Membrane Fouling Mechanisms in Combined Microfiltration-Coagulation of Algal Rich Water Applying Ceramic Membranes
Abstract
:1. Introduction
2. Materials and Methods
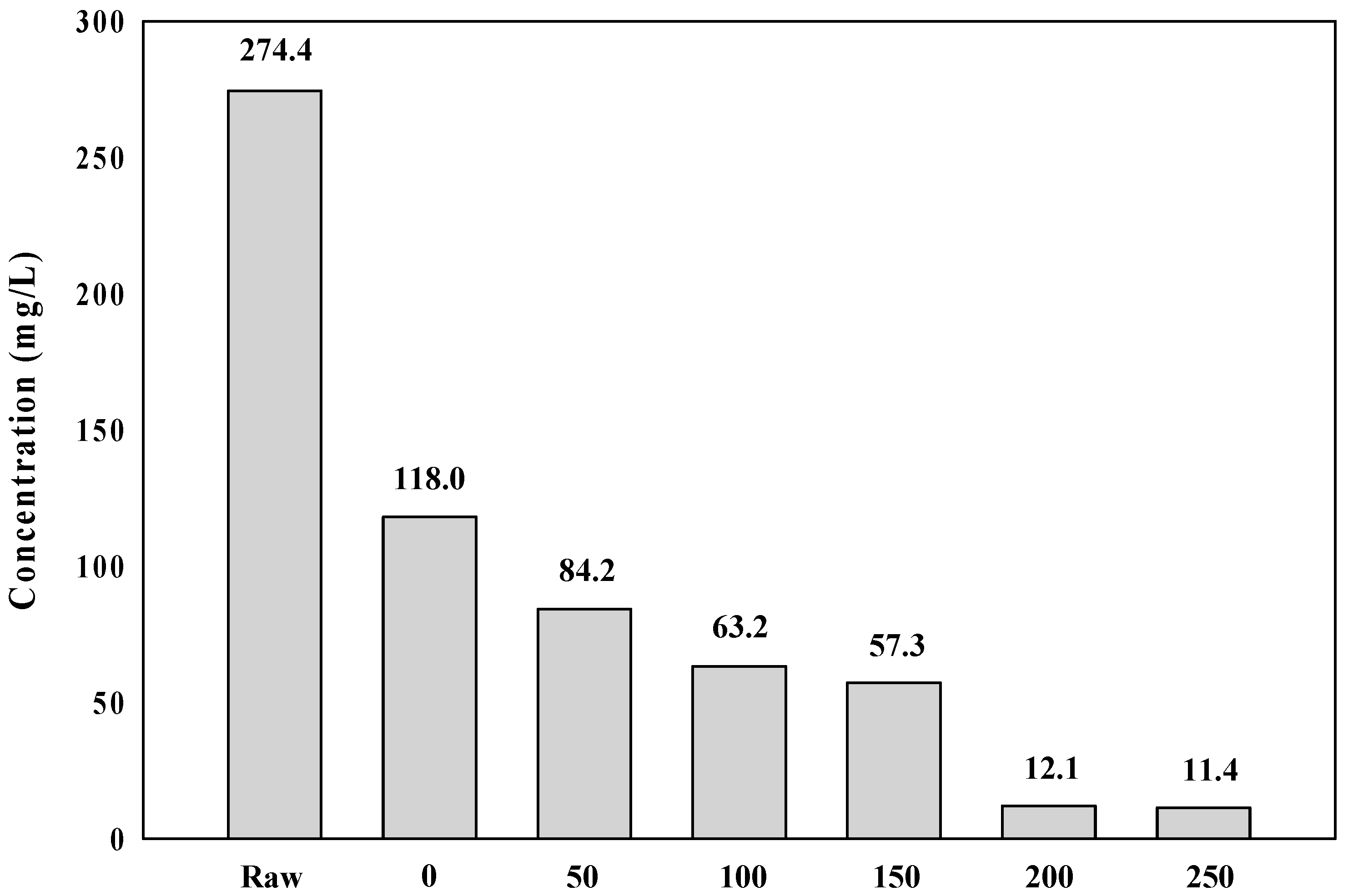
2.1. Charateristics of the Raw Water
2.2. Experimental Set-Up
2.3. Operating Conditions
2.4. Measurement of Resistance
| Rc | the resistance of algal cake (1/m) |
| ΔP | the transmembrane pressure (Pa) |
| J | the filtration flux (m3/m2/s) |
| Rm | the resistance of membrane (1/m) |
| α0 | Empirical constant |
