011, 37, 226–238. [Google Scholar] [CrossRef]
- Ong, Y.K.; Shi, G.M.; Le, N.L.; Tang, Y.P.; Zuo, J.; Nunes, S.P.; Chung, T.-S. Recent membrane development for pervaporation processes. Prog. Polym. Sci. 2016, 57, 1–31. [Google Scholar] [CrossRef]
- Wan, C.F.; Yang, T.; Lipscomb, G.G.; Stookey, D.J.; Chung, T.-S. Design and fabrication of hollow fiber membrane modules. J. Membrane Sci. 2017, 538, 96–107. [Google Scholar] [CrossRef]
- Fini, M.N.; Soroush, S.; Montazer-Rahmati, M.M. Synthesis and optimization of chitosan ceramic-supported membranes in pervaporation ethanol dehydration. Membranes 2018, 8, 119. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, Q.; Wu, C.; Wang, H.; Wang, H. Viscosity-driven in situ self-assembly strategy to fabricate cross-linked ZIF-90/PVA hybrid membranes for ethanol dehydration via pervaporation. Sep. Sci. Technol. 2018, 201, 256–267. [Google Scholar] [CrossRef]
- Yoon, K.; Kim, K.; Wang, X.; Fang, D.; Hsiao, B.S.; Chu, B. High flux ultrafiltration membranes based on electrospun nanofibrous PAN scaffolds and chitosan coating. Polymer 2006, 47, 2434–2441. [Google Scholar] [CrossRef]
- Yoon, K.; Hsiao, B.S.; Chu, B. High flux ultrafiltration nanofibrous membranes based on polyacrylonitrile electrospun scaffolds and crosslinked polyvinyl alcohol coating. J. Membr. Sci. 2009, 338, 145–152. [Google Scholar] [CrossRef]
- Tang, Z.; Wei, J.; Yung, L.; Ji, B.; Ma, H.; Qiu, C.; Yoon, K.; Wan, F.; Fang, D.; Hsiao, B.S.; et al. UV-cured poly(vinyl alcohol) ultrafiltration nanofibrous membrane based on electrospun nanofiber scaffolds. J. Membr. Sci. 2009, 328, 1–5. [Google Scholar] [CrossRef]
- Yoon, K.; Hsiao, B.S.; Chu, B. High flux nanofiltration membranes based on interfacially polymerized polyamide barrier layer on polyacrylonitrile nanofibrous scaffolds. J. Membr. Sci. 2009, 326, 484–492. [Google Scholar] [CrossRef]
- Yung, L.; Ma, H.; Wang, X.; Yoon, K.; Wang, R.; Hsiao, B.S.; Chu, B. Fabrication of thin-film nanofibrous composite membranes by interfacial polymerization using ionic liquids as additives. J. Membr. Sci. 2010, 365, 52–58. [Google Scholar] [CrossRef]
- Song, X.; Liu, Z.; Sun, D.D. Nano gives the answer: Breaking the bottleneck of internal concentration polarization with a nanofiber composite forward osmosis membrane for a high water production rate. Adv. Mater. 2011, 23, 3256–3260. [Google Scholar] [CrossRef]
- Reid, C.E.; Breton, E.J. Water and ion flow across cellulosic membranes. J. Appl. Polym. Sci. 1959, 1, 133–143. [Google Scholar] [CrossRef]
- Loeb, G.S.; Sourirajan, S. (Eds.) Sea Water Demineralization by Means of an Osmotic Membrane; Advances in Chemistry Series; American Chemical Society: Washington, DC, USA, 1963; Volume 38, p. 117. [Google Scholar]
- Kober, P.A. Pervaporation, perstillation and percrystallization. J. Am. Chem. Soc. 1917, 39, 944–948. [Google Scholar] [CrossRef]
- Binning, R.; Lee, R.; Jennings, J.; Martin, E. Separation of liquid mixtures by permeation. Ind. Eng. Chem. 1961, 53, 45–50. [Google Scholar] [CrossRef]
- Aptel, P.; Cuny, J.; Jozefonvicz, J.; Morel, G.; Neel, J. Liquid transport through membranes prepared by grafting of polar monomers onto poly (tetrafluoroethylene) films. II. some factors determining pervaporation rate and selectivity. J. Appl. Polym. Sci. 1974, 18, 351–364. [Google Scholar] [CrossRef]
- Vane, L.M.; Mairal, A.P.; Ng, A.; Alvarez, F.R.; Baker, R.W. Separation Process Using Pervaporation and Dephlegmation. U.S. Patent US6755975B2, 29 June 2004. [Google Scholar]
- Cox, G.; Baker, R. Pervaporation. Ind. Wastewater 1998, 6, 35–37. [Google Scholar]
- Elma, M.; Yacou, C.; Wang, D.K.; Smart, S.; Da Costa, J.C.D. Microporous silica based membranes for desalination. Water 2012, 4, 629–649. [Google Scholar] [CrossRef]
- Xie, Z.; Ng, D.; Hoang, M.; Duong, T.; Gray, S. Separation of aqueous salt solution by pervaporation through hybrid organic-inorganic membrane: Effect of operating conditions. Desalination 2011, 273, 220–225. [Google Scholar] [CrossRef]
- Huth, E.; Muthu, S.; Ruff, L.; Brant, J.A. Feasibility assessment of pervaporation for desalinating high-salinity brines. J. Water Reuse Desal. 2014, 4, 109–124. [Google Scholar] [CrossRef]
- Sule, M.; Jiang, J.; Templeton, M.; Huth, E.; Brant, J.; Bond, T. Salt rejection and water flux through a tubular pervaporative polymer membrane designed for irrigation applications. Environ. Technol. 2013, 34, 1329–1339. [Google Scholar] [CrossRef]
- Peng, F.; Jiang, Z.; Hu, C.; Wang, Y.; Xu, H.; Liu, J. Removing benzene from aqueous solution using CMS-filled PDMS pervaporation membranes. Sep. Purif. Technol. 2016, 48, 229–234. [Google Scholar] [CrossRef]
- Peng, F.; Hub, C.; Jiang, Z. Novel poly(vinyl alcohol)/carbon nanotube hybrid membranes for pervaporation separation of benzene/cyclohexane mixtures. J. Membr. Sci. 2007, 297, 236–242. [Google Scholar] [CrossRef]
- Peng, F.; Lu, L.; Hu, C.; Wu, H.; Jiang, Z. Significant increase of permeation flux and selectivity of poly(vinyl alcohol) membranes by incorporation of crystalline flake graphite. J. Membr. Sci. 2005, 259, 65–73. [Google Scholar] [CrossRef]
- Pan, F.; Peng, F.; Jiang, Z. Diffusion behavior of benzene/cyclohexane molecules in poly(vinyl alcohol)-graphite hybrid membranes by molecular dynamics simulation. Chem. Eng. Sci. 2007, 62, 703–710. [Google Scholar] [CrossRef]
- An, W.; Zhou, X.; Liu, X.; Chai, P.W.; Kuznicki, T.; Kuznicki, S.M. Natural zeolite clinoptilolite-phosphate composite membranes for water desalination by pervaporation. J. Membr. Sci. 2014, 470, 431–438. [Google Scholar] [CrossRef]
- Malekpour, A.; Millani, M.R.; Kheirkhah, M. Synthesis and characterization of a NaA zeolite membrane and its applications for desalination of radioactive solutions. Desalination 2008, 225, 199–208. [Google Scholar] [CrossRef]
- Wijaya, S.; Duke, M.C.; de Costa, J.C.D. Carbonized template silica membranes for desalination. Desalination 2009, 236, 291–298. [Google Scholar] [CrossRef]
- Li, D.; He, L.; Dong, D.; Forsyth, M.; Wang, H. Preparation of silicalite-polyamide composite membranes for desalination. Asia-Pac. J. Chem. Eng. 2011, 7, 434–441. [Google Scholar] [CrossRef]
- Malekpour, A.; Samadi-Maybodi, A.; Sadati, M.R. Desalination of aqueous solutions by LTA and MFI zeolite membranes using pervaporation method. Braz. J. Chem. Eng. 2011, 28, 669–677. [Google Scholar] [CrossRef]
- Ladewig, B.P.; Tan, Y.H.; Lin, C.X.; Ladewig, K.; Diniz Da Costa, J.C.; Smart, S. Preparation, characterisation and performance of templated silica membranes in non-osmotic desalination. Materials 2011, 4, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Swenson, P.; Tanchuk, B.; Gupta, A.; An, W.; Kuznicki, S.M. Pervaporative desalination of water using natural zeolite membranes. Desalination 2012, 285, 68–72. [Google Scholar] [CrossRef]
- Chaudhri, S.G.; Rajai, B.H.; Singh, P.S. Preparation of ultra-thin poly(vinyl alcohol) membranes supported on polysulfone hollow fiber and their application for production of pure water from seawater. Desalination 2015, 367, 272–284. [Google Scholar] [CrossRef]
- Singh, P.S.; Chaudhri, S.G.; Kansara, A.M.; Schetieger, W.; Selvam, T.; Reuss, S.; Kaswal, V. Cetyltrimethylammonium bromide-silica membrane for seawater desalination through pervaporation. Bull. Mater. Sci. 2015, 38, 565–572. [Google Scholar] [CrossRef]
- Feng, B.; Xu, K.; Huang, A. Covalent synthesis of three-dimensional graphene oxide framework (GOF) membrane for seawater desalination. Desalination 2016, 394, 123–130. [Google Scholar] [CrossRef]
- Feng, B.; Xu, K.; Huang, A. Synthesis of graphene oxide/polyimide mixed matrix membranes for desalination. RSC Adv. 2017, 7, 2211–2217. [Google Scholar] [CrossRef]
- Chaudhri, S.G.; Chaudhari, J.C.; Singh, P.S. Fabrication of efficient pervaporation desalination membrane by reinforcement of poly(vinyl alcohol)-silica film on porous polysulfone hollow fiber. J. Appl. Polym. Sci. 2018, 135, 45718. [Google Scholar] [CrossRef]
- Marian, S.A.; Asghari, M.; Amini, Z. Desalination of Kashan City’s water using PEBA-based nanocomposite membranes via pervaporation. J. Water Environ. Nanotechnol. 2017, 2, 96–102. [Google Scholar]
- Nigiz, F.U.; Veli, S.; Hilmioglu, N.D. Deep purification of seawater using a novel zeolite 3A incorporated polyetherblock-amide composite membrane. Sep. Purif. Technol. 2017, 188, 90–97. [Google Scholar] [CrossRef]
- Nigiz, F.U. Preparation of high-performance graphene nanoplate incorporated polyether block amide membrane and application for seawater desalination. Desalination 2018, 433, 164–171. [Google Scholar] [CrossRef]
- Zhang, R.; Liang, B.; Qu, T.; Cao, B.; Li, P. High-performance sulfosuccinic acid crosslinked PVA composite pervaporation membrane for desalination. Environ. Technol. 2019, 40, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hou, J.; Ye, Y.; Mansouri, J.; Chen, V. Composite PVA/PVDF pervaporation membrane for concentrated brine desalination: Salt rejection, membrane fouling and defect control. Desalination 2017, 422, 49–58. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, K.; Lu, R.; Jiang, J. Water desalination and biofuel dehydration through a thin membrane of polymer of intrinsic microporosity: Atomistic simulation study. J. Membr. Sci. 2018, 545, 49–56. [Google Scholar] [CrossRef]
- Yilman, B.; Nigiz, F.U.; Aytaç, A.; Hilmioglu, N.D. Multi-walled carbon nanotube doped PVA membrane for desalination. Water Supply 2018. [Google Scholar] [CrossRef]
- Huang, A.; Feng, B. Synthesis of novel graphene oxide-polyimide hollow fiber membranes for seawater desalination. J. Membr. Sci. 2018, 548, 59–65. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, X.; Cao, B.; Li, P. Fabrication of high-performance PVA/PAN composite pervaporation membranes crosslinked by PMDA for wastewater desalination. Petrol. Sci. 2018, 15, 146–156. [Google Scholar] [CrossRef]
- Liang, B.; Li, Q.; Cao, B.; Li, P. Water permeance, permeability and desalination properties of the sulfonic acid functionalized composite pervaporation membranes. Desalination 2018, 433, 132–140. [Google Scholar] [CrossRef]
- Teow, Y.H.; Mohammad, A.W. New generation nanomaterials for water desalination: A review. Desalination 2019, 451, 2–17. [Google Scholar] [CrossRef]
- Paugam, M.-F.; Buffle, J. Comparison of carrier-facilitated copper(II) ion transport mechanisms in a supported liquid membrane and in a plasticized cellulose triacetate membrane. J. Membr. Sci. 1998, 147, 207–215. [Google Scholar] [CrossRef]
- Kuznetsov, Y.P.; Kruchinina, E.V.; Baklagina, Y.G.; Khripunov, A.K.; Tulupova, O.A. Deep desalination of water by evaporation through polymeric membranes. Russ. J. Appl. Chem. 2007, 80, 790–798. [Google Scholar] [CrossRef]