| n | cake compressibility factor |
2.5. Measure of EPS
3. Results and Discussion
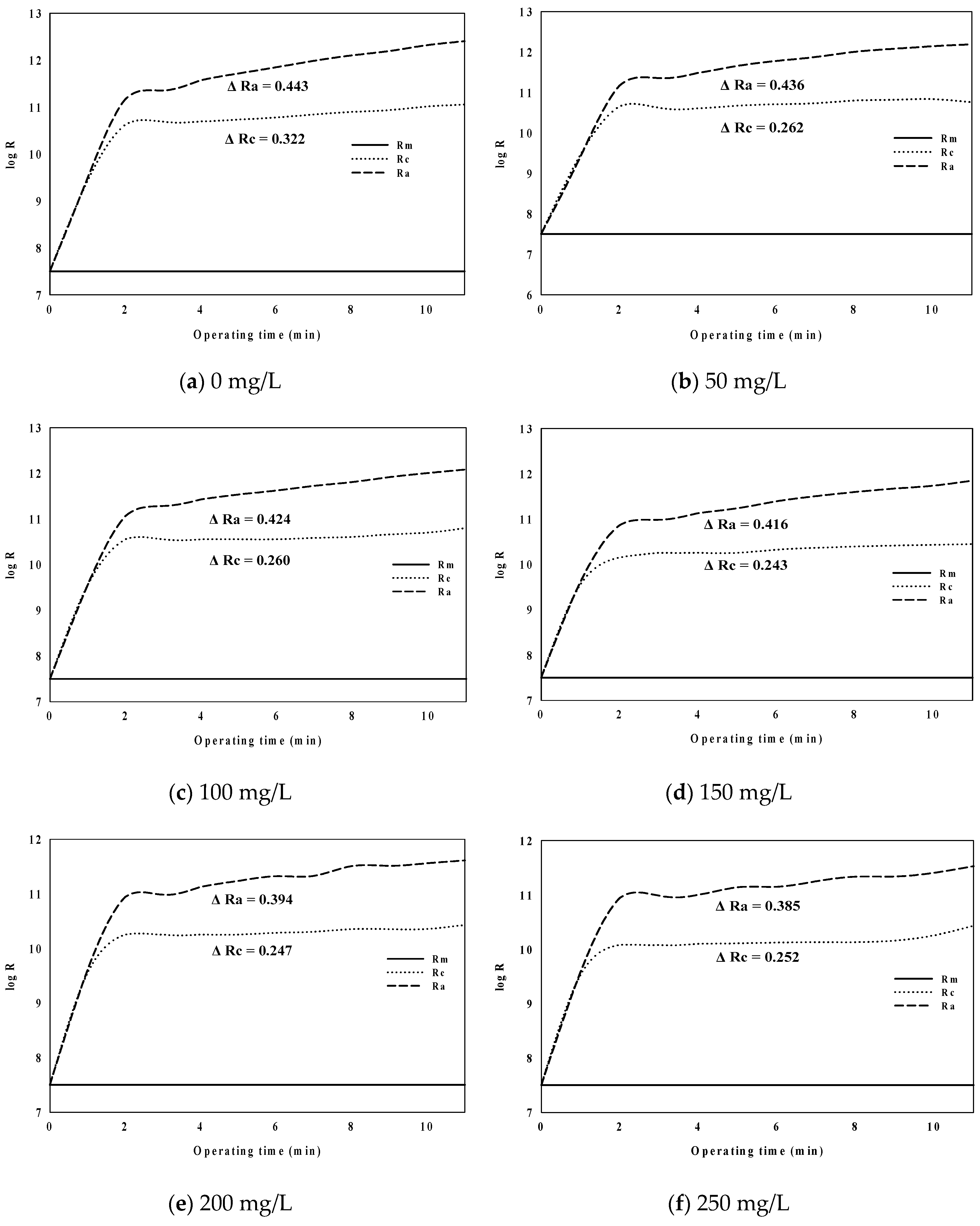
3.1. Flux Curves and Reversibility Analyses
3.2. Mechanisms of Membrane Fouling Caused by Coagulation Dosage in Algal Rich Water
3.3. Specific Cake Resistance and Compressibility
4. Conclusions
- The rate of increase in TMP decreased with increasing PaCl dosage when filtering membranes in algal rich water. It was confirmed that PaCl dosage rate and TMP were important to each other.