- Naim, M.; Elewa, M.; El-Shafei, A. Desalination of simulated seawater by purge-air pervaporation using an innovative fabricated membrane. Water Sci. Technol. 2015, 72, 785–793. [Google Scholar] [CrossRef]
- Liu, G.; Shen, J.; Liu, Q.; Liu, G.; Xiong, J.; Yang, J.; Jin, W. Ultrathin two-dimensional MXene membrane for pervaporation desalination. J. Membr. Sci. 2018, 548, 548–558. [Google Scholar] [CrossRef]
- Li, L.; Hou, J.; Ye, Y.; Mansouri, J.; Zhang, Y.; Chen, V. Suppressing salt transport through composite pervaporation membranes for brine desalination. Appl. Sci. 2017, 7, 856. [Google Scholar] [CrossRef]
- Li, Q.; Cao, B.; Li, P. Fabrication of high performance pervaporation desalination composite membranes by optimizing the support layer structures. Ind. Eng. Chem. Res. 2018, 57, 11178–11185. [Google Scholar] [CrossRef]
- Qian, X.; Li, N.; Wang, Q.; Ji, S. Chitosan/graphene oxide mixed matrix membrane with enhanced water permeability for high-salinity water desalination by pervaporation. Desalination 2018, 438, 83–96. [Google Scholar] [CrossRef]
- Quiňones-Bolaňos, E.; Zhou, H.D.; Soundararajan, R.; Otten, L. Water and solute transport in pervaporation hydrophilic membranes to reclaim contaminated water for micro-irrigation. J. Membr. Sci. 2005, 252, 19–28. [Google Scholar] [CrossRef]
- Kujawski, W.; Krajewska, S.; Kujawski, M.; Gazagnes, L.; Larbot, A.; Persin, M. Pervaporation properties of fluoroalkylsilane (FAS) grafted ceramic membranes. Desalination 2007, 205, 75–86. [Google Scholar] [CrossRef]
- Li, D.; Wang, H. Recent developments in reverse osmosis desalination membranes. J. Mater. Chem. 2010, 20, 4551–4566. [Google Scholar] [CrossRef]
- Xie, Z.; Hoang, M.; Ng, D.; Doherty, C.; Hill, A.; Gray, S. Effect of heat treatment on pervaporation separation of aqueous salt solution using hybrid PVA/MA/TEOS membrane. Sep. Purif. Technol. 2014, 127, 10–17. [Google Scholar] [CrossRef]
- Volkov, A.G.; Paula, S.; Deamer, D.W. Two mechanism of permeation of small neutral molecules and hydrated ions across phospholipid bilayers. Bioelectrochem. Bioenerg. 1997, 42, 153–160. [Google Scholar] [CrossRef]
- Lin, J.; Murad, S. A computer simulation study of the separation of aqueous solutions using thin zeolite membranes. Mol. Phys. 2001, 99, 1175–1181. [Google Scholar] [CrossRef]
- Wijmans, J.G.; Baker, R.W. The solution diffusion model: A review. J. Membr. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]
- Feng, X.; Huang, R.Y.M. Estimation of activation for permeation in pervaporation processes. J. Membr. Sci. 1996, 118, 127–131. [Google Scholar] [CrossRef]
- Barique, M.A.; Tsuchida, E.; Ohira, A.; Tashiro, K. Effect of elevated temperatures on the states of water and their correlation with the proton conductivity of Nafion. ACS Omega 2018, 3, 349–360. [Google Scholar] [CrossRef]
- Ravindra, R.; Rao, A.K.; Khan, A. Processing of liquid propellant reaction liquors by pervaporation. J. Appl. Polym. Sci. 1999, 72, 141–149. [Google Scholar] [CrossRef]
- Weng, Y.; Qiu, S.; Ma, L.; Liu, Q.; Ding, M.; Zhang, Q.; Zhang, Q.; Wang, T. Jet-fuel range hydrocarbons from biomass-derived sorbitol over Ni-HZSM-5/SBA-15 catalyst. Catalysts 2015, 5, 2147–2160. [Google Scholar] [CrossRef]
- Moermans, B.; DeBeuckelaer, W.; Vankelecom, I.F.J.; Ravishankar, R.; Martens, J.A.; Jacobs, P.A. Incorporation of nano-sized zeolites in membranes. Chem. Commun. 2000, 0, 2467–2468. [Google Scholar] [CrossRef]
- Zhan, X.; Lu, J.; Tan, T.; Li, J. Mixed matrix membranes with HF acid etched ZSM-5 for ethanol/water separation: Preparation and pervaporation performance. Appl. Surf. Sci. 2012, 259, 547–556. [Google Scholar] [CrossRef]
- Khosravi, T.; Mosleh, S.; Bakhtiari, O.; Mohammadi, T. Mixed matrix membranes of Matrimid 5218 loaded with zeolite 4A for pervaporation separation of water-isopropanol mixtures. Chem. Eng. Res. Des. 2012, 90, 2353–2363. [Google Scholar] [CrossRef]
- Qiu, S.; Wu, L.; Shi, G.; Zhang, L.; Chen, H.; Gao, C. Preparation and pervaporation property of chitosan membrane with functionalized multiwalled carbon nanotubes. Ind. Eng. Chem. Res. 2010, 49, 11667–11675. [Google Scholar] [CrossRef]
- Jiang, L.Y.; Chung, T.-S.; Rajagopalan, R. Matrimid (R)/MgO mixed matrix membranes for pervaporation. AIChE J. 2007, 53, 1745–1757. [Google Scholar] [CrossRef]
- Bhat, S.D.; Aminabhavi, T.M. Novel sodium alginate composite membranes incorporated with SBA-15 molecular sieves for the pervaporation dehydration of aqueous mixtures of isopropanol and 1,4-dioxane at 30 °C. Microporous Mesoporous Mater. 2006, 91, 206–214. [Google Scholar] [CrossRef]
- Flynn, E.J.; Keane, D.A.; Tabari, P.M.; Morris, M.A. Pervaporation performance enhancement through the incorporation of mesoporous silica spheres into PVA membranes. Sep. Purif. Technol. 2013, 118, 73–80. [Google Scholar] [CrossRef]
- Kudasheva, A.; Sorribas, S.; Zornoza, B.; Téllez, C.; Coronas, J. Pervaporation of water/ethanol mixtures through polyimide based mixed matrix membranes containing ZIF-8, ordered mesoporous silica and ZIF-8-silica core-shell spheres. J. Chem. Technol. Biotechnol. 2015, 90, 669–677. [Google Scholar] [CrossRef]
- Jeazet, H.B.T.; Staudt, C.; Janiak, C. Metal–organic frameworks in mixed matrix membranes for gas separation. Dalton Trans. 2012, 41, 14003–14027. [Google Scholar] [CrossRef]
- Zornoza, B.; Tellez, C.; Coronas, J.; Gascon, J.; Kapteijn, F. Metal organic framework based mixed matrix membranes: An increasingly important field of research with a large application potential. Microporous Mesoporous Mater. 2013, 166, 67–78. [Google Scholar] [CrossRef]
- Kang, C.-H.; Lin, Y.-F.; Huang, Y.-S.; Tung, K.-L.; Chang, K.-S.; Chen, J.-T.; Hung, W.-S.; Lee, K.-R.; Lai, J.-Y. Synthesis of ZIF-7/chitosan mixed-matrix membranes with improved separation performance of water/ethanol mixtures. J. Membr. Sci. 2013, 438, 105–111. [Google Scholar] [CrossRef]
- Hua, D.; Ong, Y.K.; Wang, Y.; Yang, T.; Chung, T.-S. ZIF-90/P84 mixed matrix membranes for pervaporation dehydration of isopropanol. J. Membr. Sci. 2014, 453, 155–167. [Google Scholar] [CrossRef]
- Kasik, A.; Lin, Y.S. Organic solvent pervaporation properties of MOF-5 membranes. Sep. Purif. Technol. 2014, 121, 38–45. [Google Scholar] [CrossRef]
- Liu, X.; Jin, H.; Li, Y.; Bux, H.; Hu, Z.; Ban, Y.; Yang, W. Metal-organic framework ZIF-8 nanocomposite membrane for efficient recovery of furfural via pervaporation and vapor permeation. J. Membr. Sci. 2013, 428, 498–506. [Google Scholar] [CrossRef]
- Liu, X.L.; Li, Y.S.; Zhu, G.Q.; Ban, Y.J.; Xu, L.Y.; Yang, W.S. An organophilic pervaporation membrane derived from metal-organic framework nanoparticles for efficient recovery of bio-alcohols. Angew. Chem. Int. Ed. 2011, 50, 10636–10639. [Google Scholar] [CrossRef]
- Sorribas, S.; Kudasheva, A.; Almendro, E.; Zornoza, B.; la Iglesia, Ó.; Téllez, C.; Coronas, J. Pervaporation and membrane reactor performance of polyimide based mixed matrix membranes containing MOF HKUST-1. Chem. Eng. Sci. 2015, 124, 37–44. [Google Scholar] [CrossRef]
- Wu, D.; Gao, A.; Zhao, H.; Feng, X. Pervaporative desalination of high-salinity water. Chem. Eng. Res. Des. 2018, 136, 154–164. [Google Scholar] [CrossRef]
- Louie, J.S.; Pinnau, I.; Ciobanu, I.; Ishida, K.P.; Ng, A.; Reinhard, M. Effects of polyether-polyamide block copolymer coating on performance and fouling of reverse osmosis membranes. J. Membr. Sci. 2006, 280, 762–770. [Google Scholar] [CrossRef]
- Orgaz-Orgaz, F. Gel to glass conversion: Densification kinetics and controlling mechanisms. J. Non-Cryst. Solids 1988, 100, 115–141. [Google Scholar] [CrossRef]
- Ye, L.Y.; Liu, Q.L.; Zhang, Q.G.; Zhu, A.M.; Zhou, G.B. Pervaporation characteristics and structure of poly(vinyl alcohol)/poly(ethylene glycol)/tetraethoxysilane hybrid membranes. J. Appl. Polym. Sci. 2007, 105, 3640–3648. [Google Scholar] [CrossRef]
- Uragami, T.; Okazaki, K.; Matsugi, H.; Miyata, T. Structure and permeation characteristics of an aqueous ethanol solution of organic-inorganic hybrid membranes composed of poly(vinyl alcohol) and tetraethoxysilane. Macromolecules 2002, 35, 9156–9163. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Zhang, G.; Meng, H.; Ji, S. Hydrolysis differences of polyacrylonitrile support membrane and its influences on polyacrylonitrile-based membrane performance. Desalination 2009, 242, 313–324. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Destaye, A.G.; Lin, C.K.; Lee, C.K. Glutaraldehyde vapor cross-linked nanofibrous PVA Mat with in situ formed silver nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 4745–4752. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, P.; Luo, Y. Separation of caprolactam-water system by pervaporation through crosslinked PVA membranes. Sep. Purif. Technol. 2006, 52, 77–83. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Kittur, A.A.; Aralaguppi, M.I.; Kariduraganavar, M.Y. Synthesis and characterization of hybrid membranes using poly(vinyl alcohol) and tetraethylorthosilicate for the pervaporation separation of water-isopropanol mixtures. J. Appl. Polym. Sci. 2004, 94, 1304–1315. [Google Scholar] [CrossRef]
- Dlamini, D.S.; Mamba, B.B.; Li, J. The role of nanoparticles in the performance of nano-enabled composite membranes–A critical scientific perspective. Sci. Total Environ. 2019, 656, 723–731. [Google Scholar] [CrossRef]
- Wang, Z.H.; Yu, H.R.; Xia, J.F.; Zhang, F.F.; Li, F.; Xia, Y.Z.; Li, Y.H. Novel GO-blended PVDF ultrafiltration membranes. Desalination 2012, 299, 50–54. [Google Scholar] [CrossRef]
- Hung, W.; Tsou, C.; Guzman, M.D.; An, Q.; Liu, Y.; Zhang, Y.; Hu, C.; Lee, K.; Lai, J. Cross-Linking with Diamine Monomers to Prepare Composite Graphene Oxide-Framework Membranes with Varying d-spacing. Chem. Mater. 2014, 26, 2983–2990. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Singha, N.R.; Dutta, A.; Mahapatra, M.; Karmakar, M.; Mondal, H.; Chattopadhyay, P.K.; Maiti, D.K. Guar Gum-Grafted Terpolymer Hydrogels for Ligand-Selective Individual and Synergistic Adsorption: Effect of Comonomer Composition. ACS Omega 2018, 3, 472–494. [Google Scholar] [CrossRef]
- Mondal, H.; Karmakar, M.; Dutta, A.; Mahapatra, M.; Deb, M.; Mitra, M.; Roy, J.S.D.; Roy, C.; Chattopadhyay, P.K.; Singha, N.R. Tetrapolymer network hydrogels via gum ghatti-grafted and N–H/C–H-activated allocation of monomers for composition-dependent superadsorption of metal ions. ACS Omega 2018, 3, 10692–10708. [Google Scholar] [CrossRef]
- Singha, N.R.; Roy, C.; Mahapatra, M.; Dutta, A.; Roy, J.S.D.; Mitra, M.; Chattopadhyay, P.K. Scalable synthesis of collagenic-waste and natural rubber-based biocomposite for removal of Hg(II) and dyes: Approach for cost-friendly waste management. ACS Omega 2019, 4, 421–436. [Google Scholar] [CrossRef]
- Chatterjee, S.; Gupta, A.; Mohanta, T.; Mitra, R.; Samanta, D.; Mandal, A.B.; Majumder, M.; Rawat, R.; Singha, N.R. Scalable synthesis of hide substance-chitosan-hydroxyapatite: Novel biocomposite from industrial wastes and its efficiency in dye removal. ACS Omega 2018, 3, 11486–11496. [Google Scholar] [CrossRef]
- Roy, C.; Dutta, A.; Mahapatra, M.; Karmakar, M.; Roy, J.S.D.; Mitra, M.; Chattopadhyay, P.K.; Singha, N.R. Collagenic waste and rubber based resin-cured biocomposite adsorbent for high-performance removal(s) of Hg(II), safranine, and brilliant cresyl blue: A cost-friendly waste management approach. J. Hazard. Mater. 2019, 369, 199–213. [Google Scholar] [CrossRef]
- Kim, D.S.; Park, H.B.; Rhim, J.W.; Lee, Y.M. Preparation and characterization of crosslinked PVA/SiO2 hybrid membranes containing sulfonic acid groups for direct methanol fuel cell applications. J. Membr. Sci. 2004, 240, 37–48. [Google Scholar] [CrossRef]
- Singha, N.R.; Dutta, A.; Mahapatra, M.; Roy, J.S.D.; Mitra, M.; Deb, M.; Chattopadhyay, P.K. in situ attachment of acrylamido sulfonic acid-based monomer in terpolymer hydrogel optimized by response surface methodology for individual and/or simultaneous removal(s) of M(III) and cationic dyes. ACS Omega 2019, 4, 1763–1780. [Google Scholar] [CrossRef]
- Mahapatra, M.; Karmakar, M.; Dutta, A.; Mondal, H.; Roy, J.S.D.; Chattopadhyay, P.K.; Singha, N.R. Microstructural analyses of loaded and/or unloaded semisynthetic porous material for understanding of superadsorption and optimization by response surface methodology. J. Environ. Chem. Eng. 2018, 6, 289–310. [Google Scholar] [CrossRef]
- Karmakar, M.; Mahapatra, M.; Dutta, A.; Chattopadhyay, P.K.; Singha, N.R. Fabrication of semisynthetic collagenic materials for mere/synergistic adsorption: A model approach of determining dye allocation by systematic characterization. Int. J. Biol. Macromol. 2017, 102, 438–456. [Google Scholar] [CrossRef]
- Mitra, M.; Mahapatra, M.; Dutta, A.; Roy, J.S.D.; Karmakar, M.; Deb, M.; Mondal, H.; Chattopadhyay, P.K.; Bandyopadhyay, A.; Singha, N.R. Carbohydrate and collagen-based doubly-grafted interpenetrating terpolymer hydrogel via N–H activated in situ allocation of monomer for superadsorption of Pb(II), Hg(II), dyes, vitamin-C, and p-nitrophenol. J. Hazard. Mater. 2018. [Google Scholar] [CrossRef]
- Elakkiya, T.; Sheeja, R.; Ramadhar, K.; Natarajan, T.S. Biocompatibility studies of electrospun nanofibrous membrane of PLLA-PVA blend. J. Appl. Polym. Sci. 2013, 128, 2840–2846. [Google Scholar] [CrossRef]
- Park, J.; Park, J.; Ruckenstein, E. Thermal and dynamic mechanical analysis of PVA/MC blend hydrogels. Polymer 2001, 42, 4271–4280. [Google Scholar] [CrossRef]
- Lai, D.; Wei, Y.; Zou, L.; Xu, Y.; Lu, H. Wet spinning of PVA composite fibers with a large fraction of multi-walled carbon nanotubes. Prog. Nat. Sci. Mater. 2015, 25, 445–452. [Google Scholar] [CrossRef]
- Chanthad, C.; Wootthikanokkhan, J. Effects of crosslinking time and amount of sulfophthalic acid on properties of the sulfonated poly(vinyl alcohol) membrane. J. Appl. Polym. Sci. 2006, 101, 1931–1936. [Google Scholar] [CrossRef]
- Bolto, B.; Tran, T.; Hoang, M.; Xie, Z. Crosslinked poly(vinyl alcohol) membranes. Prog. Polym. Sci. 2009, 34, 969–981. [Google Scholar] [CrossRef]
- Mahendia, S.; Heena, G.; Kandhol, U.P.; Deshpande, S.K. Determination of glass transition temperature of reduced graphene oxide-poly(vinyl alcohol) composites using temperature dependent Fourier transform infrared spectroscopy. J. Mol. Struct. 2016, 1111, 46–54. [Google Scholar] [CrossRef]
- Kim, H.M.; Lee, H.S. Water and oxygen permeation through transparent ethylene vinyl alcohol/(graphene oxide) membranes. Carbohydr. Lett. 2014, 15, 50–56. [Google Scholar] [CrossRef]
- Zhao, C.; Xu, X.; Chen, J.; Yang, F. Optimization of preparation conditions of poly (vinylidene fluoride)/graphene oxide microfiltration membranes by the Taguchi experimental design. Desalination 2014, 334, 17–22. [Google Scholar] [CrossRef]
- Achaby, M.E.; Arrakhiz, F.Z.; Vaudreuil, S.; Essassi, E.M.; Qaiss, A. Piezoelectric β-polymorph formation and properties enhancement in graphene oxide–PVDF nanocomposite films. Appl. Surf. Sci. 2012, 258, 7668–7677. [Google Scholar] [CrossRef]
- Shao, L.; Chang, X.; Zhang, Y.; Huang, Y.; Yao, Y.; Guo, Z. Graphene oxide cross-linked chitosan nanocomposite membrane. Appl. Surf. Sci. 2013, 280, 989–992. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Xiao, P.; Li, J.; Tian, E.; Zhao, Y.; Ren, Y. High water permeable free-standing cellulose triacetate/graphene oxide membrane with enhanced antibiofouling and mechanical properties for forward osmosis. Colloids Surf. A 2016, 508, 327–335. [Google Scholar] [CrossRef]
- Panahian, S.; Raisi, A.; Aroujalian, A. Multilayer mixed matrix membranes containing modified-MWCNTs for dehydration of alcohol by pervaporation process. Desalination 2015, 355, 45–55. [Google Scholar] [CrossRef]
- Peppas, N.A.; Merrill, E.W. Crosslinked poly(vinyl alcohol) hydrogels as swollen elastic networks. J. Appl. Polym. Sci. 1977, 21, 1763–1770. [Google Scholar] [CrossRef]
- Xie, W.; Cook, J.; Park, H.B.; Freeman, B.D.; Lee, C.H.; McGrath, J.E. Fundamental salt and water transport properties in directly copolymerized disulfonated poly(arylene ether sulfone) random copolymers. Polymer 2011, 52, 2032–2043. [Google Scholar] [CrossRef]
- Suhas, D.P.; Raghu, A.V.; Jeong, H.M.; Aminabhavi, T.M. Graphene-loaded sodium alginate nanocomposite membranes with enhanced isopropanol dehydration performance via a pervaporation technique. RSC Adv. 2013, 3, 17120–17130. [Google Scholar] [CrossRef]
- Dharupaneedi, S.P.; Anjanapura, R.V.; Han, J.M.; Aminabhavi, T.M. Functionalized graphene sheets embedded in chitosan nanocomposite membranes for ethanol and isopropanol dehydration via pervaporation. Ind. Eng. Chem. Res. 2014, 53, 14474–14484. [Google Scholar] [CrossRef]
- Choi, J.; Jegal, J.; Kim, W.; Choi, H. Incorporation of multiwalled carbon nanotubes into poly(vinyl alcohol) membranes for use in the pervaporation of water/ethanol mixtures. J. Appl. Polym. Sci. 2009, 111, 2186–2193. [Google Scholar] [CrossRef]
- Dassios, K.G.; Galiotis, C. Polymer–nanotube interaction in MWCNT/poly(vinyl alcohol) composite mats. Carbon 2012, 50, 4291–4294. [Google Scholar] [CrossRef]
- Maiti, J.; Kakati, N.; Lee, S.H.; Jee, S.H.; Viswanathan, B.; Yoon, Y.S. Where do poly(vinyl alcohol) based membranes stand in relation to Nafion® for direct methanol fuel cell applications? J. Power Sources 2012, 216, 48–66. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Moore, R.B. State of understanding of Nafion. Chem. Rev. 2004, 104, 4535–4585. [Google Scholar] [CrossRef]
- Xue, Y.l.; Lau, C.H.; Cao, B.; Li, P. Elucidating the impact of polymer crosslinking and fixed carrier on enhanced water transport during desalination using pervaporation membranes. J. Membr. Sci. 2019, 575, 135–146. [Google Scholar] [CrossRef]
- Jiraratananon, R.; Chanachai, A.; Huang, R.Y.M.; Uttapap, D. Pervaporation dehydration of ethanol—water mixtures with chitosan/hydroxyethylcellulose (CS/HEC) composite membranes: I. Effect of operating conditions. J. Membr. Sci. 2002, 195, 143–151. [Google Scholar] [CrossRef]
- Elewa, M.M.; El-Shafei, A.A.; Moneer, A.A.; Naim, M.M. Effect of cell hydrodynamics in desalination of saline water by sweeping air pervaporation technique using innovated membrane. Desalin. Water Treat. 2016, 57, 23293–23307. [Google Scholar] [CrossRef]
- Geng, Y.; Zhang, P.; Wang, Q.; Liu, Y.; Pan, K. Novel PAN/PVP Janus ultrafine fiber membrane and its application for biphasic drug release. J. Mater. Chem. B 2017, 5, 5390–5396. [Google Scholar] [CrossRef]
- Peng, P.; Fane, A.G.; Li, X. Desalination by membrane distillation adopting a hydrophilic membrane. Desalination 2005, 173, 45–54. [Google Scholar] [CrossRef]
- Dotrement, C.; Van den Ende, S.; Vandemmele, H.; Vandecsteele, C. Concentration polarization and other boundary layer effects in the pervaporation of chlorinated hydrocarbons. Desalination 1994, 95, 91–113. [Google Scholar] [CrossRef]
- Martínez, L. Comparison of membrane distillation performance using different feeds. Desalination 2004, 168, 359–365. [Google Scholar] [CrossRef]
- Lawson, K.W.; Lloyd, D.R. Membrane distillation. J. Membr. Sci. 1997, 124, 1–25. [Google Scholar] [CrossRef]
- Qtaishat, M.; Matsuura, T.; Kruczek, B.; Khayet, M. Heat and mass transfer analysis in direct contact membrane distillation. Desalination 2008, 219, 272–292. [Google Scholar] [CrossRef]
- Wang, Q.; Li, N.; Bolto, B.; Hoang, M.; Xie, Z. Desalination by pervaporation: A review. Desalination 2016, 387, 46–60. [Google Scholar] [CrossRef]
- Fu, Y.; Lai, C.; Chen, J.; Liu, C.; Huang, S. Hydrophobic composite membranes for separating of water–alcohol mixture by pervaporation at high temperature. Chem. Eng. Sci. 2014, 111, 203–210. [Google Scholar] [CrossRef]
- Peters, T.A.; Poeth, C.H.S.; Benes, N.E.; Buijs, H.C.W.M.; Vercauteren, F.F.; Keurentjes, J.T.F. Ceramic-supported thin PVA pervaporation membranes combining high flux and high selectivity; contradicting the flux-selectivity paradigm. J. Membr. Sci. 2006, 276, 42–50. [Google Scholar] [CrossRef]
- Thomson, G.W. The Antoine equation for vapour-pressure data. Chem. Rev. 1946, 38, 1–39. [Google Scholar] [CrossRef]
- Burshe, M.C.; Sawant, S.B.; Joshi, J.B.; Pangarkar, V.G. Sorption and permeation of binary water-alcohol systems through PVA membranes crosslinked with multifunctional crosslinking agents. Sep. Purif. Technol. 1997, 12, 145–156. [Google Scholar] [CrossRef]
- Fujita, H.; Kishimoto, A.; Matsumo, K. Concentration and temperature dependence of diffusion coefficients. Trans. Faraday Soc. 1960, 56, 424–437. [Google Scholar] [CrossRef]
- Isklan, N.; Sanl, O. Separation characteristics of acetic acid–water mixtures by pervaporation using poly(vinyl alcohol) membranes modified with malic acid. Chem. Eng. Process. 2005, 44, 1019–1027. [Google Scholar] [CrossRef]
- Hyder, M.N.; Huang, R.Y.M.; Chen, P. Effect of selective layer thickness on pervaporation of composite poly(vinyl alcohol)-poly(sulfone) membranes. J. Membr. Sci. 2008, 318, 387–396. [Google Scholar] [CrossRef]
- Zhang, C.; Fu, L.; Tian, Z.; Cao, B.; Li, P. Post-crosslinking of triptycene-based Tröger’s base polymers with enhanced natural gas separation performance. J. Membr. Sci. 2018, 556, 277–284. [Google Scholar] [CrossRef]
- George, S.C.; Thomas, S. Transport phenomena through polymeric systems. Prog. Polym. Sci. 2001, 26, 985–1017. [Google Scholar] [CrossRef]
- Boukhvalov, D.W.; Katsnelson, M.I.; Son, Y. Origin of anomalous water permeation through graphene oxide membrane. Nano Lett. 2013, 13, 3930–3935. [Google Scholar] [CrossRef]