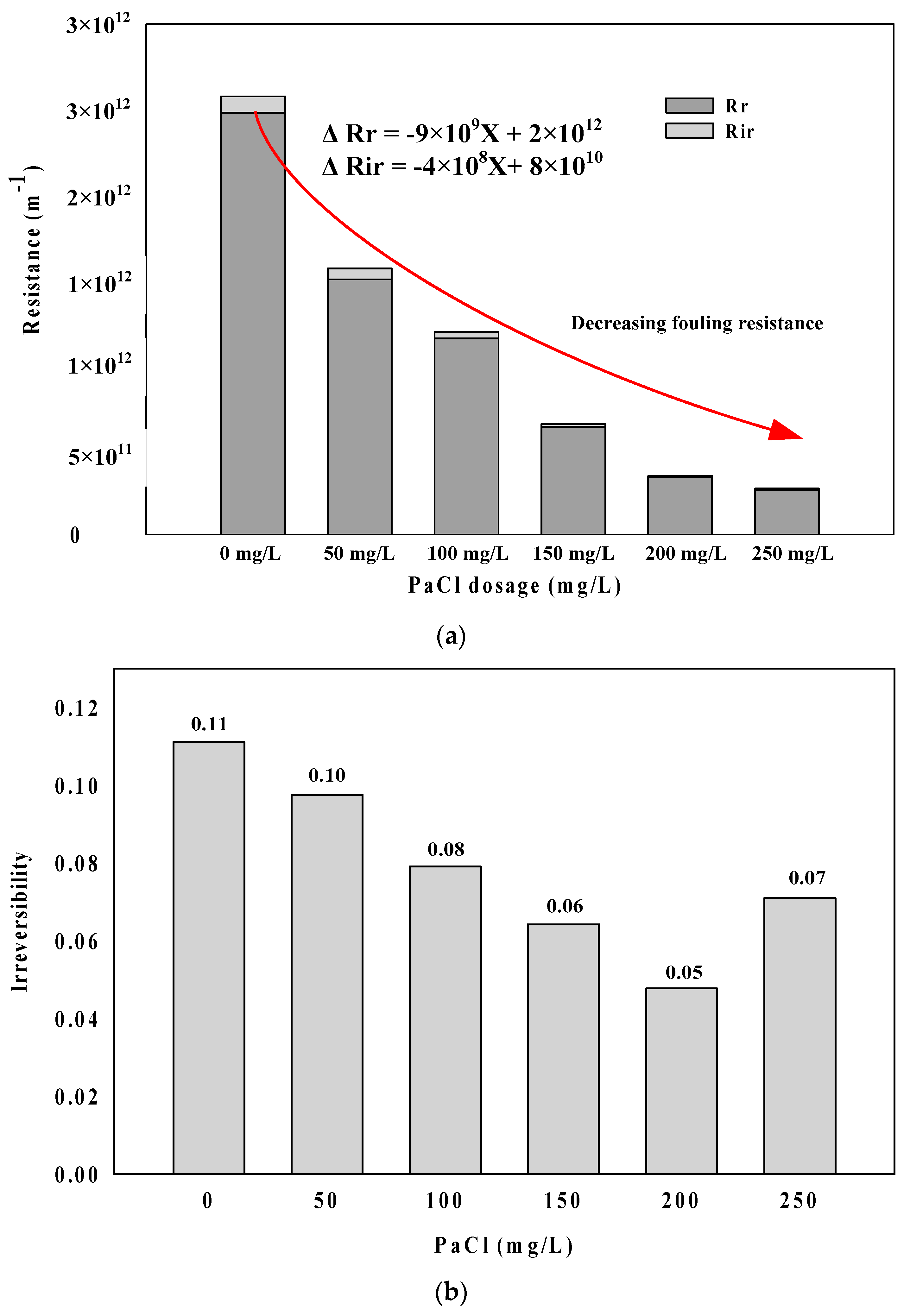
- The reversible and irreversible fouling resistance decreased with an increasing PaCl dosage rate. The irreversible rate increased above the optimal PaCl dosage (200 mg/L as PaCl).