- Xu, C.; Cui, A.J.; Xu, Y.L.; Fu, X.Z. Graphene oxide–TiO2 composite filtration membranes and their potential application for water purification. Carbon 2013, 62, 465–471. [Google Scholar] [CrossRef]
- Hung, W.; An, Q.; Guzman, M.D.; Lin, H.; Huang, S.; Liu, W.; Hu, C.; Lee, K.; Lai, J. Pressure-assisted self-assembly technique for fabricating composite membranes consisting of highly ordered selective laminate layers of amphiphilic graphene oxide. Carbon 2014, 68, 670–677. [Google Scholar] [CrossRef]
- Wei, Y.; Xu, Z.; Qusay, F.A.; Wu, K. Polyvinyl alcohol/polysulfone (PVA/PSF) hollow fiber composite membranes for pervaporation separation of ethanol/water solution. J. Appl. Polym. Sci. 2005, 98, 247–254. [Google Scholar] [CrossRef]
- Inamuddin; Thomas, S.; Mishra, R.K.; Asiri, A.M. Sustainable Polymer Composites and Nanocomposites; Springer: Cham, Switzerland, 2019. [Google Scholar]
- van Gemert, R.W.; Petrus-Cuperus, F. Newly developed ceramic membranes for dehydration and separation of organic mixtures by pervaporation. J. Membr. Sci. 1995, 105, 287–291. [Google Scholar] [CrossRef]
- Verkerk, A.W.; van Male, P.; Vorstman, M.A.G.; Keurentjes, J.T.F. Properties of high flux ceramic pervaporation membranes for dehydration of alcohol/water mixtures. Sep. Purif. Technol. 2001, 22– 23, 689–695. [Google Scholar] [CrossRef]
- Wynn, N. Dehydration with silica pervaporation membranes. Membr. Technol. 2001, 2001, 10–11. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, D.; Jiang, L.; Jin, J. Recent progress in developing advanced membranes for emulsified oil/water separation. NPG Asia Mater. 2014, 6, 1–11. [Google Scholar] [CrossRef]
- Zakrzewska-Trznadel, G. A dvances in membrane technologies for the treatment of liquid radioactive waste. Desalination 2013, 321, 119–130. [Google Scholar] [CrossRef]
- Rana, D.; Matsuura, T.; Kassim, M.A.; Ismail, A.F. Radioactive decontamination of water by membrane processes–A review. Desalination 2013, 321, 77–92. [Google Scholar] [CrossRef]
- Mustafa, G.; Wyns, K.; Buekenhoudt, A.; Meynen, V. Antifouling grafting of ceramic membranes validated in a variety of challenging wastewaters. Water Res. 2016, 104, 242–253. [Google Scholar] [CrossRef]
- Amin, S.K.; Abdallah, H.A.M.; Roushdy, M.H.; El-Sherbiny, S.A. An overview of production and development of ceramic membranes. Int. J. Appl. Eng. Res. 2016, 11, 7708–7721. [Google Scholar]
- Dong, H.; Zhao, L.; Zhang, L.; Chen, H.; Gao, C.; Ho, W.S.W. High-flux reverse osmosis membranes incorporated with NaY zeolite nanoparticles for brackish water desalination. J. Membr. Sci. 2015, 476, 373–383. [Google Scholar] [CrossRef]
- Lee, K.P.; Arnot, T.C.; Mattia, D. A review of reverse osmosis membrane materials for desalination—Development to date and future potential. J. Membr. Sci. 2011, 370, 1–22. [Google Scholar] [CrossRef]
- Uhlhorn, R.J.R.; Keizer, K.; Burggraaf, A.J. Gas transport and separation with ceramic membranes. Part II. Synthesis and separation properties of microporous membranes. J. Membr. Sci. 1992, 66, 271–287. [Google Scholar] [CrossRef]
- De Lange, R.S.A.; Hekkink, J.H.A.; Keizer, K.; Burggraaf, A.J. Formation and characterization of supported microporous ceramic membranes prepared by sol–gel modification techniques. J. Membr. Sci. 1995, 99, 57–75. [Google Scholar] [CrossRef]
- Lorente-Ayza, M.M.; Perez-Fernandez, O.; Alcala, R.; Sanchez, E.; Mestre, S.; Coronas, J.; Menéndez, M. Comparison of porosity assessment techniques for low-cost ceramic membranes. Bol. Soc. Esp. Ceram. Vidrio 2016, 56, 29–38. [Google Scholar] [CrossRef]
- Lorente-Ayza, M.M.; Mestre, S.; Sanz, V.; Sanchez, E. On the underestimated effect of the starch ash on the characteristics of low cost ceramic membranes. Ceram. Int. 2016, 42, 18944–18954. [Google Scholar] [CrossRef]
- Kaur, H.; Bulasara, V.K.; Gupta, R.K. Effect of carbonates composition on the permeation characteristics of low-cost ceramic membrane supports. J. Ind. Eng. Chem. 2016, 44, 185–194. [Google Scholar] [CrossRef]
- Hedfi, I.; Hamdi, N.; Rodriguez, M.A.; Srasra, E. Development of a low cost microporous ceramic membrane from kaolin and alumina, using the lignite as porogen agent. Ceram. Int. 2016, 42, 5089–5093. [Google Scholar] [CrossRef]
- Lorente-Ayza, M.M.; Sánchez, E.; Sanz, V.; Mestre, S. Influence of starch content on the properties of low-cost microfiltration ceramic membranes. Ceram. Int. 2015, 41, 13064–13073. [Google Scholar] [CrossRef]
- Quinones-Bolanos, E.; Zhou, H.D.; Parkin, G. Membrane pervaporation for wastewater reuse in microirrigation. J. Environ. Eng. ASCE 2005, 131, 1633–1643. [Google Scholar] [CrossRef]
- Michaels, A.S.; Bixler, H.J.; Hodges Jr., R. M. Kinetics of water and salt transport in cellulose acetate reverse osmosis desalination membranes. J. Colloid Sci. 1965, 20, 1034–1056. [Google Scholar] [CrossRef]
- Voros, N.G.; Maroulis, Z.B.; Marinos-Kouris, D. Salt and water permeability in reverse osmosis membranes. Desalination 1996, 104, 141–154. [Google Scholar] [CrossRef]
- Zhang, X.; Cahill, D.G.; Coronell, O.; Mariñas, B.J. Partitioning of salt ions in FT30 reverse osmosis membranes. Appl. Phys. Lett. 2007, 91, 181904–181907. [Google Scholar] [CrossRef]
- Ju, H.; Sagle, A.C.; Freeman, B.D.; Mardel, J.I.; Hill, A.J. Characterization of sodium chloride and water transport in crosslinked poly(ethylene oxide) hydrogels. J. Membr. Sci. 2010, 358, 131–141. [Google Scholar] [CrossRef]
- Nigiz, F.U.; Hilmioglu, N.D. Pervaporative desalination of seawater by using composite and blended poly(vinyl alcohol) membranes. Desalin. Water Treat. 2016, 57, 4749–4755. [Google Scholar] [CrossRef]
- Duke, M.C.; Mee, S.; Diniz da Costa, J.C.D. Performance of porous inorganic membranes in nonosmotic desalination. Water Res. 2007, 41, 3998–4004. [Google Scholar] [CrossRef]
- Darmawan, A.; Karlina, L.; Astuti, Y.; Sriatun, J.M.; Wang, D.K.; da Costa, J.C.D. Structural evolution of nickel oxide silica sol-gel for the preparation of interlayer-free membranes. J. Non-Cryst. Solids 2016, 447, 9–15. [Google Scholar] [CrossRef]
- Fernández, E.; Benito, J.M.; Pazos, C.; Coca, J. Ceramic membrane ultrafiltration of anionic and nonionic surfactant solutions. J. Membr. Sci. 2005, 246, 1–6. [Google Scholar] [CrossRef]
- Larbot, A.B. Fundamentals on inorganic membranes: Present and new developments. Pol. J. Chem. Technol. 2003, 6, 8–13. [Google Scholar]
- Tavolaro, A.; Drioli, E. Zeolite Membranes. Adv. Mater. 1999, 11, 975–996. [Google Scholar] [CrossRef]
- Cot, L.; Ayral, A.; Durand, J.; Guizard, C.; Hovnanian, N.; Julbe, A.; Larbot, A. Inorganic membranes and solid state sciences. Solid State Sci. 2000, 2, 313–334. [Google Scholar] [CrossRef]
- Alami-Younssi, S.; Kiefer, C.; Larbot, A.; Persin, M.; Sarrazin, J. Grafting γ alumina microporous membranes by organosilanes: Characterisation by pervaporation. J. Membr. Sci. 1998, 143, 27–36. [Google Scholar] [CrossRef]
- Jou, J.; Yoshida, W.; Cohen, Y. A novel ceramic supported polymer membrane for pervaporation of dilute volatile organic compounds. J. Membr. Sci. 1999, 162, 269–284. [Google Scholar] [CrossRef]
- Picard, C.; Larbot, A.; Guida-Pietrasanta, F.; Boutevin, B.; Ratsimihety, A. Grafting of ceramic membranes by fluorinated silanes: Hydrophobic features. Sep. Purif. Technol. 2001, 25, 65–69. [Google Scholar] [CrossRef]
- Caro, J.; Noack, M.; Kolsch, P. Chemically modified ceramic membranes. Microporous Mesoporous Mater. 1998, 22, 321–332. [Google Scholar] [CrossRef]
- Yoshida, W.; Cohen, Y. Ceramic-supported polymer membranes for pervaporation of binary organic/organic mixtures. J. Membr. Sci. 2003, 213, 145–157. [Google Scholar] [CrossRef]
- Yoshida, W.; Cohen, Y. Removal of methyl tertbutyl ether from water by pervaporation using ceramic-supported polymer membranes. J. Membr. Sci. 2004, 229, 27–32. [Google Scholar] [CrossRef]
- Leger, C.; Lira, H.D.L.; Paterson, R. Preparation and properties of surface modified ceramic membranes. Part II. Gas and liquid permeabilities of 5 nm alumina membranes modified by a monolayer of bound polydimethylsiloxane (PDMS) silicone oil. J. Membr. Sci. 1996, 120, 135–146. [Google Scholar] [CrossRef]
- Dafinov, A.; Garcia-Valls, R.; Font, J. Modification of ceramic membranes by alcohol adsorption. J. Membr. Sci. 2002, 196, 69–77. [Google Scholar] [CrossRef]
- Krajewski, S.R.; Kujawski, W.; Dijoux, F.; Picard, C.; Larbot, A. Grafting of ZrO2 powder and ZrO2 membrane by fluoroalkylsilanes. Colloids Surf. A 2004, 243, 43–47. [Google Scholar] [CrossRef]
- Schondelmaier, D.; Cramm, S.; Klingeler, R.; Morenzin, J.; Zilkens, C.; Eberhardt, W. Orientation and self-assembly of hydrophobic fluoroalkylsilanes. Langmuir 2002, 18, 6242–6245. [Google Scholar] [CrossRef]
- Akamatsu, Y.; Makita, K.; Inaba, H.; Minami, T. Water-repellent coating films on glass prepared from hydrolysis and polycondensation reactions of fluroalkyltrialkoxysilane. Thin Solid Films 2001, 289, 138–145. [Google Scholar] [CrossRef]
- Yoshida, W.; Cohen, Y. Topological AFM characterization of graft polymerized silica membranes. J. Membr. Sci. 2003, 215, 249–264. [Google Scholar] [CrossRef]
- Larbot, A.; Gazagnes, L.; Krajewski, S.; Bukowska, M.; Kujawski, W. Water desalination using ceramic membrane distillation. Desalination 2004, 168, 367–372. [Google Scholar] [CrossRef]
- Picard, C.; Larbot, A.; Tronel-Peyroz, E.; Berjoan, R. Characterisation of hydrophilic ceramic membranes modified by fluoroalkylsilanes into hydrophobic membranes. Solid State Sci. 2004, 6, 605–612. [Google Scholar] [CrossRef]
- Janknecht, P.; Widerer, P.A.; Picard, C.; Larbot, A. Ozone-water contacting by ceramic membranes. Sep. Purif. Technol. 2001, 25, 341–346. [Google Scholar] [CrossRef]
- Ren, C.; Fang, H.; Gu, J.; Winnubst, L.; Chen, C. Preparation and characterization of hydrophobic alumina planar membranes for water desalination. J. Eur. Ceram. Soc. 2015, 35, 723–730. [Google Scholar] [CrossRef]
- Yacou, C.; Smart, S.; da Costa, J.C.D. Mesoporous TiO2 based membranes for water desalination and brine processing. Sep. Purif. Technol. 2015, 147, 166–171. [Google Scholar] [CrossRef]
- Sakka, S. Handbook of Sol-Gel Science and Technology: Processing, Characterization and Applications; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2005. [Google Scholar]
- Chen, X.; Zhang, W.; Lin, Y.; Cai, Y.; Qiu, M.; Fan, Y. Preparation of high-flux γ-alumina nanofiltration membranes by using a modified sol-gel method. Microporous Mesoporous Mater. 2015, 214, 195–203. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, Y.; Xu, Z.; Cao, Y.; Dong, Z.; Shi, X. Preparation, characterization and solvent resistance of γ-Al2O3/α-Al2O3 inorganic hollow fiber nanofiltration membrane. J. Membr. Sci. 2016, 503, 69–80. [Google Scholar] [CrossRef]