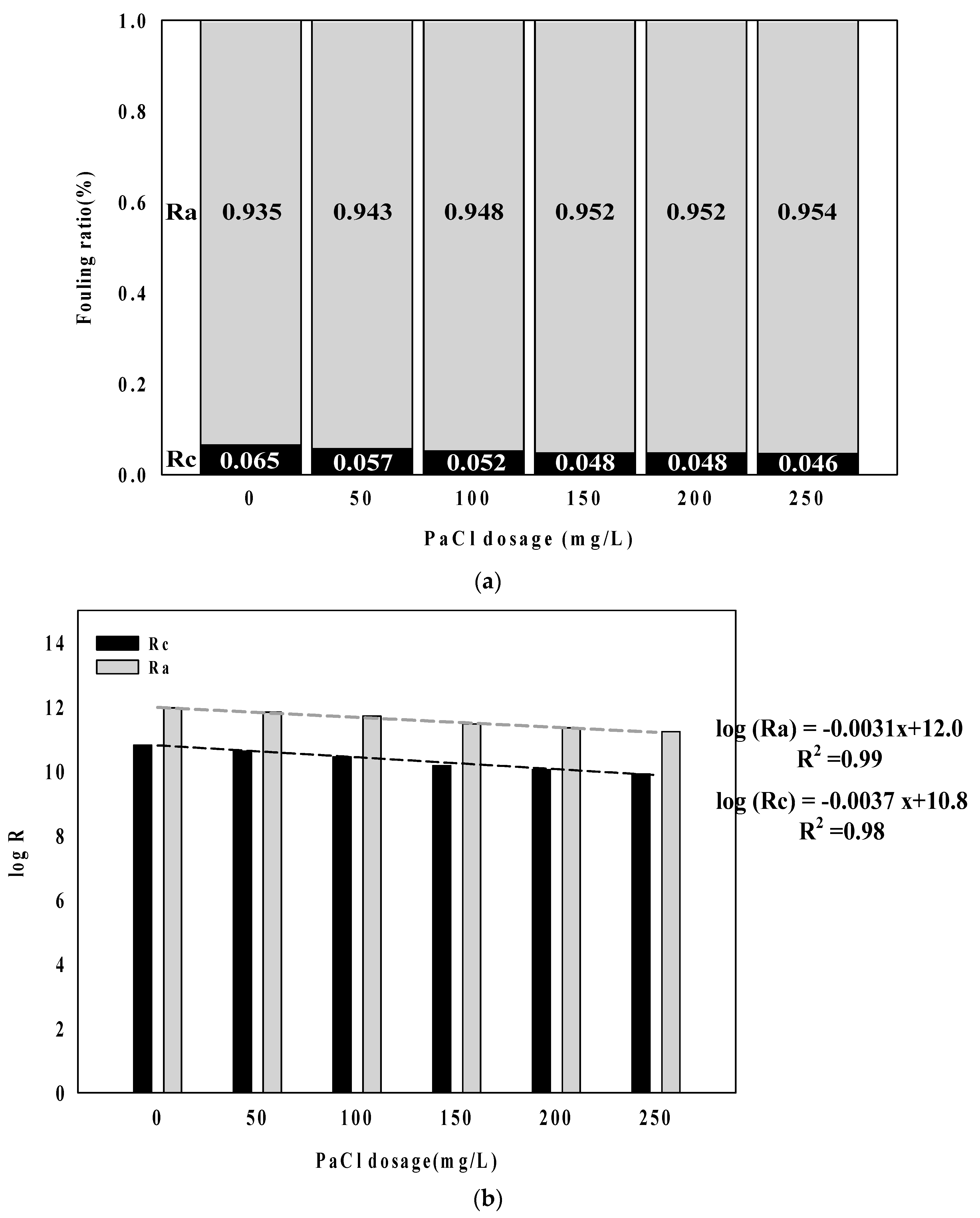
- Fouling resistance showed a tendency to decrease with an increasing PaCl dosage rate. As a form of membrane fouling, the adsorption resistant accounted for a higher proportion than cake resistance. In particular, cake resistance showed a higher decreasing trend than adsorption resistance. It is considered that an increase in the floc size according to coagulant played a causative role.