- Elma, M.; Yacou, C.; da Costa, J.C.D.; Wang, D.K. Performance and long term stability of mesoporous silica membranes for desalination. Membranes 2013, 3, 136–150. [Google Scholar] [CrossRef]
- Chua, Y.T.; Ji, G.; Birkett, G.; Lin, C.X.C.; Kleitz, F.; Smart, S. Nanoporous organosilica membrane for water desalination: Theoretical study on the water transport. J. Membr. Sci. 2015, 482, 56–66. [Google Scholar] [CrossRef]
- Chua, Y.T.; Lin, C.X.C.; Kleitz, F.; Zhao, X.S.; Smart, S. Nanoporous organosilica membrane for water desalination. Chem. Commun. 2013, 49, 4534–4536. [Google Scholar] [CrossRef]
- Elma, M.; Wang, D.K.; Yacou, C.; da Costa, J.C.D. Interlayer-free P123 carbonised template silica membranes for desalination with reduced salt concentration polarization. J. Membr. Sci. 2015, 475, 376–383. [Google Scholar] [CrossRef]
- Elma, M.; Wang, D.K.; Yacou, C.; Motuzas, J.; da Costa, J.C.D. High performance interlayer-free mesoporous cobalt oxide silica membranes for desalination applications. Desalination 2015, 365, 308–315. [Google Scholar] [CrossRef]
- Liu, J.; Dong, Y.; Dong, X.; Hampshire, S.; Zhu, L.; Zhu, Z.; Li, L. Feasible recycling of industrial waste coal fly ash for preparation of anorthite-cordierite based porous ceramic membrane supports with addition of dolomite. J. Eur. Ceram. Soc. 2016, 36, 1059–1071. [Google Scholar] [CrossRef]
- Qin, G.; Lü, X.; Wei, W.; Li, J.; Cui, R.; Hu, S. Microfiltration of kiwifruit juice and fouling mechanism using fly-ash-based ceramic membranes. Food Bioprod. Process. 2015, 96, 278–284. [Google Scholar] [CrossRef]
- Namburath, M.; Joshi, G.; Cholemari, M.; Shet, C.; Sreekrishnan, T.R.; Veeravalli, S. Feasibility study of indigenously developed fly ash membrane in municipal wastewater treatment. Aquat. Procedia 2015, 4, 1492–1499. [Google Scholar] [CrossRef]
- Verweij, H. Inorganic membranes. Curr. Opin. Chem. Eng. 2012, 1, 156–162. [Google Scholar] [CrossRef]
- Silva, K.K.O.S.; Paskocimas, C.A.; Oliveira, F.R.; Nascimento, J.H.O.; Zille, A. Development of porous alumina membranes for treatment of textile effluent. Desalin. Water Treat. 2016, 57, 2640–2648. [Google Scholar] [CrossRef]
- Tolba, G.M.K.; Bastaweesy, A.M.; Ashour, E.A.; Abdelmoez, W.; Khalil, K.A.; Barakat, N.A.M. Effective and highly recyclable ceramic membrane based on amorphous nanosilica for dye removal from the aqueous solutions. Arab. J. Chem. 2015, 9, 287–296. [Google Scholar] [CrossRef]
- Ni, L.; Meng, J.; Li, X.; Zhang, Y. Surface coating on the polyamide TFC RO membrane for chlorine resistance and antifouling performance improvement. J. Membr. Sci. 2014, 451, 205–215. [Google Scholar] [CrossRef]
- Gentleman, M.M.; Ruud, J.A. Role of hydroxyls in oxide wettability. Langmuir 2010, 26, 1408–1411. [Google Scholar] [CrossRef]
- Kujawski, W.; Kujawa, J.; Wierzbowska, E.; Cerneaux, S.; Bryjak, M.; Kujawski, J. Influence of hydrophobization conditions and ceramic membranes pore size on their properties in vacuum membrane distillation of water-organic solvent mixtures. J. Membr. Sci. 2016, 499, 442–451. [Google Scholar] [CrossRef]
- Kujawa, J.; Cerneaux, S.; Kujawski, W. Investigation of the stability of metal oxide powders and ceramic membranes grafted by perfluoroalkylsilanes. Colloids Surf. A 2014, 443, 109–117. [Google Scholar] [CrossRef]
- Kujawa, J.; Cerneaux, S.; Kujawski, W. Characterization of the surface modification process of Al2O3, TiO2 and ZrO2 powders by PFAS molecules. Colloids Surf. A 2014, 447, 14–22. [Google Scholar] [CrossRef]
- Kujawa, J.; Kujawski, W.; Koter, S.; Rozicka, A.; Cerneaux, S.; Persin, M.; Larbot, A. Efficiency of grafting of Al2O3, TiO2 and ZrO2 powders by perfluoroalkylsilanes. Colloids Surf. A 2013, 420, 64–73. [Google Scholar] [CrossRef]
- Pazokifard, S.; Mirabedini, S.M.; Esfandeh, M.; Farrokhpay, S. Fluoroalkylsilane treatment of TiO2 nanoparticles in difference pH values: Characterization and mechanism. Adv. Powder Technol. 2012, 23, 428–436. [Google Scholar] [CrossRef]
- Nataraj, S.K.; Roy, S.; Patil, M.B.; Nadagouda, M.N.; Rudzinski, W.E.; Aminabhavi, T.M. Cellulose acetate-coated α-alumina ceramic composite tubular membranes for wastewater treatment. Desalination 2011, 281, 348–353. [Google Scholar] [CrossRef]
- Liu, G.; Wei, W.; Jin, W.; Xu, N. Polymer/ceramic composite membranes and their application in pervaporation process. Chin. J. Chem. Eng. 2012, 20, 62–70. [Google Scholar] [CrossRef]
- Wei, W.; Xia, S.; Liu, G.; Dong, X.; Jin, W.; Xu, N. Effects of polydimethylsiloxane (PDMS) molecular weight on performance of PDMS/ceramic composite membranes. J. Membr. Sci. 2011, 375, 334–344. [Google Scholar] [CrossRef]
- Wu, T.; Wang, N.; Li, J.; Wang, L.; Zhang, W.; Zhang, G.; Ji, S. Tubular thermal crosslinked-PEBA/ceramic membrane for aromatic/aliphatic pervaporation. J. Membr. Sci. 2015, 486, 1–9. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, G.; Ji, S. Covalent crosslinked assembly of tubular ceramic-based multilayer nanofiltration membranes for dye desalination. AIChE J. 2013, 59, 3834–3842. [Google Scholar] [CrossRef]
- Castro, R.P.; Monbouquette, H.G.; Cohen, Y. Polyvinylpyrrolidone-silica membranes for the treatment of oil-in-water emulsions. J. Membr. Sci. 1996, 115, 179–190. [Google Scholar] [CrossRef]
- Wang, Z.; Ge, O.; Shao, J.; Yan, Y. High performance zeolite LTA pervaporation membranes on ceramic hollow fibers by dipcoating-wiping seed deposition. J. Am. Chem. Soc. 2009, 131, 6910–6911. [Google Scholar] [CrossRef]
- Carreon, M.A.; Li, S.; Falconer, J.L.; Noble, R.D. Alumina-supported SAPO-34 membranes for CO2/CH4 separation. J. Am. Chem. Soc. 2008, 130, 5412–5413. [Google Scholar] [CrossRef]
- Ockwig, N.W.; Nenoff, T.M. Membranes for hydrogen separation. Chem. Rev. 2007, 107, 4078–4110. [Google Scholar] [CrossRef]
- Rana, D.; Matsuura, T. Surface modifications for antifouling membranes. Chem. Rev. 2010, 110, 2448–2471. [Google Scholar] [CrossRef]
- Samei, M.; Iravaninia, M.; Mohammadi, T.; Asadi, A.A. Solution diffusion modeling of a composite PVA/fumed silica ceramic supported membrane. Chem. Eng. Process. 2016, 109, 11–19. [Google Scholar] [CrossRef]
- Randon, J.; Blanc, P.; Paterson, R. Modification of ceramic membrane surfaces using phosphoric acid and alkyl phosphonic acids and its effects on ultrafiltration of BSA protein. J. Membr. Sci. 1995, 98, 119–129. [Google Scholar] [CrossRef]
- Nakajima, A.; Fujishima, A.; Hashimoto, K.; Watanabe, T. Preparation of transparent superhydrophobic boehmite and silica films by sublimation of aluminum acetylacetonate. Adv. Mater. 1999, 11, 1365–1368. [Google Scholar] [CrossRef]
- Yoshino, N.; Sasaki, A.; Seto, T. Synthesis of a silane coupling agent containing a 4-(perfluoroalkyl)phenyl group and its application to the surface modification of glass. J. Fluor. Chem. 1995, 71, 21–29. [Google Scholar] [CrossRef]
- Pesek, J.J.; Leigh, I. (Eds.) Chemically Modified Surfaces; The Royal Society of Chemistry: London, UK, 1994. [Google Scholar]
- Strathmann, H.; Kock, K.; Amar, P.; Bake, R.W. The formation mechanism of asymmetric membranes. Desalination 1975, 16, 179–203. [Google Scholar] [CrossRef]
- Bottino, A.; Camera-Roda, G.; Capannelli, G.; Munari, S. The formation of microporous polyvinylidene difluoride membranes by phase separation. J. Membr. Sci. 1991, 57, 1–20. [Google Scholar] [CrossRef]
- Tsay, C.S.; McHugh, A.J. Mass transfer modeling of asymmetric membrane formation by phase inversion. J. Polym. Sci. Part B Polym. Phys. 1990, 28, 1327–1365. [Google Scholar] [CrossRef]
- Kang, Y.S.; Kim, H.J.; Kim, U.Y. Asymmetric membrane formation via immersion precipitation method. I. Kinetic effect. J. Membr. Sci. 1991, 60, 219–232. [Google Scholar]
- Young, T.H.; Chen, L.W. A diffusion-controlled model for wet-casting membrane formation. J. Membr. Sci. 1991, 59, 169–181. [Google Scholar] [CrossRef]
- Rezac, M.E.; Roux, J.D.L.; Chen, H.; Paul, D.R.; Koros, W.J. Effect of mild solvent posttreatments on the gas transport properties of glassy polymer membranes. J. Membr. Sci. 1994, 90, 213–229. [Google Scholar] [CrossRef]
- Vasarhelyi, K.; Ronner, J.A.; Mulder, M.H.V.; Smolders, C.A. Development of wet-dry reversible reverse osmosis membranes with high performance from cellulose acetate and cellulose triacetate blends. Desalination 1987, 61, 211–235. [Google Scholar] [CrossRef]
- Pinnau, I.; Hellums, M.W.; Koros, W.J. Gas transport through homogeneous and asymmetric polyestercarbonate membranes. Polymer 1991, 32, 2612–2617. [Google Scholar] [CrossRef]
- Wijmans, J.G.; Baaij, J.P.B.; Smolders, C.A. The mechanism of formation of microporous or skinned membranes produced by immersion precipitation. J. Membr. Sci. 1983, 14, 263–274. [Google Scholar] [CrossRef]
- Mulder, M.H.V.; Hendrikman, J.O.; Wijmans, J.G.; Smolders, C.A. A rationale for the preparation of asymmetric pervaporation membranes. J. Appl. Polym. Sci. 1985, 30, 2805–2820. [Google Scholar] [CrossRef]
- Venna, S.R.; Carreon, M.A. Highly Permeable Zeolite Imidazolate Framework-8 Membranes for CO2/CH4 Separation. J. Am. Chem. Soc. 2010, 132, 76–78. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, C.; Peng, L.; Wang, X.; Kong, Q.; Gu, X. Enhanced Stability of MFI Zeolite Membranes for Separation of Ethanol/Water by Eliminating Surface Si–OH Groups. ACS Appl. Mater. Interfaces 2018, 10, 3175–3180. [Google Scholar] [CrossRef]
- Garofalo, A.; Carnevale, M.C.; Donato, L.; Drioli, E.; Alharbi, O.; Aljlil, S.A.; Criscuoli, A.; Algieri, C. Scale-up of MFI zeolite membranes for desalination by vacuum membrane distillation. Desalination 2016, 397, 205–212. [Google Scholar] [CrossRef]
- Kazemimoghadam, M.; Mohammadi, T. Synthesis of MFI zeolite membrane for water desalination. Desalination 2007, 206, 547–553. [Google Scholar] [CrossRef]
- Li, L.X.; Dong, J.H.; Nenoff, T.M.; Lee, R. Desalination by reverse osmosis using MFI zeolite membranes. J. Membr. Sci. 2004, 243, 401–404. [Google Scholar] [CrossRef]
- Palinkas, G.; Radnai, T.; Szasz, G.I.; Heinzinger, K. The structure of an aqueous ammonium chloride solution. J. Chem. Phys. 1981, 84, 3522–3526. [Google Scholar] [CrossRef]
- Li, L.; Liu, N.; McPherson, B.; Lee, R. Enhanced water permeation of reverse osmosis through MFI-type zeolite membrane with high aluminum contents. Ind. Eng. Chem. Res. 2007, 46, 1584–1589. [Google Scholar] [CrossRef]
- Xia, S.; Peng, Y.; Lu, H.; Wang, Z. The influence of nanoseeds on the pervaporation performance of MFI-type zeolite membranes on hollow fibers. Microporous Mesoporous Mater. 2016, 222, 128–137. [Google Scholar] [CrossRef]
- Zhu, B.; Hong, Z.; Milne, N.; Doherty, C.M.; Zou, L.; Lin, Y.S.; Hill, A.J.; Gu, X.; Duke, M. Desalination of sea water ion complexes by MFI-type zeolite membranes: Temperature and long term stability. J. Membr. Sci. 2014, 453, 126–135. [Google Scholar] [CrossRef]
- Duke, M.C.; O’brien-Abraham, J.; Milne, N.; Zhu, B.; Lin, J.Y.S.; da Costa, J.C.D. Seawater desalination performance of MFI type membranes made by secondary growth. Sep. Purif. Technol. 2009, 68, 343–350. [Google Scholar] [CrossRef]
- Zhu, B.; Myat, D.T.; Shin, J.W.; Na, Y.H.; Moon, I.S.; Connor, G.; Maeda, S.; Morris, G.; Gray, S.; Duke, M. Application of robust MFI-type zeolite membrane for desalination of saline wastewater. J. Membr. Sci. 2015, 475, 167–174. [Google Scholar] [CrossRef]
- Li, L.; Dong, J.H.; Nenoff, T.M.; Lee, R. Reverse osmosis of ionic aqueous solutions on a MFI zeolite membrane. Desalination 2004, 170, 309–316. [Google Scholar] [CrossRef]
- Zhu, B.; Morris, G.; Moon, I.; Gray, S.; Duke, M. Diffusion behaviour of multivalent ions at low pH through a MFI-type zeolite membrane. Desalination 2018, 440, 88–98. [Google Scholar] [CrossRef]
- Shin, W.; Tye, L.; Thian, C.; Bhatia, S. Synthesis, characterization and pervaporation properties of microwave synthesized zeolite A membrane. Desalination 2011, 277, 383–389. [Google Scholar]
- McLeary, E.E.; Jansen, J.C.; Kaptijn, F. Zeolite based films, membranes and membrane reactors: Progress and prospects. Microporous Mesoporous Mater. 2006, 90, 198–220. [Google Scholar] [CrossRef]
- van Bekkum, H.; Cejka, J.; Corma, A.; Schuth, F. (Eds.) Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Bowen, T.C.; Noble, R.D.; Falconer, J.L. Fundamentals and applications of pervaporation through zeolite membranes. J. Membr. Sci. 2004, 245, 1–33. [Google Scholar] [CrossRef]
- Tuan, V.A.; Li, S.; Falconer, J.L.; Noble, R.D. Separating organics from water by pervaporation with isomorphously-substituted MFI zeolite membranes. J. Membr. Sci. 2002, 196, 111–123. [Google Scholar] [CrossRef]
- Noack, M.; Kӧlsch, P.; Dittmar, A.; Stӧhr, M.; Georgi, G.; Eckelt, R.; Caro, J. Effect of crystal intergrowth supporting substances (ISS) on the permeation properties of MFI membranes with enhanced Al-content. Microporous Mesoporous Mater. 2006, 97, 88–96. [Google Scholar] [CrossRef]
- Shah, D.; Kissick, K.; Ghorpade, A.; Hannah, R.; Bhattacharyya, D. Pervaporation of alcohol-water and dimethyl formamide-water mixtures using hydrophilic zeolite NaA membranes: Mechanisms and experimental results. J. Membr. Sci. 2000, 179, 185–205. [Google Scholar] [CrossRef]
- Lai, Z.; Bonilla, G.; Diaz, I.; Nery, J.G.; Sujaoti, K.; Amat, M.A.; Kokkoli, E.; Terasaki, O.; Thompson, R.W.; Tsapatsis, M.; et al. Microstructural optimization of a zeolite membrane for organic vapor separation. Science 2003, 300, 456–460. [Google Scholar]
- Xu, C.; Lu, X.; Wang, Z. Effects of sodium ions on the separation performance of pure-silica MFI zeolite membranes. J. Membr. Sci. 2017, 524, 124–131. [Google Scholar] [CrossRef]
- Humplik, T.; Raj, R.; Maroo, S.C.; Laoui, T.; Wang, E.N. Effect of hydrophilic defects on water transport in MFI zeolites. Langmuir 2014, 30, 6446–6453. [Google Scholar] [CrossRef]
- Zhu, B.; Kim, J.H.; Na, Y.H.; Moon, I.S.; Connor, G.; Maeda, S.; Morris, G.; Gray, S.; Duke, M. Temperature and pressure effects of desalination using a MFI-type zeolite membrane. Membranes 2013, 3, 155–168. [Google Scholar] [CrossRef]
- Zhou, C.; Zhou, J.; Huang, A. Seeding-free synthesis of zeolite FAU membrane for seawater desalination by pervaporation. Microporous Mesoporous Mater. 2016, 234, 377–383. [Google Scholar] [CrossRef]
- Kumakiri, I.; Yamaguchi, T.; Nakao, S. Preparation of zeolite A and faujasite membranes from a clear solution. Ind. Eng. Chem. Res. 1999, 38, 4682–4688. [Google Scholar] [CrossRef]
- Kumakiri, I.; Hashimoto, K.; Nakagawa, Y.; Inoue, Y.; Kanehiro, Y.; Tanaka, K.; Kita, H. Application of FAU zeolite membranes to alcohol/acrylate mixture systems. Catal. Today 2014, 236, 86–91. [Google Scholar] [CrossRef]
- Mastropietro, T.F.; Drioli, E.; Candamano, S.; Poerio, T. Crystallization and assembling of FAU nanozeolites on porous ceramic supports for zeolite membrane synthesis. Microporous Mesoporous Mater. 2016, 228, 141–146. [Google Scholar] [CrossRef]
- Naskar, M.K.; Das, A.; Kundu, D.; Chatterjee, M. Emulsion-based synthesis of NaA zeolite nanocrystals and its integration towards NaA membranes. Bull. Mater. Sci. 2011, 34, 651–659. [Google Scholar] [CrossRef]
- Tsapatsis, M.; Lovallo, M.C.; Okubo, T.; Davis, M.E.; Sadakata, M. Characterization of zeolite L nanoclusters. Chem. Mater. 1995, 7, 1734–1741. [Google Scholar] [CrossRef]
- Xu, X.C.; Yang, W.S.; Liu, J.; Lin, L.W. Synthesis and perfection evaluation of NaA zeolite membrane. Sep. Purif. Technol. 2001, 25, 475–485. [Google Scholar] [CrossRef]
- Warzywoda, J.; Edelman, R.D.; Thompson, R.W. Crystallization of high-silica ZSM-5 in the presence of seeds. Zeolites 1991, 11, 318–324. [Google Scholar] [CrossRef]
- Tsokanis, E.A.; Thompson, R.W. Further investigations of nucleation by initial breeding in the Al-free NH4-ZSM-5 system. Zeolites 1992, 12, 369–373. [Google Scholar] [CrossRef]
- Gora, L.; Thompson, R.W. Controlled addition of aged mother liquor to zeolite NaA synthesis solution. Zeolites 1997, 18, 132–141. [Google Scholar] [CrossRef]
- Lai, R.; Gavalas, G.R. Surface seeding in ZSM-5 membrane preparation. Ind. Eng. Chem. Res. 1998, 37, 4275–4283. [Google Scholar] [CrossRef]
- Boudreau, L.C.; Kuck, J.A.; Tsapatsis, M. Deposition of oriented zeolite A films: In situ and secondary growth. J. Membr. Sci. 1999, 152, 41–59. [Google Scholar] [CrossRef]
- Xu, X.C.; Yang, W.S.; Liu, J.; Lin, L.W. Synthesis of NaA zeolite membranes from clear solution. Microporous Mesoporous Mater. 2001, 43, 299–311. [Google Scholar] [CrossRef]
- Engstrom, V.; Mihailova, B.; Hedlund, J.; Holmgren, A.; Sterte, J. The effect of seed size on the growth of silicalite-1 films on gold surfaces. Microporous Mesoporous Mater. 2000, 38, 51–60. [Google Scholar] [CrossRef]
- Kusakabe, K.; Kuroda, T.; Murata, A.; Morooka, S. Formation of a Y-type zeolite membrane on a porous -alumina tube for gas separation. Ind. Eng. Chem. Res. 1997, 36, 649–655. [Google Scholar] [CrossRef]
- Zhang, X.F.; Wang, J.Q.; Yin, D.H.; Liu, C.H. Synthesis of ZSM-5 zeolite membrane and its influencing factors by a novel method. Chin. J. Catal. 2000, 21, 451. [Google Scholar]
- Mintova, S.; Bein, T. Microporous films prepared by spin-coating stable colloidal suspensions of zeolites. Adv. Mater. 2001, 13, 1880–1883. [Google Scholar] [CrossRef]
- Balkus, K.J., Jr.; Munoz, T.; Gimon-Kinsel, M.E. Preparation of zeolite UTD-1 films by pulsed laser ablation: Evidence for oriented crystal growth. Chem. Mater. 1998, 10, 464–466. [Google Scholar] [CrossRef]
- Balkus, K.J., Jr.; Scott, A.S.; Gimon-Kinsel, M.E.; Blanco, J.H. Oriented films of mesoporous MCM-41 macroporous tubules via pulsed laser deposition. Microporous Mesoporous Mater. 1999, 11, 97–105. [Google Scholar]
- Bernal, M.P.; Xomeritakis, G.; Tsapatsis, M. Tubular MFI zeolite membranes made by secondary (seeded) growth. Catal. Today 2001, 67, 101–107. [Google Scholar] [CrossRef]
- Li, Y.; Yang, W. Microwave synthesis of zeolite membranes: A review. J. Membr. Sci. 2008, 316, 3–17. [Google Scholar] [CrossRef]
- Garofalo, A.; Donato, L.; Drioli, E.; Criscuoli, A.; Carnevale, M.C.; Alharbi, O.; Aljlil, S.A.; Algieri, C. Supported MFI zeolite membranes by cross flow filtration for water treatment. Sep. Purif. Technol. 2014, 137, 28–35. [Google Scholar] [CrossRef]
- Li, L.; Liu, N.; McPherson, B.; Lee, R. Influence of counter ions on the reverse osmosis through MFI zeolite membranes: Implications for produced water desalination. Desalination 2008, 228, 217–225. [Google Scholar] [CrossRef]
- Skluzacek, J.M.; Tejedor, M.I.; Anderson, M.A. An iron-modified silica nanofiltration membrane: Effect of solution composition on salt rejection. Microporous Mesoporous Mater. 2006, 94, 288–294. [Google Scholar] [CrossRef]
- Alami-Younssi, S.; Larbot, A.; Persin, M.; Sarrazin, J.; Cot, L. Gamma alumina nanofiltration membrane applications to the rejection of metallic cations. J. Membr. Sci. 1994, 91, 87–95. [Google Scholar] [CrossRef]
- de Lint, W.B.S.; Zivkovic, T.; Benes, N.E.; Buwmeester, H.J.M.; Blank, D.H.A. Electrolyte retention of supported bi-layered nanofiltration membranes. J. Membr. Sci. 2006, 277, 18–27. [Google Scholar]
- Gazagnes, L.; Cerneaux, S.; Persin, M.; Prouzet, E.; Larbot, A. Desalination of sodium chloride solutions and seawater with hydrophobic ceramic membranes. Desalination 2007, 217, 260–266. [Google Scholar] [CrossRef]
- Khajavi, S.; Jansen, J.C.; Kapteijn, F. Performance of hydroxyl sodalite membranes as absolute water selective materials under acidic and basic conditions. J. Membr. Sci. 2010, 356, 1–6. [Google Scholar] [CrossRef]
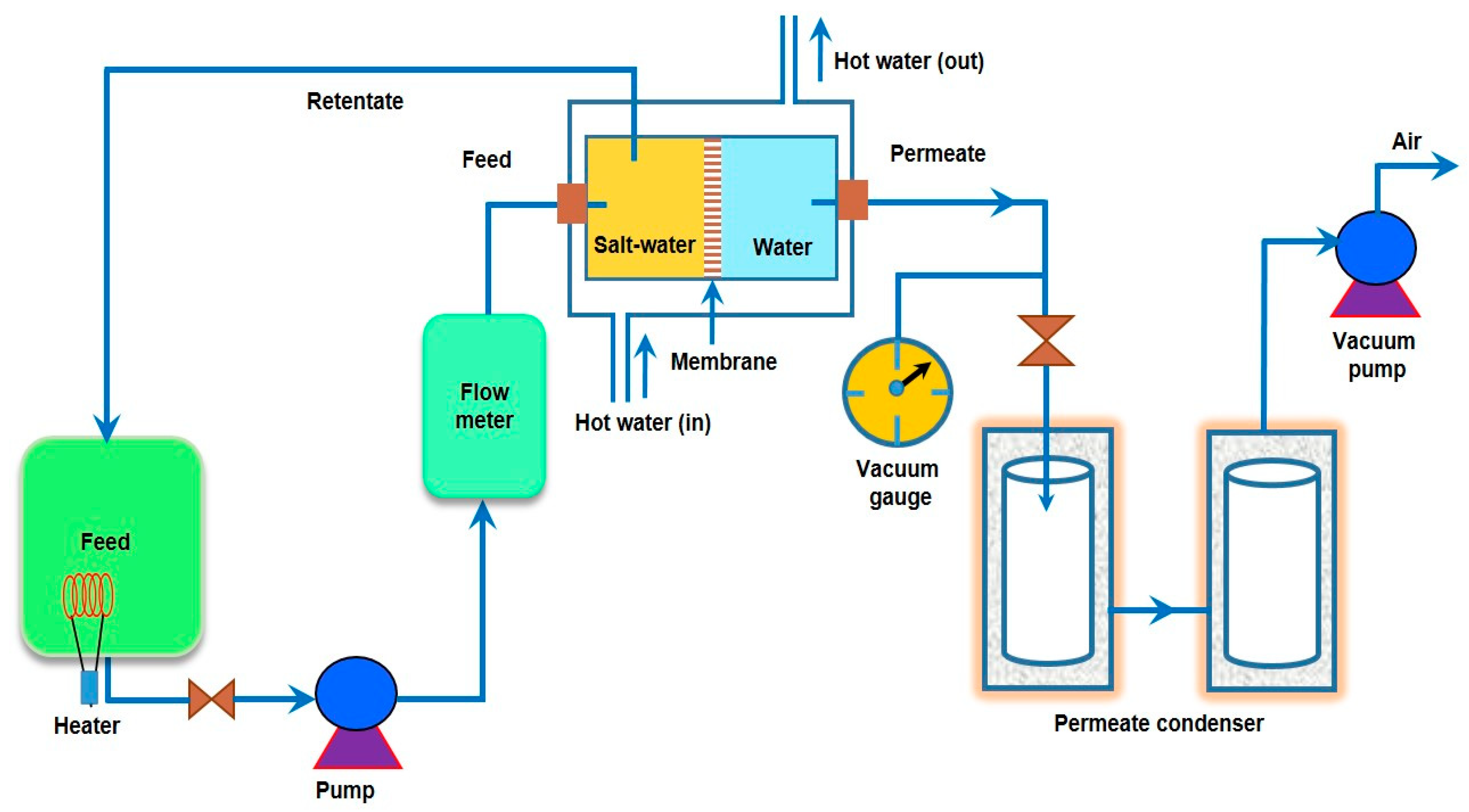

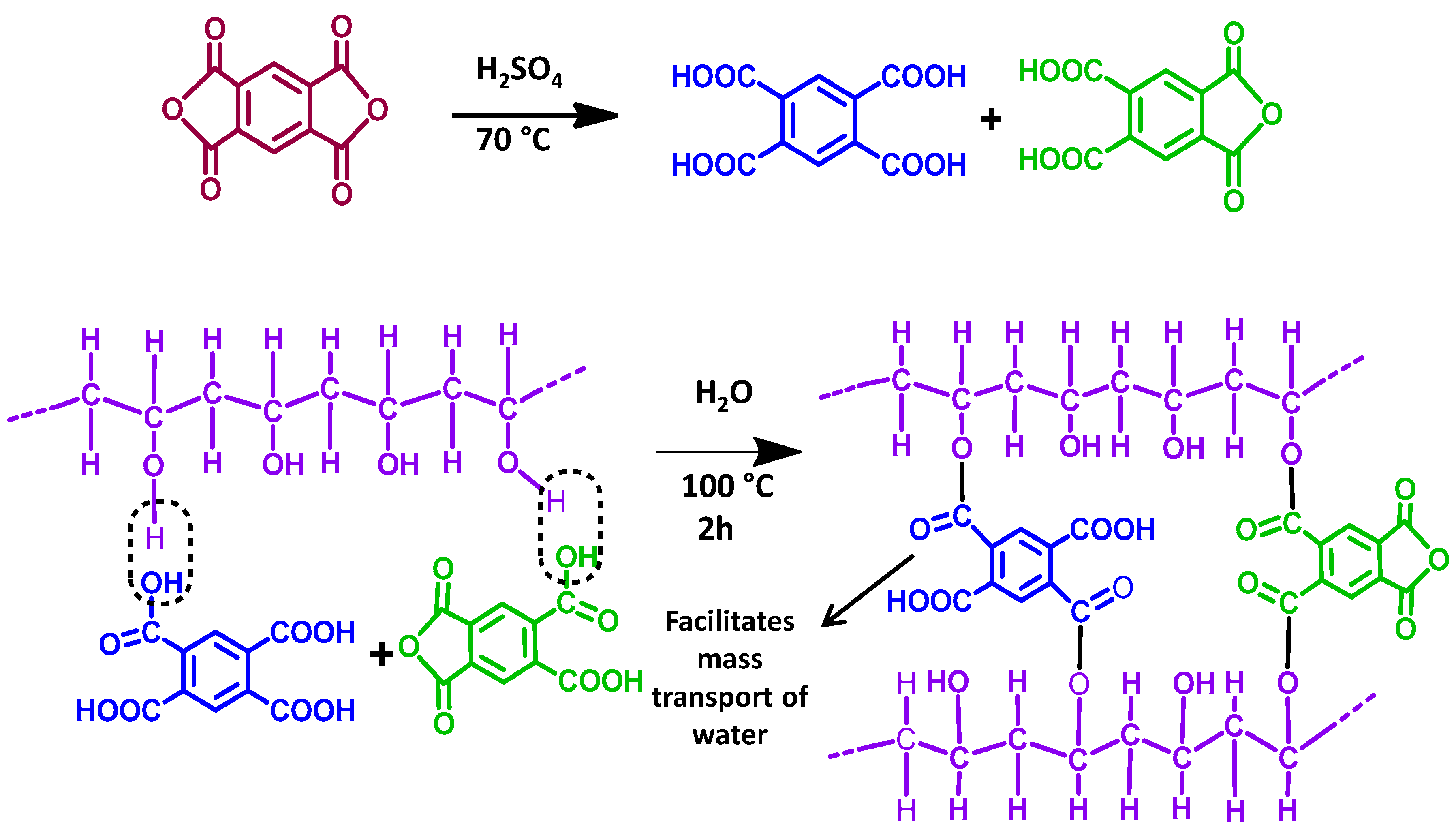
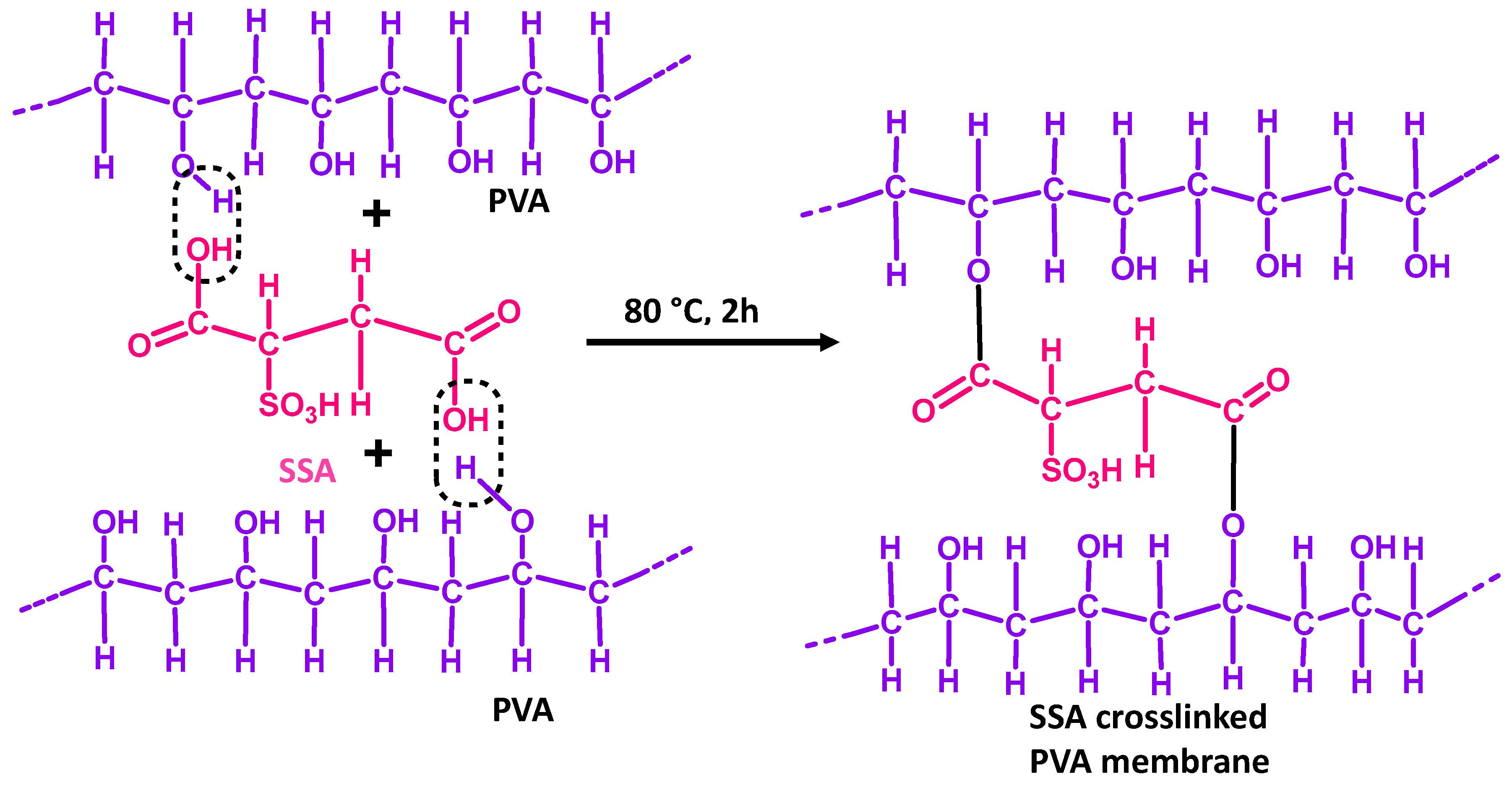
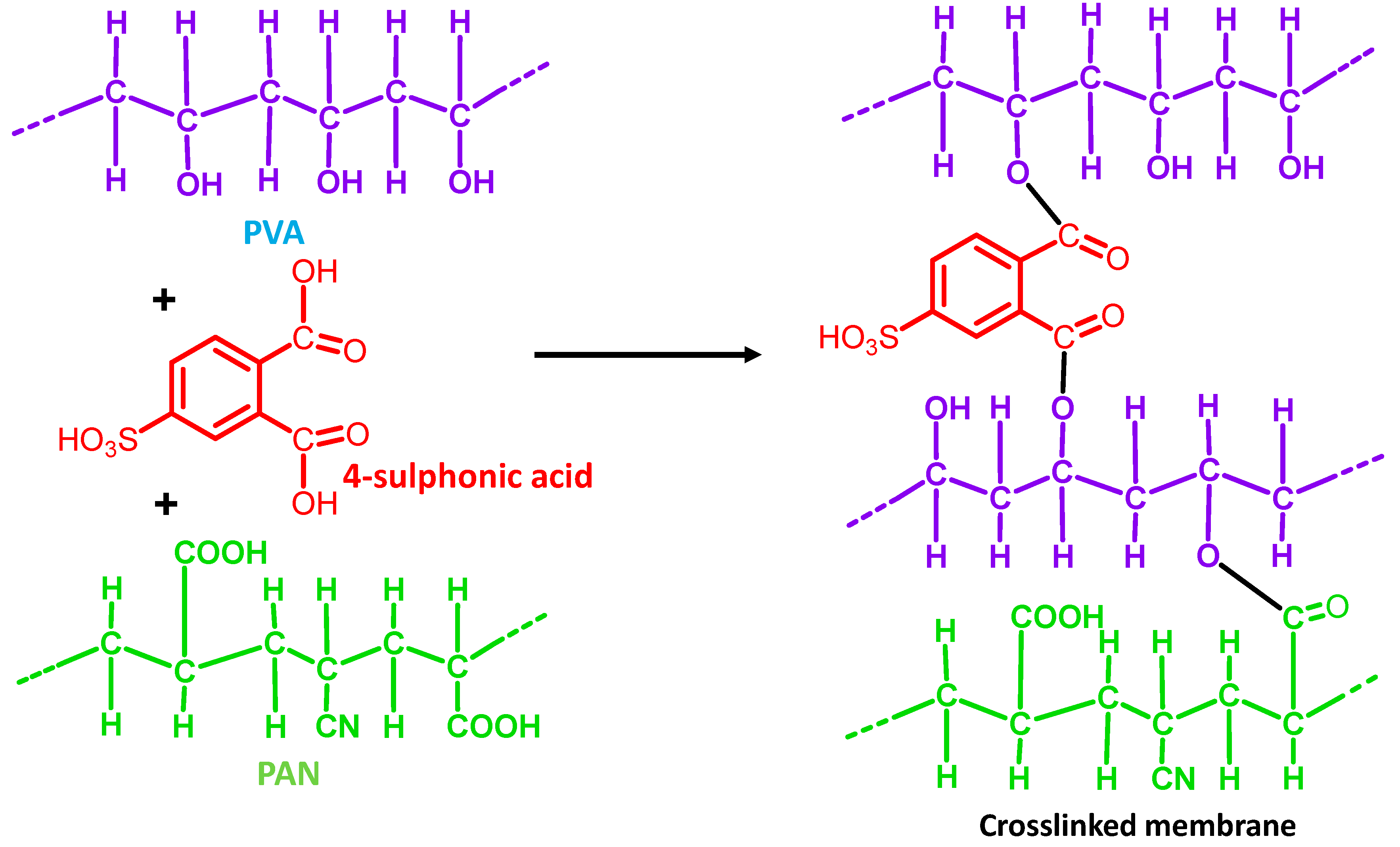
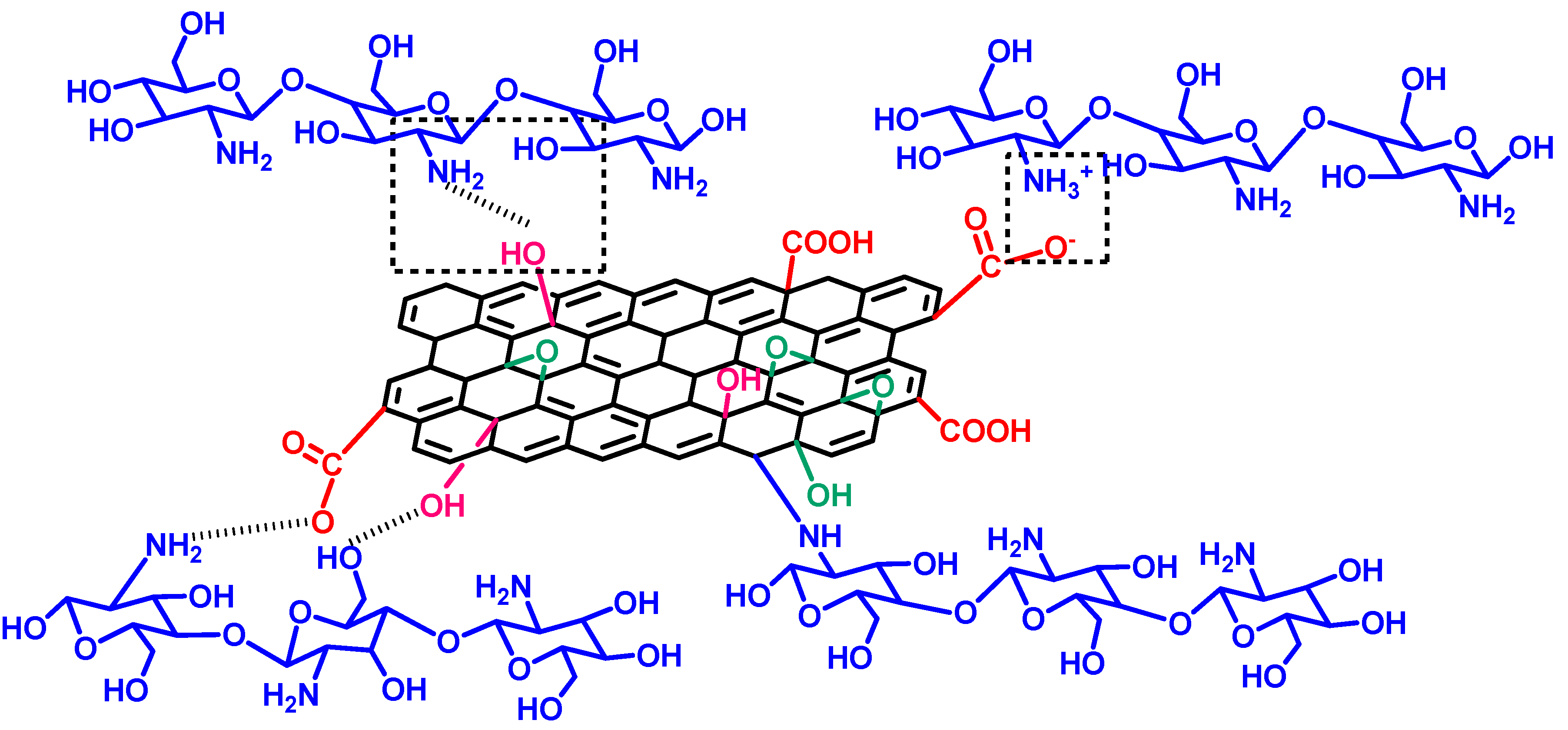
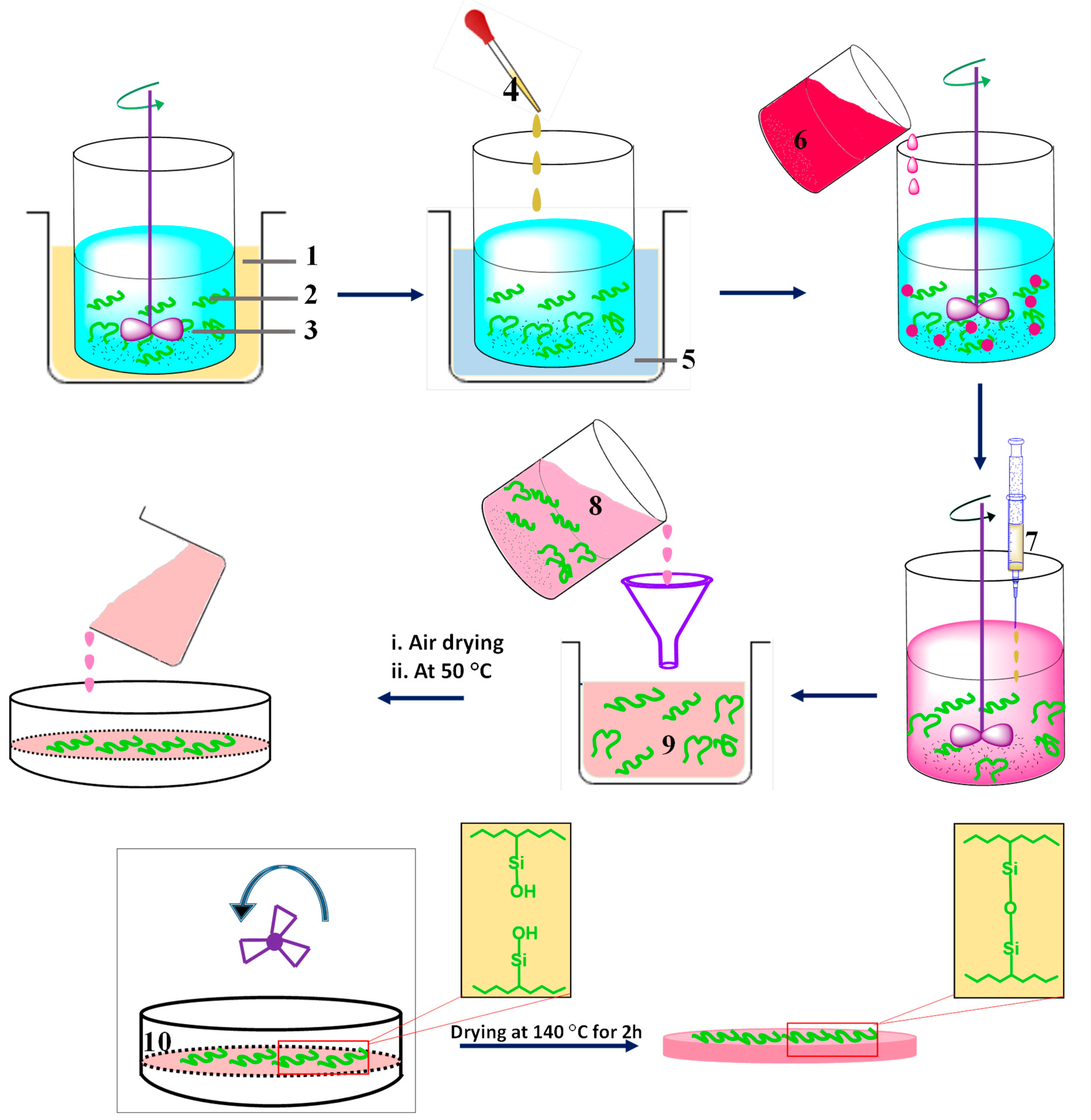
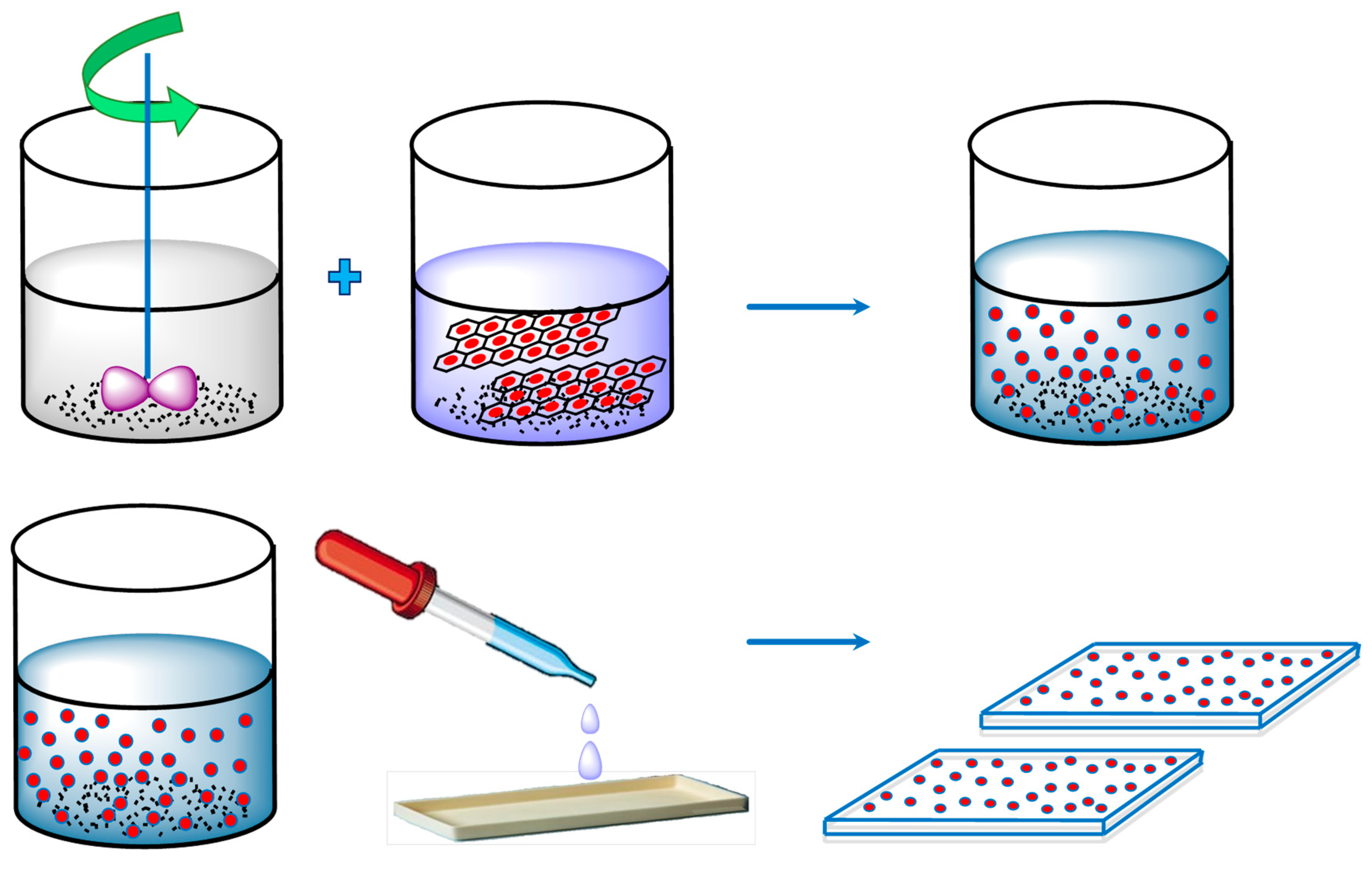
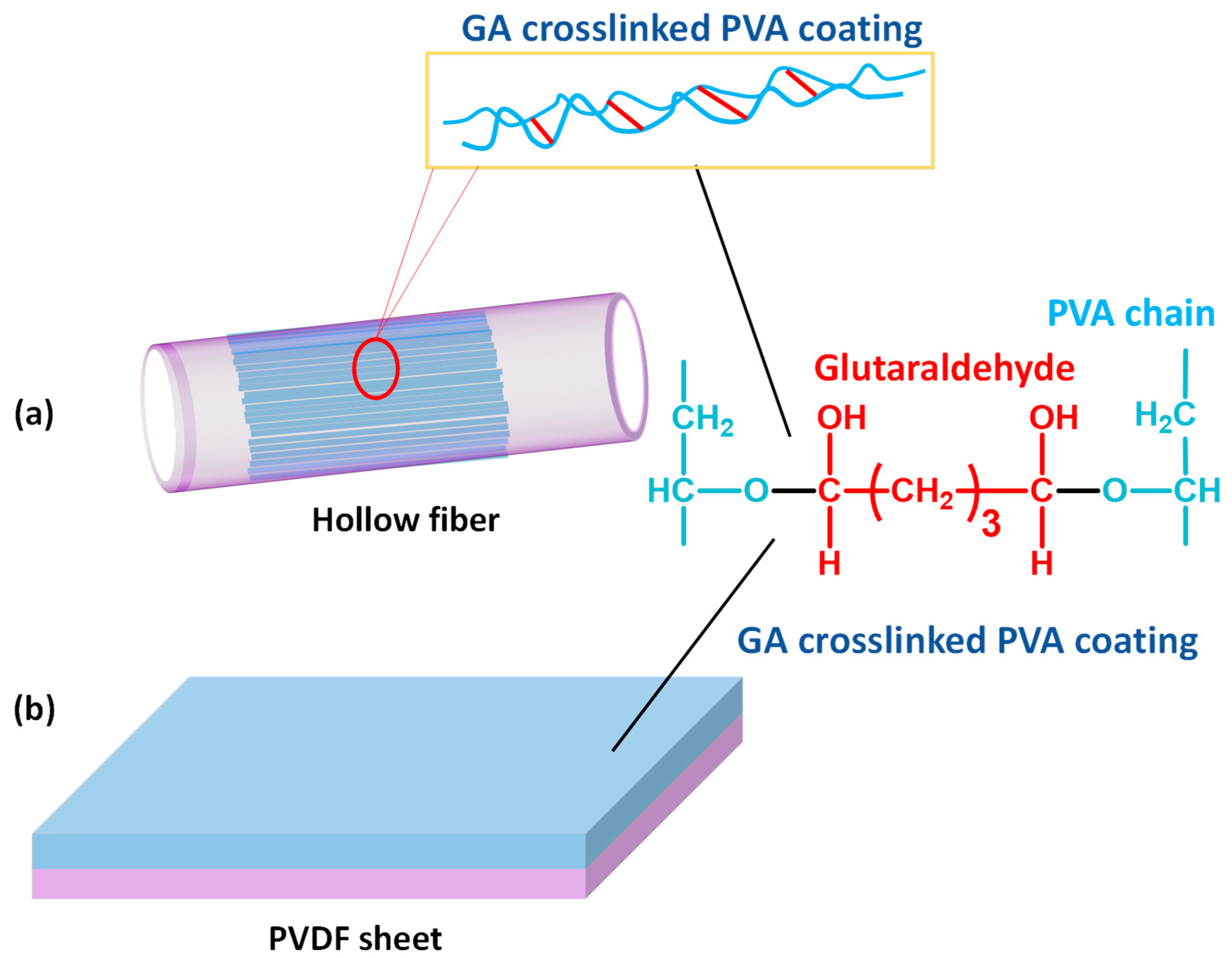
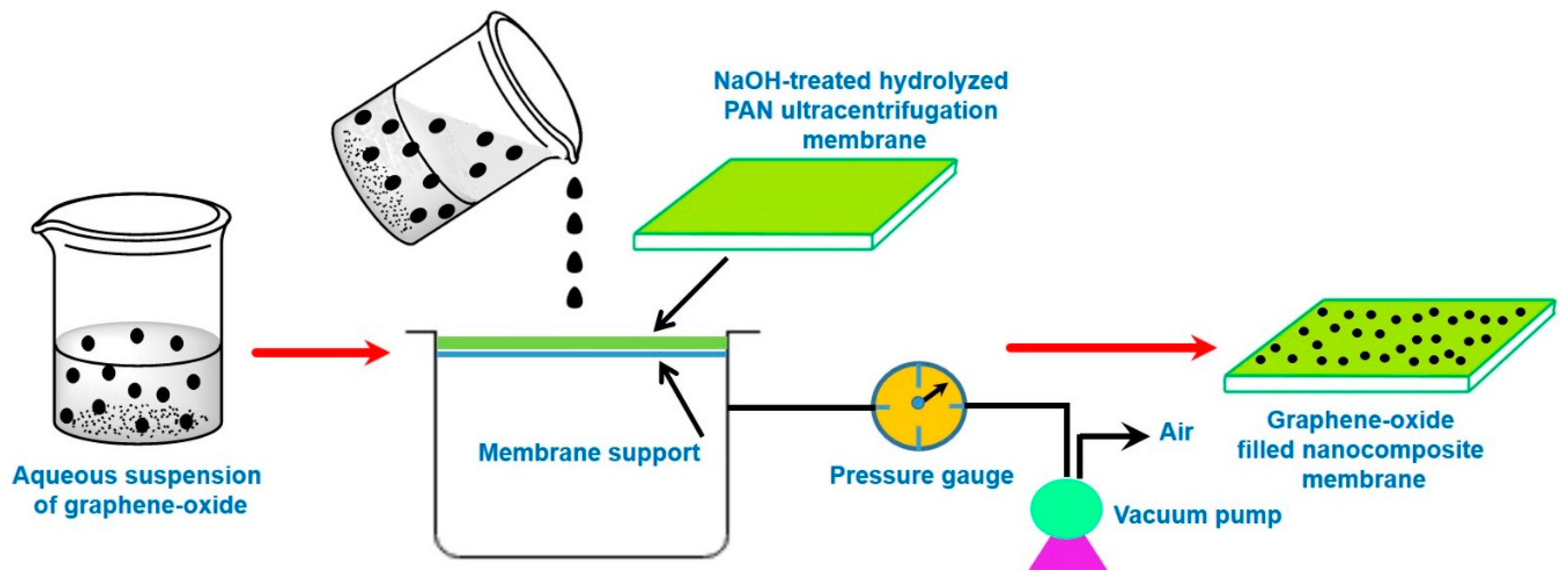
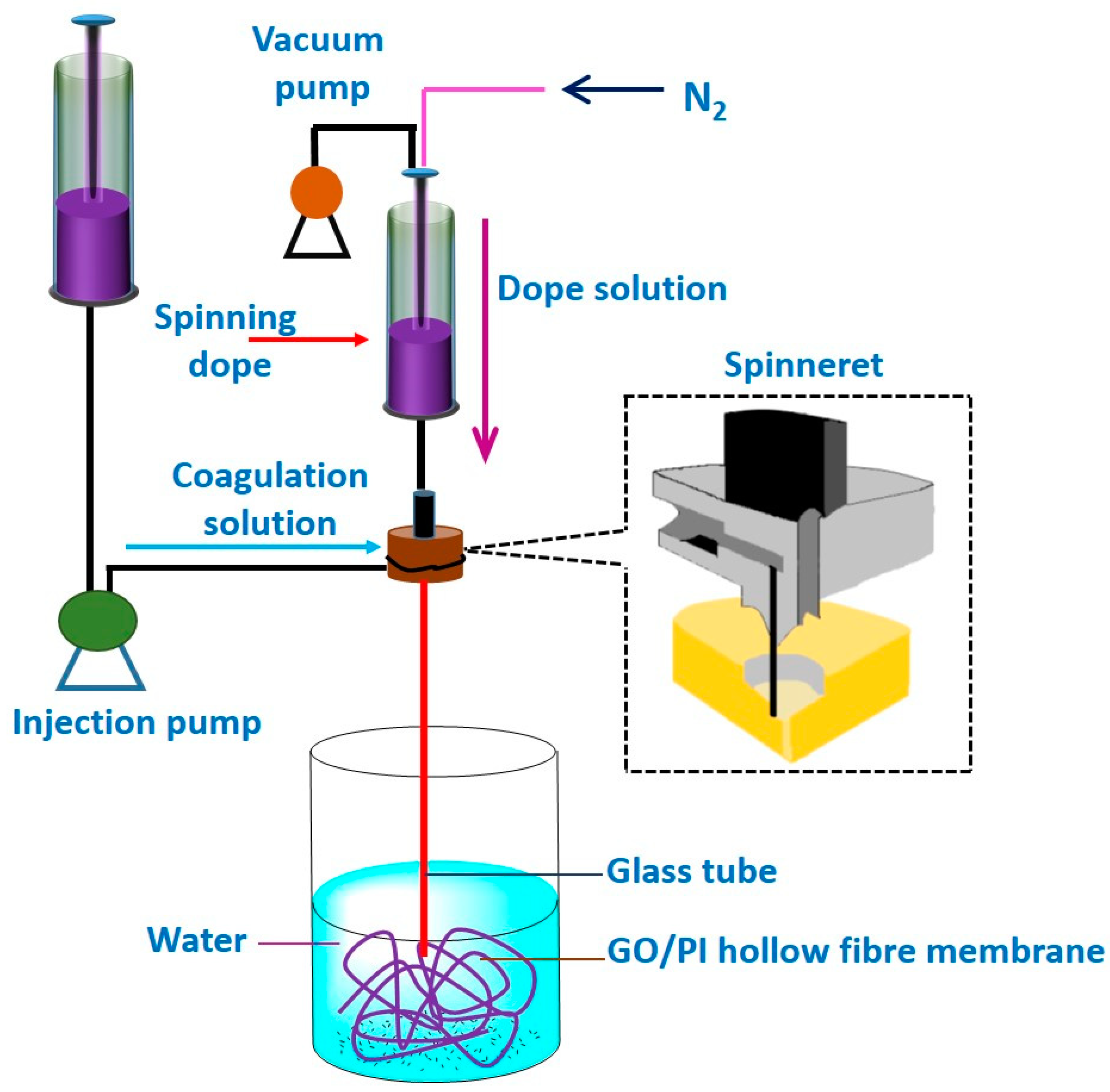


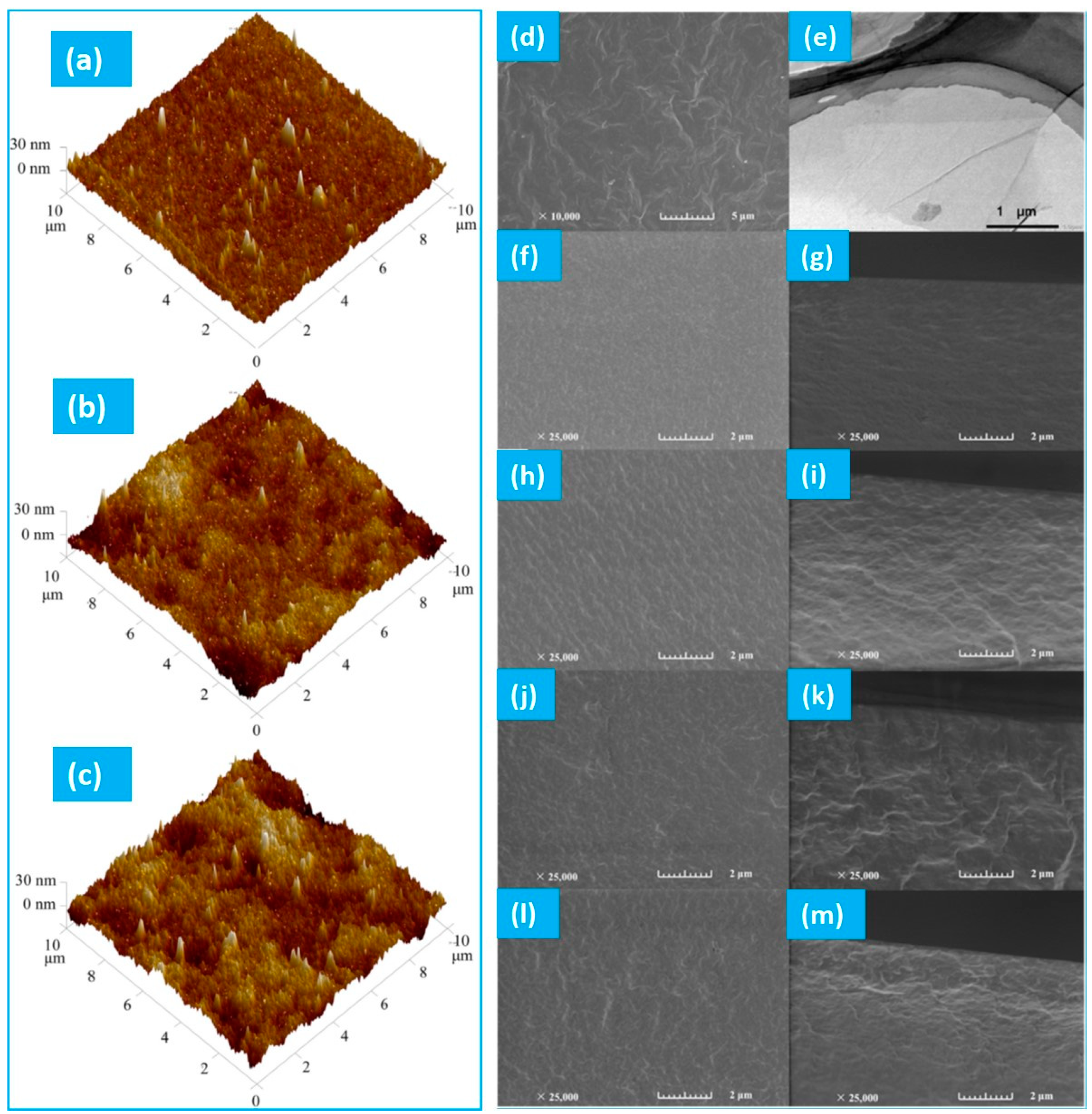
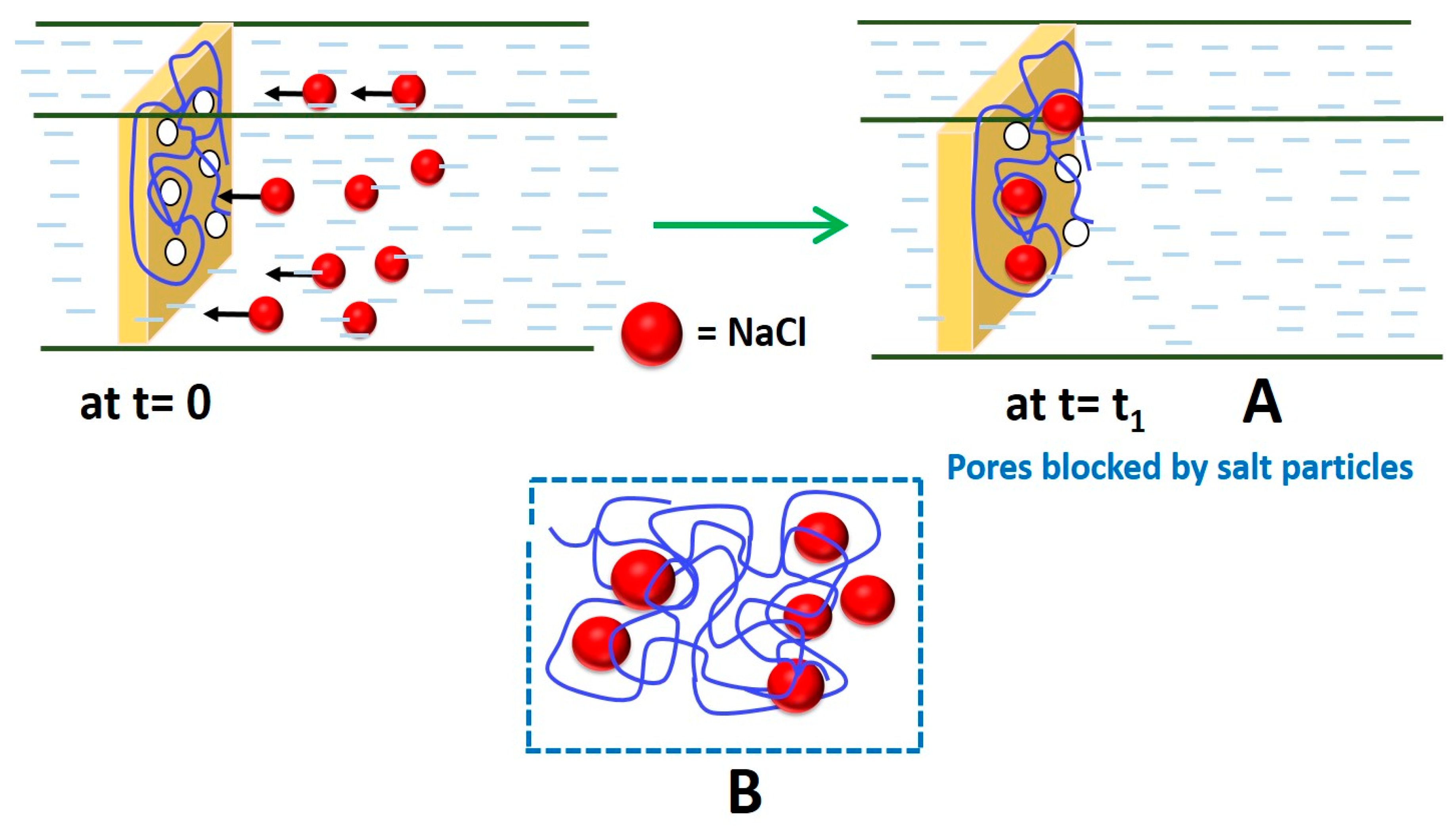
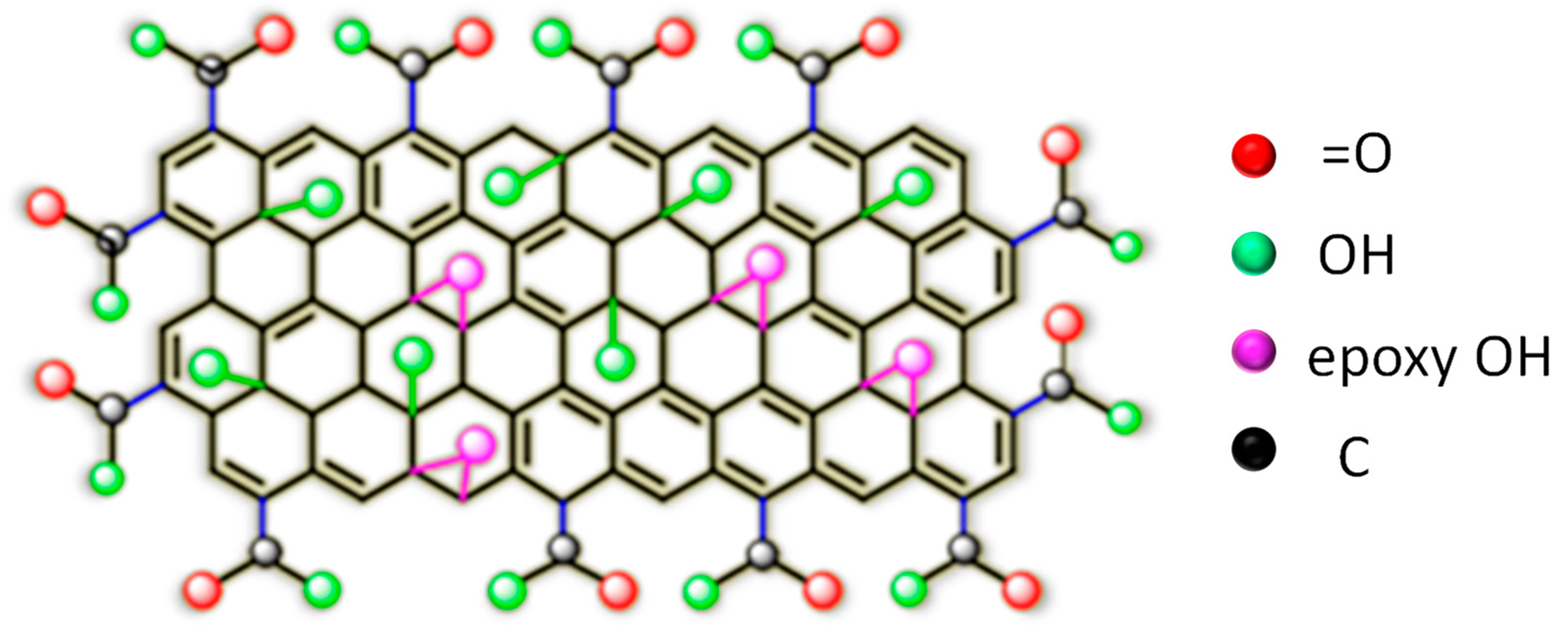
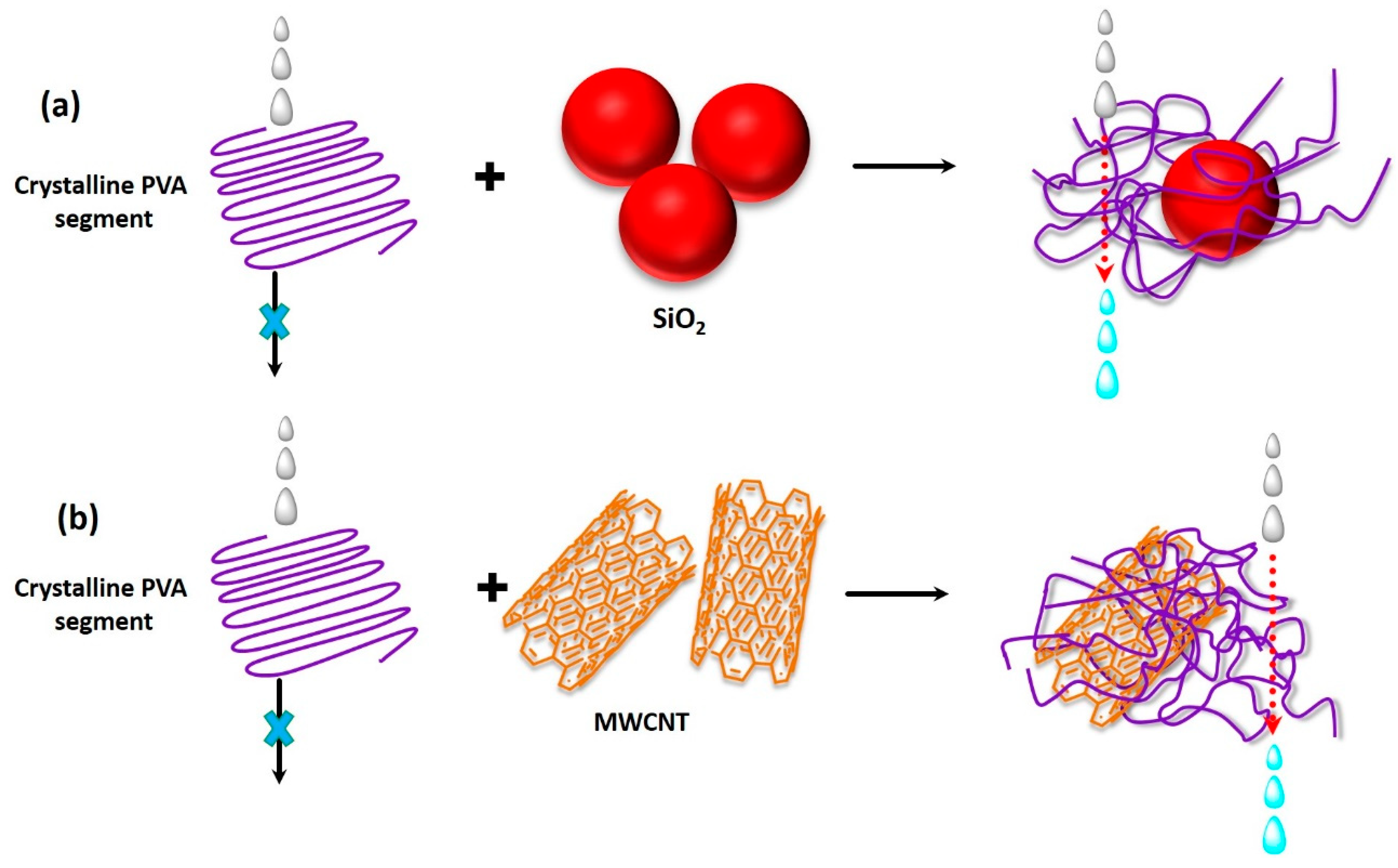
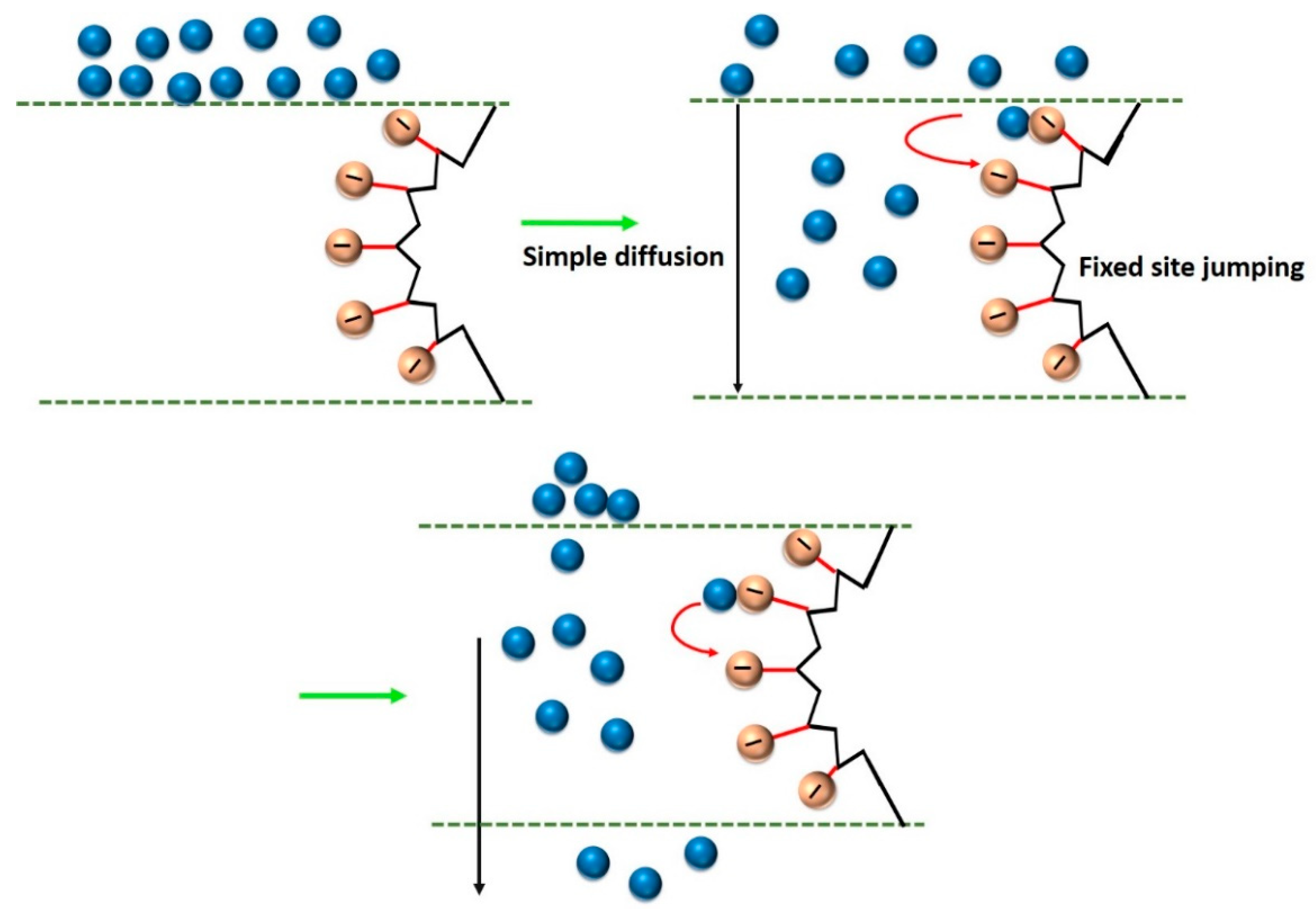
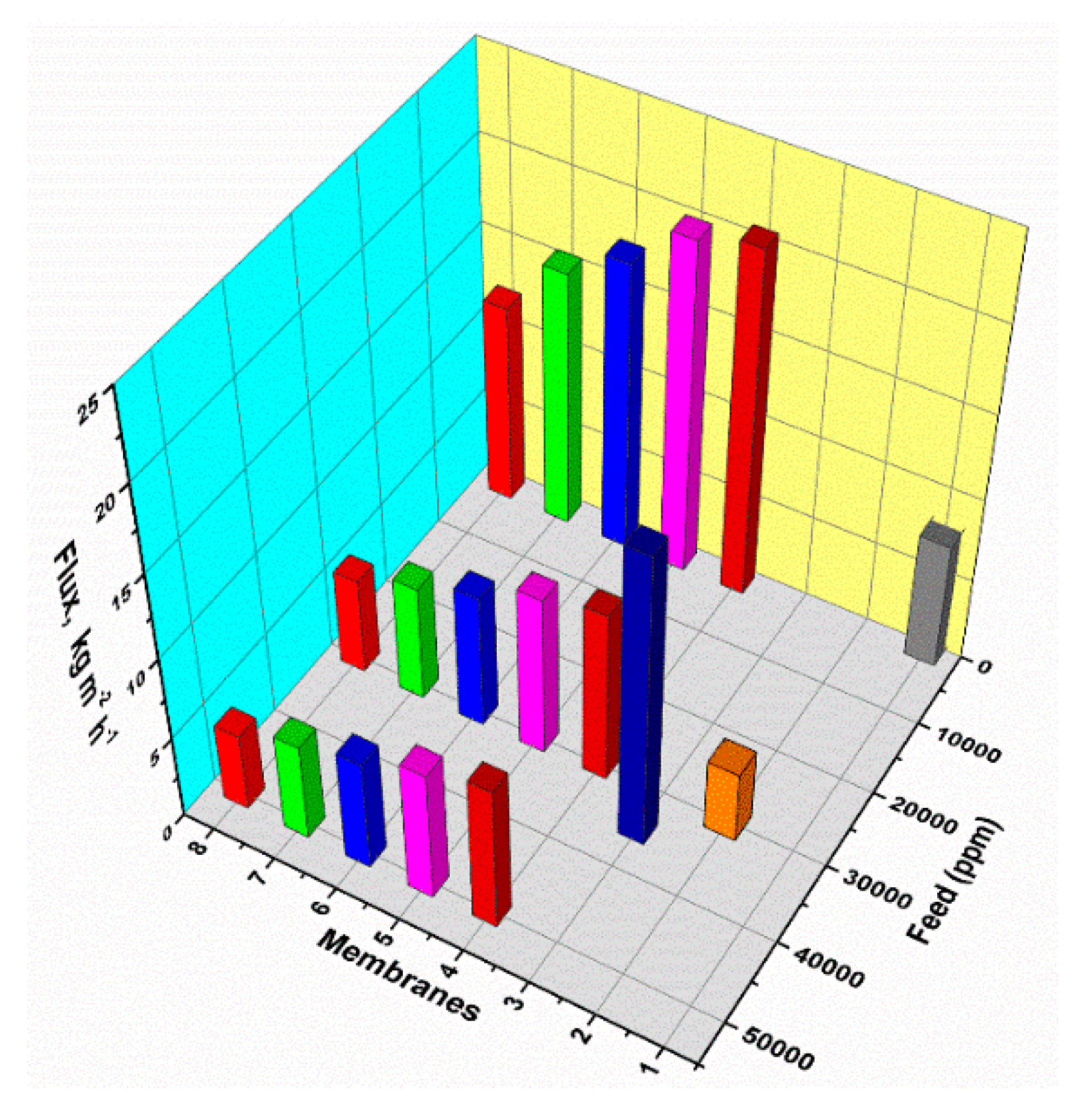
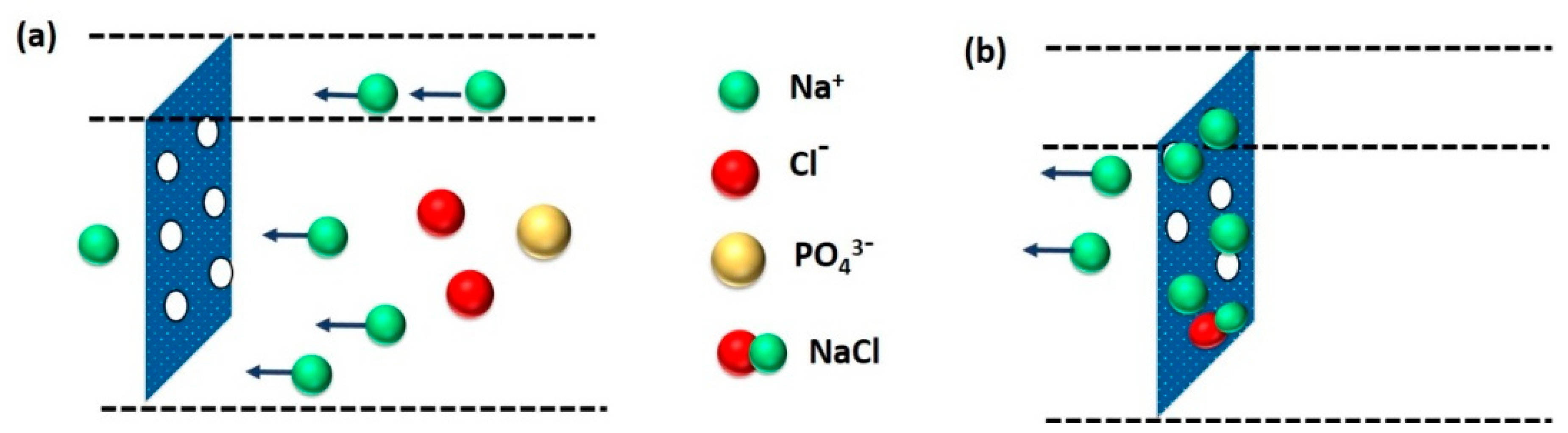
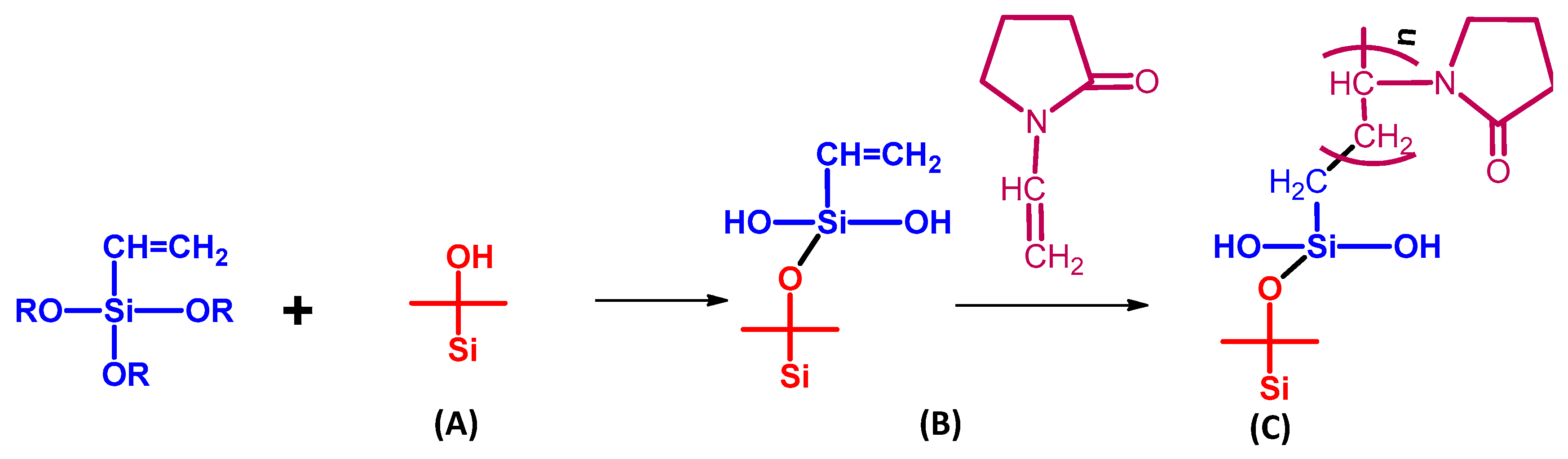
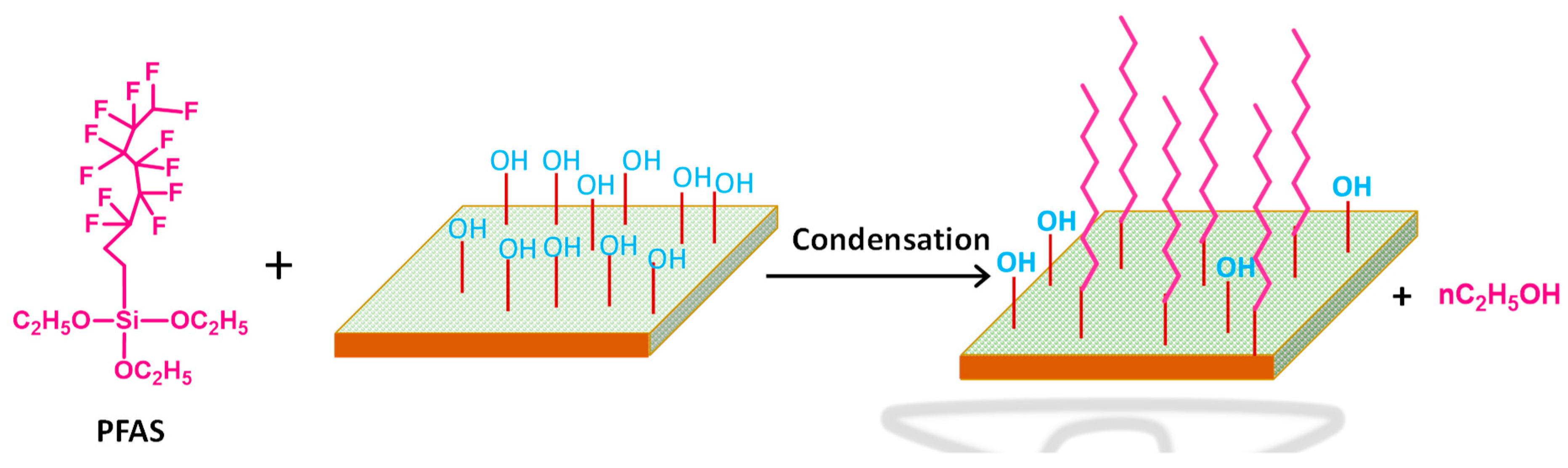
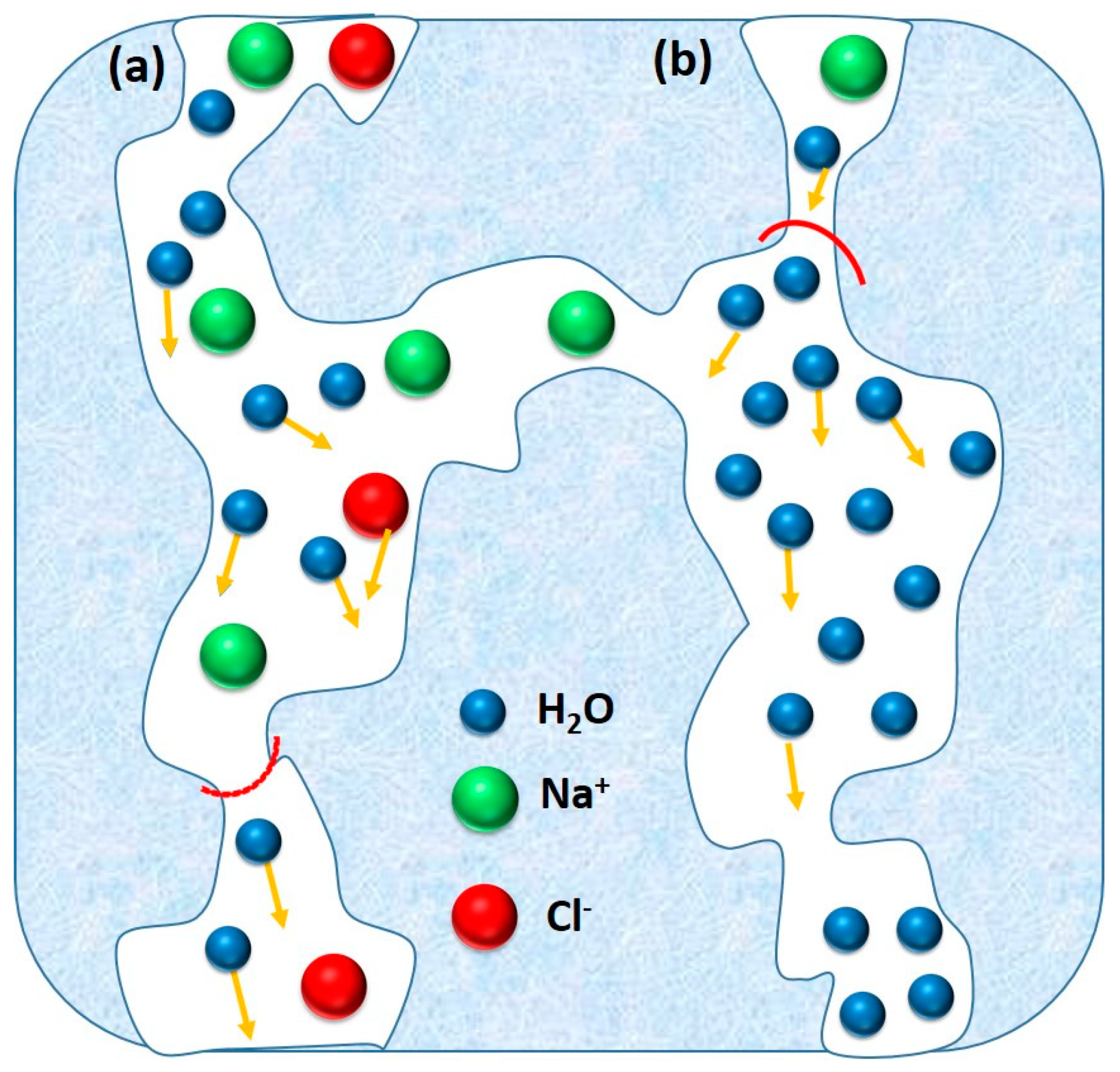
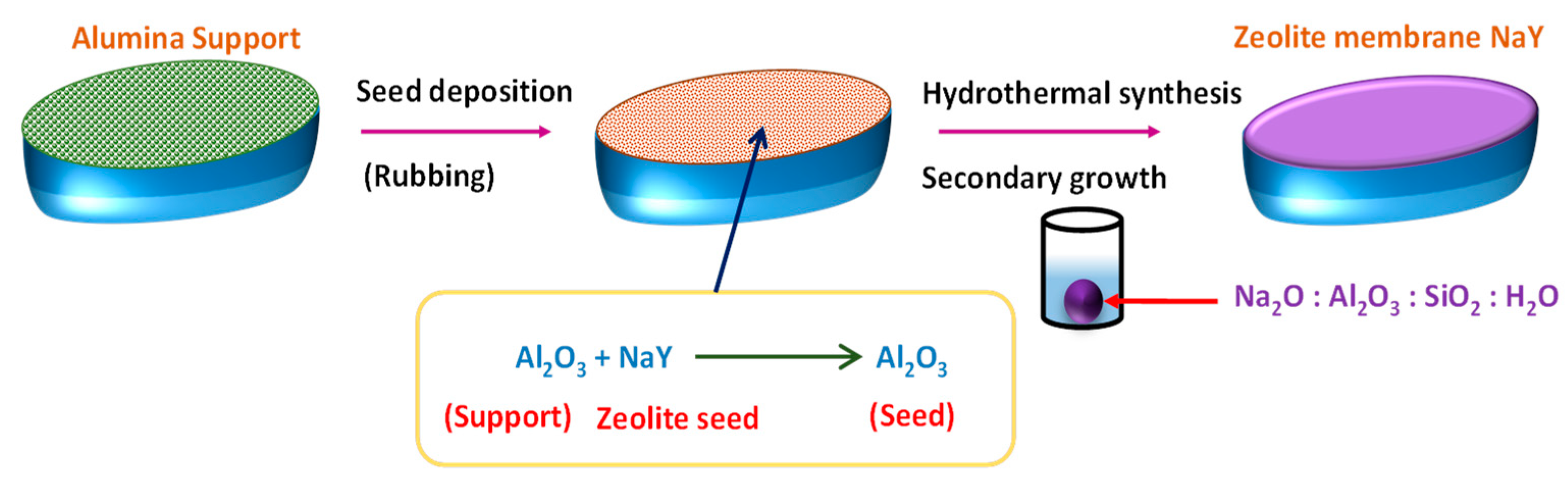
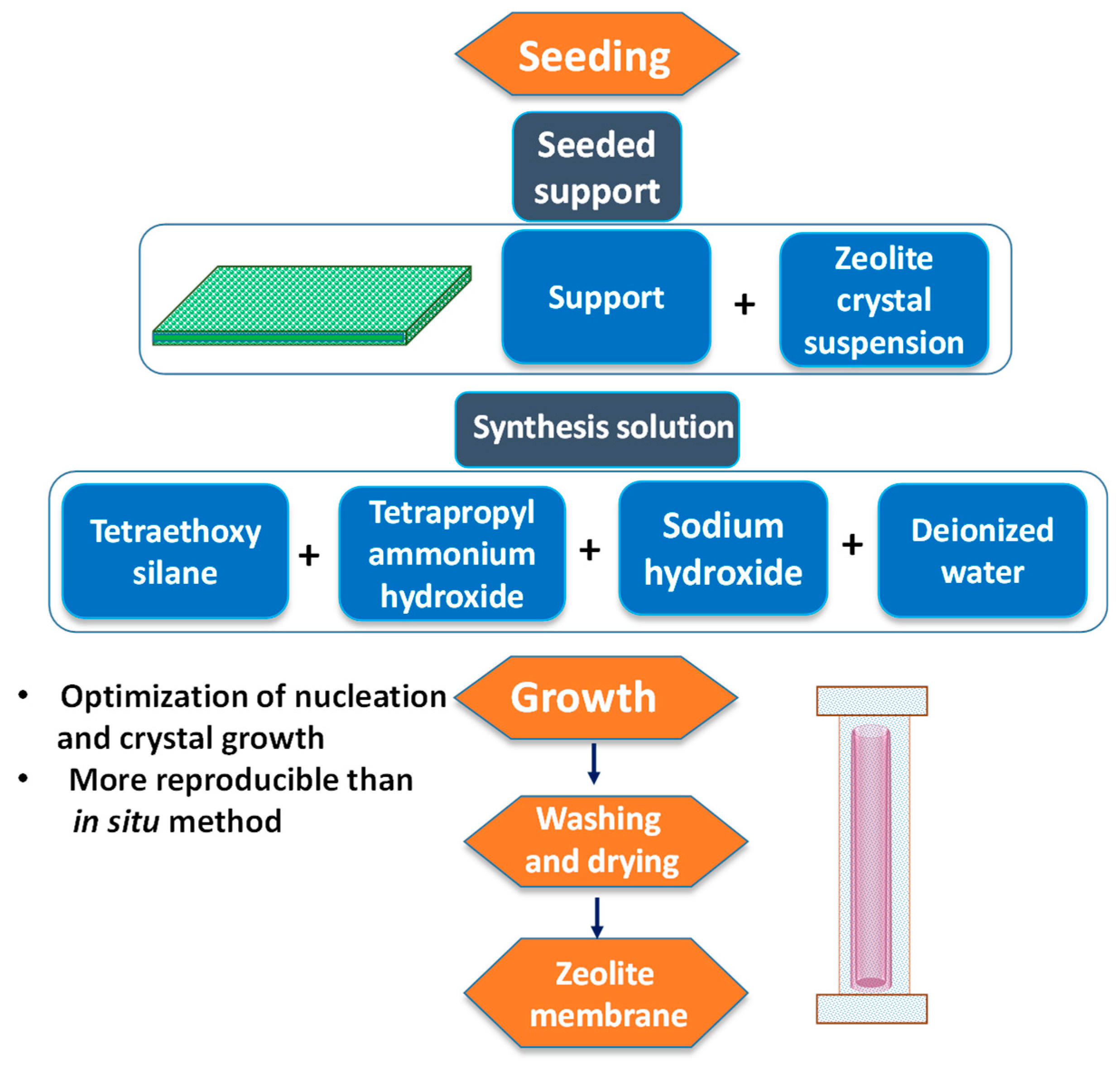
| Year | Polymer/Composite/ NCP Membranes | Fabrication Process | Advantages | Drawbacks | Ref |
|---|---|---|---|---|---|
| 1996 | Sulfonated polyethylene hollow fibers | Not reported | a. Superior control of operational parameters; b. Optimized pressure drop and reduced energy for water/ air circulation c. Desalinated water is free of organic substances | a. low production rates per unit area | [41] |
| 2005 | polyetheramide-based polymer film | Yet to be disclosed | a. Utilization of renewable, non-conventional energy resources b. Simple construction, operation, and maintenance c. Utilization of dense membranes prevent wetting induced salt leakage and pore-plugging problems are anticipated not to occur | a. yet to be commercialized b. low production rates per unit area | [43] |
| 2008 | NaA zeolite | Direct hydrothermal synthesis on porous α-alumina support | a. Higher thermal, chemical and mechanical resistance b. Improved SR ab efficiency | higher production cost | [108] |
| 2009 | CTS a membranes | Two-step sol-gel catalyzed process | Maximum NaCl rejection and flux are 97% and 3 kg m−2 h−1, respectively for membrane derived from the longest carbon chain (C16) surfactant | a. unstable during desalination, as water interacted with the silanol groups and enlarged pore sizes of microporous silica film. | [109] |
| 2010 | Hydroxy sodalite membrane | Surface of a polished-alumina disk (25 mm diameter, 2 mm thickness, 80 nm top layer pore size, and 150 nm bottom layer pore size) | – | a. poor particle dispersion driven inferior polymer-inorganic interactions and structural defects in the membrane | [79] |
| 2011 | NaA zeolite | Secondary growth process, with a single-channel porous α-alumina tube applied as a support. | a. Improved thermal, chemical, and mechanical resistance b. Appreciable SR efficiency | a. higher production cost b. poor particle dispersion driven inferior polymer-inorganic interactions and structural defects in the membrane | [81] |
| 2011 | Hybrid PVA b/MA c/silica membrane | Aqueous sol-gel route | a. Crosslinking among three components resulting higher crosslinking density and better SR | – | [46] |
| 2011 | Silicalite-polyamide composite membranes | Interfacial polymerization | a. Capable to desalinate saline water of exceptionally high initial concentration at a significantly higher flux and SR | – | [110] |
| 2011 | LTAd and MFI zeolite | Hydrothermal synthesis on the surface of an α-alumina porous support. | a. Better thermal, chemical, mechanical resistances b. Improved SR efficiency | – | [111] |
| 2011 | Templated silica | Interfacial polymerization on a commercial polysulfone substrate | – | – | [112] |
| 2012 | hydrophilic polyester tubular pervaporative membrane | grown hydrothermally on the surface of an α-alumina porous support | – | – | [102] |
| 2012 | S-1 e and ZSM-5 f membranes | Secondary growth on tubular ceramic supports | a. Mechanically stronger and durable | a. higher production cost | [55] |
| 2012 | Dense natural zeolite | Membranes were sliced as sheets from the as-mined material after a visual inspection | a. High temperature stability b. Higher thermal, chemical, mechanical resistances, along with significant SR efficiency | a. poor particle dispersion driven inferior polymer-inorganic interactions and structural defects in the membrane | [113] |
| 2014 | Cellulose triacetate membrane | Dip-coating membrane | – | – | [101] |
| 2014 | Natural zeolite clinoptilolite-phosphate composite | Dry powder pressing followed by high temperature steaming | a. Higher thermal, chemical, mechanical resistances, along with significant SR efficiency | – | [107] |
| 2014 | PVA b(uncrosslinked)/PAN g/PET h | Electrospraying and electrospinning | a. PAN nanofiber provided necessary mechanical strength | – | [80] |
| 2015 | MA c crosslinked PVA b/PVSF j hollow fiber | Direct spinning and phase inversion | a. PVSF hollow fiber provided mechanical strength, chemical resistance, and thermal stability | – | [114] |
| 2015 | Mesostructured CTAB k-silica membrane | – | – | – | [115] |
| 2015 | GO l/PAN g composite membrane | Vacuum filtration-assisted assembly method | a. Exfoliated distribution of GO particles | a. higher production cost b. poor particle dispersion driven inferior polymer-inorganic interactions and structural defects in the membrane | [50] |
| 2016 | GOF m membrane | Vacuum filtration of GOF suspension | a. Outstanding water permeability b. Preferential water adsorption ability and fast water diffusivity | a. higher production cost b. poor mechanical strength and susceptible to destruction during practical applications. | [116] |
| 2016 | GOF m membranes PDI n-modified α-Al2O3 | Vacuum filtration of GOF suspension | a. Thick GO membranes equipped with enhanced mechanical stability. | – | [116] |
| 2017 | nanohybrid GO l/PI o MMMs p | Phase inversion in a water coagulation bath | a. higher desalination performance b. stable under harsh conditions | a. low packing density. b. higher production cost | [117] |
| 2017 | PVA b-SiO2/PVSF j hollow fiber | Direct spinning and phase inversion | a. PVSF hollow fiber provided mechanical strength, chemical resistance, and thermal stability b. SiO2 filler mediated crosslinks | a. complicated and time-consuming to fabricate | [118] |
| 2017 | PEBA q/PAN g/PE r, PEBA q/PSF s/PE r, PEBA q + NaX/PSF s/PE r | Solution mixing and casting | a. Soft and flexible segments b. High sorption of water vapor | a. yet to be scaled up and commercialized | [119] |
| 2017 | zeolite 3A/PEBA q | Solution mixing and casting | a. Molecular sieving effect of the zeolite 3A cages improved SR b. Better chemical resistance and higher SR efficiency | a. higher production cost | [120] |
| 2017 | GNPs t/PEBA q | Solution mixing and casting | a. superior antifouling property | – | [121] |
| 2017 | SSA u crosslinked PVA b/PAN g | Solution mixing and casting | a. Improved flux owing to Sulfonic acid bearing crosslinks b. PAN provided mechanical strength and thermal stability | water-soluble PVA is to be crosslinked to increase the stability in water | [122] |
| 2017 | GA i crosslinked PVA b/PVDF v | Dip coating and cast-coating. | a. Excellent storage stability, anti-fouling properties, and cleaning efficiency | a. low desalination productivity and poor operational stability with brine feeds. | [123] |
| 2018 | thin PIM-1 w membrane | Dispersing GO into PI through wet phase inversion method | a. PI possesses good thermal and mechanical stability, easy processing and good solubility in various common solvents | a. PI membrane was affected by polymer concentration, evaporation time and post casting annealing, substantiated by the molecular weight cut-off curve | [124] |
| 2018 | MWCNT-PVA x | Solution mixing and casting | Good film formability; higher hydrophilicity introduced by OH and COOH; superior antifouling property; improved SR, durability, electrical conductivity; higher adsorption and water fluxes | costly | [125] |
| 2018 | GO l/PI o hollow fiber | Direct spinning and phase inversion | – | – | [126] |
| 2018 | GO l/CS y | Solution mixing and casting | a. mechanically stable via covalent crosslinking between epoxy of GO with amine of CS | – | [114] |
| 2018 | PMDA z crosslinked PVA b/PAN g | Solution mixing and casting | a. PAN provided mechanical strength | – | [127] |
| 2018 | SPTA aa crosslinked PVA b/PAN g | Solution mixing and casting | a. Sulfonic acid containing crosslinks improved flux b. PAN provided mechanical strength | – | [128] |
| Composite/NCP Membranes | Components | Tg | Thickness (μm) | Degree of Swelling (%) | Contact Angle | Ref |
|---|---|---|---|---|---|---|
| PEBA a/PAN b/PE c | PEBA a, PAN b, PE c | – | 11.0 | 21.8 | 53.0 | [119] |
| PEBA a/PSF d/PE c | PEBA a, PSF d, PE c | – | – | 21.8 | 53.0 | [119] |
| PEBA a + NaX e/PSF d/PE c | PEBA a + NaX e, PSF d, PE c | – | – | 21.2 | 53.0 | [119] |
| PVA noncrosslinked TFNPVC f | PVA g, PAN b, PET h | – | 0.6 | 180.5 | 48.2 | [80] |
| PVA crosslinked TFNPVC f | PVA g, PAN b, PET h | – | 0.7 | 14.5 | 63.5 | [80] |
| S-PVA i (1:10)/PAN films | PAN b, PVA g, SPTA j | – | 0.8 | 150.0 | 46.4 ± 3.4 | [128] |
| cellulose triacetate | cellulose triacetate | – | 10.0 | – | 46.1 ± 3.0 | [101] |
| cellulose acetate | cellulose acetate | – | 20.0–25.0 | 99.7 | – | [130] |
| PE c | PE c | – | 750.0 | 60.0 | – | [102] |
| PVA g/MA k (M-1) | PVA g, MA k | 366.0–401.0 | 0.1 | – | 71.5 | [114] |
| PVA g/MA k (M-2) | PVA g, MA k | 366.0–401.0 | 0.1 | – | 65.1 | [114] |
| PVA g/MA k (M-3) | PVA g, MA k | 366.0–401.0 | 0.3 | – | 63.2 | [114] |
| PVA g/MA k (M-4) | PVA g, MA k | 366.0–401.0 | 0.4 | – | 61.1 | [114] |
| PVA g/MA k (M-5) | PVA g, MA k | 366.0–401.0 | 0.9 | – | 56.5 | [114] |
| PVA g/MA k (M-6) | PVA g, MA k | 366.0–401.0 | 1.0 | – | 52.7 | [114] |
| clinoptilolite-phosphate | MKP l, MgO | – | 1300.0 | – | – | [107] |
| CTAB m-silica | CTAB m, SiO2 | – | 0.2 | – | – | [115] |
| polyether amide | polyether amide | – | 40.0 | – | – | [43] |
| NaA zeolite membrane | NaA zeolite membrane | – | – | – | – | [81] |
| GO n/PAN b | GO n, PAN b | – | 0.1–1.4 | – | – | [50] |
| 2D MXene | Mn+1XnTx, where n=1, 2, or 3, M=early transition metal, X=C/N, and T=surface group (OH, O, or F) | – | 0.1 | – | 45.9 | [133] |
| GO n/PVA b/PVDF | 0.2 wt% GO incorporated PVA/PVDM composite | 60.0 | 100.0 | 38.2 ± 2.1 | 37.1 ± 2.3 | [134] |
| LiCl-SPVA | LiCl, SPTA, PVA | – | 33.1 | – | – | [135] |
| PEBA o | PEBA o | – | 100.0 | – | 86.0 | [120] |
| Zeolite 3A incorporated PEBA composite membrane | Zeolite 3A, PEBA o | – | 100.0 | – | 63.0–71.0 | [120] |
| CS o membrane | CS | 140.6 | 10.0–13.0 | – | 85.0 | [136] |
| CS/GO MMM p | CS, GO | 143.5–145.3 | 10.0–13.0 | – | 77.5 | [136] |
| PVA g dense film | PMDA q, PVA g | – | 2.0 | – | 42.1 | [127] |
| PVA g/PAN b composite PV membrane (M-a) | 0% of the mole concentrations of –COOH group of the hydrolyzed PMDA q, PVA g, PAN b | 75.0 | 2.0 | – | – | [127] |
| PVA g/PAN b composite PV membrane (M-b) | 5% of the mole concentrations of –COOH group of the hydrolyzed PMDA q, PVA g, PAN b | 75.0 | 2.0 | 170.5 | 42.1 | [127] |
| PVA g/PAN b composite PV membrane (M-c) | 10% of the mole concentrations of –COOH group of the hydrolyzed PMDA q, PVA g, PAN b | 75.0 | 2.0 | 110.9 | 57.4 | [127] |
| PVA g/PAN b composite PV membrane (M-d) | 20% of the mole concentrations of –COOH group of the hydrolyzed PMDA q, PVA g, PAN b | – | 2.0 | 88.0 | 55.0 | [127] |
| PVA g/PAN b composite PV membrane (M-e) | 30% of the mole concentrations of –COOH group of the hydrolyzed PMDA q, PVA g, PAN b | – | 2.0 | 90.0 | 52.5 | [127] |
| PVA g/MA k/ Silica | PVA g, MA k, Silica | – | 10.0 | 36.0 ± 5.0 | 51.5 | [100] |
| Polyether ester | Polyether ester | – | 160.0 | – | – | [137] |
| FAS r-Al2O3 s | 1H,1H,2H,2H-perfluorodecyltriethoxysilane, alumina | – | 23.0 | – | – | [138] |
| FAS-TiO2 t | 1H,1H,2H,2H-perfluorodecyltriethoxysilane, titania | – | 23.0 | – | – | [138] |
| CC u membrane | Cotton | – | 30.0 | 44.0 | – | [131] |
| WC v membrane | Wood | – | 30.0 | 28.0 | – | [131] |
| CDA w membrane | Cellulose diacetate | – | 3.0–5.0 | 6.5 | – | [131] |
| GFBC-10x | Bacterial cellulose | – | 240.0 | – | – | [131] |
| BC-D y | Bacterial cellulose | – | 40.0 | – | – | [131] |
| Sulfonated PE z | Sulfonated polyethylene | – | 100.0 | 25.0–36.0 | – | [41] |
| Quaternized PE z | Quaternized polyethylene | – | 70.0 | 31.5 | – | [42] |
| Quaternized PE z | Quaternized polyethylene | – | 170.0 | 36.5 | – | [42] |
| S-PVA/PAN b | SSA aa, PVA a, PAN b | 113.0 | 4.9 | 83.5 ± 5.1 | 77.1 ± 3.0 | [122] |
| PVA g | PVA g | 84.0 | 4.9 | 194.3 ± 10.7 | 44.3 ± 2.7 | [122] |
| PVA g/MAk | PVA g, 20% MA k | 94.0 | 5.0 | 61.0 ± 5.0 | 59.4 ± 2 | [139] |
| PVA g/20MA k/ 10Silica | PVA g, 20% MA k, 10% Silica | 103.0 | 5.0 | 22.0 ± 2.0 | 63.5 ± 2 | [139] |
| PVA g/20MA k/ 25Silica | PVA g, 20% MA k, 25% Silica | 107.0 | 5.0 | 11.0 ± 1.0 | 79.4 ± 2 | [139] |
| PEBA a | – | 0.1 | – | 86.0 | [121] | |
| 2GNPs ab/PEBA a | 2.0 wt% GNP, PEBA a | – | 0.1/0.2 | – | 80.0 | [121] |
| 5GNPs ab/PEBA a | 5.0 wt% GNP, PEBA a | – | 0.2 | – | 75.0 | [121] |
| 0.3MWCNT/PVA ac | 0.3 wt% MWCNT ac, PVA g | – | 100.0 | 275.0–350.0 | [100] | |
| PI ad hollow fiber | 14.6 wt% PI ad, 2.0 wt% PVP ae, 9.1 wt% EtOH, 74.3 wt% NMP af | – | 1000.0 | – | 92.0 | [126] |
| GO n/PI ad hollow fiber | 1.0 wt% GO, 14.4 wt% PI ad, 2.0 wt% PVP af, 9.0 wt% EtOH, 73.6 wt% NMP af | – | 1000.0 | – | 59.0 | [126] |
| CS o | 0.1 wt% GO n, CS o | 140.6 | – | – | 85.0 | [136] |
| 1GO n/CS o | 1.0 wt% GO n, CS o | 143.5 | – | – | 77.5 | [136] |
| 2GO n/CS o | 2.0 wt% GO n, CS o | 145.3 | – | – | 77.0 | [136] |
| PVA g-SiO2/ PVSF ag hollow fiber | 52.4:1:1:(1000-67) = C2H5OH: TEOS: PVAg:H2O (w/w) | 396.0 | 0.2–1.1 | – | – | [118] |
| Composite/NCP Membranes | Thickness (μm) | Feed Temperature (°C) | Feed Flow Rate (mL min−1) | Feed Concentration (ppm) | Activation Energy (kJ mol−1) | Permeate Pressure | Flux (kg m−2h−1) | Salt Rejection (%) | Ref |
|---|---|---|---|---|---|---|---|---|---|
| PVA a/20MA b/10Silica | 5 | 22 | – | – | – | 0.80 | 5.51 | >95.50 | [139] |
| PVA a/20MA b/25Silica | 5 | 22 | – | – | – | 0.80 | >3.65 | >95.50 | [139] |
| PVA a/5MA b/10Silica | 20 ± 1 | 22–65 | 30 | 2000–50,000 NaCl | 23.80–20.10 | 0.80 | 2.50–11.70 | 99.90 (max) | [100] |
| GO c/PAN d | – | 30 | – | 2000/35,000/50,000/100,000 | 22.19 | – | 16.84/14.31/13.56/ 11.23 | >99.80 | [50] |
| GO c/PAN d | 0.003–1.4 | 90 | – | 35,000 | 22.19 | – | 65.10 | >99.80 | [50] |
| PEBA e | 120 ± 5 | 65 | – | – | – | 0.50 | – | 99.12 | [121] |
| 3GNPs f/PEBA e | 72/120 ± 5/181 | 65 | – | – | – | 0.50 | 5.12/3.61/2.73 | >99.89 | [121] |
| 3GNPs f/PEBA e | 120 ± 5 | 35 | – | – | – | 0.50 | 2.58 | 99.94 | [121] |
| MWCNT-PVA g | 100 | 40 | – | NaCl (1000/1500/2000) + MgCl2 (200) + KCl (200) | – | – | – | >92.00 | [125] |
| GO c/PI h hollow fiber | 1000 | 45 | – | 35,000 sea water [Na+ (2067) K+ (323) Mg2+(872) Ca2+(247), F− (188), Cl− (3132), PO3−(1025)] | 18.76 | – | 6.40 | >99.80 [Na+ (99.90) K+ (99.80) Mg2+(99.90) Ca2+(99.90), F− (99.80), Cl− (99.90), PO3− (99.80)] | [126] |
| GO c/PI h hollow fiber | 1000 | 60 | – | Do | Do | – | 8.10 | Do | [126] |
| GO c/PI h hollow fiber | 1000 | 75 | – | 20,000/35,000/100,000 | Do | – | 17.50/11.50/4.20 | Do | [126] |
| GO c/PI h hollow fiber | 1000 | 90 | – | 35,000 sea water [Na+ (2067) K+ (323) Mg2+(872) Ca2+(247), F− (188), Cl− (3132), PO3−(1025)] | Do | – | 15.60 | Do | [126] |
| PI h | 1000 | 75 | – | Do | – | – | 1.90 | – | [126] |
| PEBA e/PAN d/PE i | – | 40/50/60 | – | 760 NaCl | – | 4.00 | >3.00/4.93/7.63 | >93.00 | [119] |
| PEBA e/PSF j/PE i | – | 50 | – | Do | – | Do | 1.24 | >93.00 | [119] |
| PEBA e+NaX/PSF j/PE i | – | 50 | – | Do | – | Do | 1.30 | >93.00 | [119] |
| PVA a(GA k crosslinked)/PAN d/PET l | – | 25 | – | 5000/35,000/50,000 | – | 0.10 | 8.53/7.36/5.81 | 99.90/99.80/99.80 | [80] |
| SPTA m crosslinked PVA a/PAN d | 0.8 (thickness of SPTA m crosslinked PVA a) | 70 | – | 35,000 NaCl | – | 0.10 | 49.30 ± 1.10 | 99.80 | [128] |
| SPTA m crosslinked PVA a/PAN d | 0.8/1.06/1.84/2.56/4.48/4.86/17.2/33/46.3/76 (thickness of SPTA m crosslinked PVA a) | 30 | – | 35,000 NaCl | – | 0.10 | 14.11/11.23/10.08/9.50/9.42/8.64/8.35/6.76/5.18/3.74 | 99.80 ± 0.10 | [128] |
| SPTA m crosslinked PVA a/PAN d | 0.8 | 30 | – | 100,000 NaCl | – | 0.10 | 7.80 ± 0.30 | [128] | |
| 20zeolite 3A/PEBA e | 100 | 40 | – | 10,000/30,000/100,000 NaCl | – | – | 3.30/3.10/1.98 | 99.63 | [120] |
| 20zeolite 3A/PEBA e | 100 | 60 | – | 30,000 NaCl | – | – | 4.33 | 99.16 | [120] |
| PEBA e | 100 | 40 | – | 30,000 NaCl | – | – | 2.07 | >99.50 | [120] |
| GO c/CS n | – | 81 | 1000 | 50,000/100,000 NaCl | 31.28 ± 1.09/ 32.95 ± 2.37 | 0.80 | 30.00/27.60 | 99.99 | [136] |
| GO c/CS n | – | 75 | 1000 | 50,000 NaCl | Do | 6.00 | 25.80 | 99.99 | [136] |
| GO c/CS n | – | 60 | 1000 | 35,000/100,000 NaCl | Do | 6.00 | 17.70/16.20 | 99.99 | [136] |
| CS n | – | 81 | 1000 | 50,000/ 100,000 NaCl | 37.94 ± 1.19/ 42.90 ± 1.93 | 6.00 | – | – | [136] |
| SSA o crosslinked PVA a/PAN d | 4.9 (SSA p crosslinked PVA a layer) | 70 | – | 35,000 | – | 0.10 | 27.90 | 99.80 | [122] |
| SSA o crosslinked PVA a/PAN d | 4.9/18.4 (SSA p crosslinked PVA a layer) | 30 | – | 35,000 | – | 0.10 | 7.90/6.40 | 99.80 | [122] |
| SSA o crosslinked PVA a/PAN d | 4.9 (SSA p crosslinked PVA a layer) | 30 | – | 100,000 | – | 0.10 | 4.50 | – | [122] |
| 20PMDA p crosslinked PVA a/PAN d | 2 (PMDA p crosslinked PVA a layer) | 70 | – | 35,000 | 23.60 | 0.10 | 32.26 | 99.98 | [127] |
| 5PMDA p crosslinked PVA a/PAN d | 2 (do) | 50 | – | 35,000 | – | 0.10 | 9.88 | 99.96 | [127] |
| 10PMDA p crosslinked PVA a/PAN d | 2 (do) | 50 | – | 35,000 | – | 0.10 | 12.32 | 99.98 | [127] |
| 20PMDA p crosslinked PVA a/PAN d | 2 (do) | 50 | – | 35,000 | – | 0.10 | 16.47 | 99.98 | [127] |
| 30PMDA p crosslinked PVA a/PAN d | 2 (do) | 50 | – | 35,000 | – | 0.10 | 16.02 | 99.98 | [127] |
| PVA a-SiO2/PVSF q hollow fiber | 0.22 ± 0.03 | 60 | – | 2000/30,000/50,000 | –/14.44/14.74 | – | 20.60 ± 0.45/10.40 ± 0.22/8.80 ±0.20 | 99.90 | [118] |
| PVA a-SiO2/PVSF q hollow fiber | 0.30 ± 0.03 | 60 | – | Do | –/15.05/16.47 | – | 19.80 ± 0.40/ 9.70 ± 0.24/7.90 ± 0.18 | 99.90 | [118] |
| PVA a-SiO2/PVSF q hollow fiber | 0.60 ± 0.05 | 60 | – | Do | –/16.63/20.05 | – | 17.20 ± 0.40/8.10 ± 0.22/6.70 ± 0.17 | 99.90 | [118] |
| PVA a-SiO2/PVSFq hollow fiber | 0.85 ± 0.07 | 60 | – | Do | –/18.52/23.49 | – | 15.30 ± 0.33/7.00 ± 0.23/5.90 ± 0.18 | 99.90 | [118] |
| PVA a-SiO2/PVSF q hollow fiber | 1.13 ± 0.11 | 60 | – | Do | –/22.01/24.48 | – | 11.90 ± 0.29/5.80 ± 0.25/4.70 ± 0.26 | 99.90 | [118] |
| MA b crosslinked PVA a/PVSF q hollow fiber | 0.10 | 71 | – | 30,000/40,000/50,000 | 25.80/25.40/25.39 | – | 4.60–7.40/–/– | 99.90 | [114] |
| MA b crosslinked PVA a/PVSF q hollow fiber | 0.14 | 71 | – | Do | 25.80/25.06/25.06 | – | Do | 99.90 | [114] |
| MA b crosslinked PVA a/PVSF q hollow fiber | 0.27 | 71 | – | Do | 24.51/24.60/24.41 | – | Do | 99.90 | [114] |
| MA b crosslinked PVA a/PVSF q hollow fiber | 0.40 | 71 | – | Do | 23.72/24.38/24.06 | – | Do | 99.90 | [114] |
| MA b crosslinked PVA a/PVSF q hollow fiber | 0.85 | 71 | – | Do | 23.80/23.62/23.51 | – | Do | 99.90 | [114] |
| MA b crosslinked PVA a/PVSF q hollow fiber | 1.03 | 71 | – | Do | 23.46/23.10/23.07 | – | Do | 99.90 | [114] |
| nonporous films of hydrated cellulose | – | – | – | – | – | 0.02 | 0.91–1.90 | 100.00 | [142] |
| CA r powder based membranes | 20–25 | 50–80 | – | 40,000, 48,000, 50,000, and 140,000 NaCl | 21.71, 19.83, 15.94, and 20.21 | – | 5.97–10.00 | 99.70 | [130] |
| cellulose triacetate | 10 | 50 | – | 100,000 | – | air sweep | 2.30 | 99.00 | [101] |
| quaternized PE i, anion exchanger | – | 45–65 | – | 0–176,000 | – | 1.50–3.00 | [42] | ||
| polyether amide | 40 | 68–70 | solar heating | 32,000 | – | cooler tunnel | 0.56 | 99.99 | [43] |
| deacetylated cellulose acetate | 22 | 70 | – | 120,000 | – | air sweep | 4.11 | 99.90 | [211] |
| cotton cellulose | 30 | 40 | – | 40,000 | – | vacuum; 0.02 | 4.55–6.70 | 100.00 | [131] |
| polyester | 750 | 20 | – | 35,000 | – | sand heating | 7.10 × 10−3 | 99.84 | [102] |
| Composite/ NCP Inorganic Membranes | Temperature (°C) | Conditions in Feed Side | Feed Concentration (g L−1) | Conditions in Permeate Side | Membrane Thickness (μm) | Flux (kg m−2 h−1) | Rejection (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| NaA zeolite membrane | 69 | – | – | – | – | 1.90 | – | [81] |
| PVA a/MA b/silica hybrid membrane | 22 | – | – | – | – | 6.93 | – | [46] |
| 2D MXene c | 65 | – | 35.00 | – | 0.06 | 4.74 | 99.50 | [133] |
| GO d/PAN e | 90 | – | 100.00 | – | 0.03–1.40 | 3.62 | 99.80 | [50] |
| GO d/PVA a/PVDF f | 65 | cross-flow velocity = 0.625 ms−1 | 100.00 | vacuum pressure ~24,000 Pa | 100.00 | 1.56 | 99.99 | [134] |
| LiCl-S-PVA g | 70 | flow rate = 0.2 ms−1 | 35.00 | pressure = 100 Pa | 33.10 | 60.80 | 99.80 | [135] |
| pristine PVA a | 20/30/40 | atmospheric pressure | 35.00 | 3000 Pa | – | 1.49/1.63/1.98 | 100.00/95.80/95.00 | [255] |
| zeolite 3A-loaded PVA | 20/30/40 | atmospheric pressure | 35.00 | 3000 Pa | – | 1.82/2.36/2.57 | 100.00/96.10/96.00 | [255] |
| PVA a/PAN e | 20 | – | 5.00 | vacuum | 0.62 | 9.04 | 99.50 | [80] |
| NaA zeolite membrane | 69 | – | Seawater | vacuum | – | 1.90 | 99.90 | [81] |
| NaA zeolite membrane | 77 | – | 29.00 | vacuum | – | 4.40 | 99.90 | [81] |
| LTA h and MFI zeolite | 25 | – | 0.13 | vacuum | 15.00 | 0.20 | 99.40 | [111] |
| NaA zeolite | 20 | – | 0.10 | vacuum | 10.00 | 1.43 | 99.83 | [108] |
| clinoptilolite-phosphate | 95 | – | 1.40 | vacuum | – | 15.00 | 95.00 | [107] |
| silica from TEOS i and MTES j | 25 | 700 kPa | 3.00 | vacuum | – | 4.70 | 93.00 | [256] |
| silica from TEOS i and MTES j | 25 | 700 kPa | 3.00 | vacuum | – | 2.20 | 99.90 | [256] |
| CTS k | 20 | – | 3.00 | vacuum | – | 3.20 | 97.00 | [109] |
| CTAB l-silica | 25 | – | 40.00 | vacuum | 0.21 | 2.60 | 99.90 | [115] |
| templated silica | 20 | – | 35.00 | vacuum | 0.50 | 3.70 | 98.50 | [112] |
| nickel oxide silica | 25 | – | 3.00 | vacuum | – | 7.00 | 99.90 | [257] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singha, N.R.; Karmakar, M.; Chattopadhyay, P.K.; Roy, S.; Deb, M.; Mondal, H.; Mahapatra, M.; Dutta, A.; Mitra, M.; Roy, J.S.D. Structures, Properties, and Performances—Relationships of Polymeric Membranes for Pervaporative Desalination. Membranes 2019, 9, 58. https://doi.org/10.3390/membranes9050058
Singha NR, Karmakar M, Chattopadhyay PK, Roy S, Deb M, Mondal H, Mahapatra M, Dutta A, Mitra M, Roy JSD. Structures, Properties, and Performances—Relationships of Polymeric Membranes for Pervaporative Desalination. Membranes. 2019; 9(5):58. https://doi.org/10.3390/membranes9050058
Chicago/Turabian StyleSingha, Nayan Ranjan, Mrinmoy Karmakar, Pijush Kanti Chattopadhyay, Sagar Roy, Mousumi Deb, Himarati Mondal, Manas Mahapatra, Arnab Dutta, Madhushree Mitra, and Joy Sankar Deb Roy. 2019. "Structures, Properties, and Performances—Relationships of Polymeric Membranes for Pervaporative Desalination" Membranes 9, no. 5: 58. https://doi.org/10.3390/membranes9050058
APA StyleSingha, N. R., Karmakar, M., Chattopadhyay, P. K., Roy, S., Deb, M., Mondal, H., Mahapatra, M., Dutta, A., Mitra, M., & Roy, J. S. D. (2019). Structures, Properties, and Performances—Relationships of Polymeric Membranes for Pervaporative Desalination. Membranes, 9(5), 58. https://doi.org/10.3390/membranes9050058