- The specific cake resistance and compressible index were analyzed to examine the cake layer properties according to the PaCl dosage rate. As a result, the cake resistance decreased with an increasing PaCl dosage, but the compressible index showed a tendency to increase above the proper coagulant dosage. It is considered that the calculation of the proper coagulant dosage is an important factor controlling membrane fouling in membrane process of algal rich water.
Author Contributions
Funding
Conflicts of Interest
References
- Chow, R.H.; Klingauf, J.; Heinemann, C.; Zucker, R.S.; Neher, E. Mechanisms determining the time course of secretion in neuroendocrine cells. Neuron 1996, 16, 369–376. [Google Scholar] [CrossRef]
- Gijsbertsen-Abrahamse, A.J.; Schmidt, W.; Chorus, I.; Heijman, S.G.J. Removal of cyanotoxins by ultrafiltration and nanofiltration. J. Membr. Sci. 2006, 276, 252–259. [Google Scholar] [CrossRef]
- Campinas, M.; Rosa, M.J. Evaluation of cyanobacterial cells removal and lysis by ultrafiltration. Sep. Purif. Technol. 2010, 70, 345–353. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, C.; Li, K.; Zhang, W.; Tao, Y. Probing the fouling process and mechanisms of submerged ceramic membrane ultrafiltration during algal harvesting under sub- and super-critical fluxes. Sep. Purif. Technol. 2018, 195, 199–207. [Google Scholar] [CrossRef]
- Chiou, Y.T.; Hsieh, M.L.; Yeh, H.H. Effect of algal extracellular polymer substances on UF membrane fouling. Desalination 2010, 250, 648–652. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Zhang, H.W.; Guo, W.S.; Ngo, H.H. Feasibility study on magnetic enhanced flocculation for mitigating membrane fouling. J. Ind. Eng. Chem. 2015, 26, 37–45. [Google Scholar] [CrossRef]
- Zhang, W.X.; Luo, J.Q.; Ding, L.H. A review on flux decline control strategies in pressure-driven membrane processes. Ind. Eng. Chem. Res. 2015, 54, 284361. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. Removal of natural organic matter (NOM) and its constituents from water by adsorption—A review. Chemosphere 2017, 166, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Loi-Brügger, A.; Panglisch, S.; Buchta, P.; Hattori, K.; Yonekawa, H.; Tomita, Y.; Gimbel, R. Ceramic membranes for direct river water treatment applying coagulation and microfiltration. Water Sci. Technol. Water Supply 2006, 6, 89–98. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, J.; Liang, H.; Nan, J.; Chen, Z.; Li, G. Chemical cleaning of fouled PVC membrane during ultrafiltration of algal-rich water. J. Environ. Sci. 2011, 23, 529–536. [Google Scholar] [CrossRef]
- Moon, J.; Kim, S.; Cho, J. Characterizations of natural organic matter as nano particle using flow field-flow fractionation. Colloids Surf. A Physicochem. Eng. 2006, 287, 232–236. [Google Scholar] [CrossRef]
- Barbot, E.; Moustier, S.; Bottero, J.; Moulin, P. Coagulation and ultrafiltration; understanding of the key parameters of the hybrid process. J. Membr. Sci. 2008, 325, 520–527. [Google Scholar] [CrossRef]
- Konieczny, K.; Sąkol, D.; Płonka, J.; Rajca, M.; Bodzek, M. Coagulation—Ultrafiltration system for river water treatment. Desalination 2009, 240, 151–159. [Google Scholar] [CrossRef]
- Boerlage, S.F.; Kennedy, M.D.; Aniye, M.P.; Abogrean, E.; Tarawneh, Z.S.; Schippers, J.C. The MFI-UF as a Water Quality Test and Monitor. J. Membr. Sci. 2003, 211, 271–289. [Google Scholar] [CrossRef]
- Harrison, R.G.; Todd, P.; Rudge, S.R.; Petrides, D.P. Bioseparations Science and Engineering; Oxford University Press, Inc.: New York, NY, USA, 2003. [Google Scholar]
- Ohn, T.; Jami, M.; Iritani, E.; Mukai, Y.; Katagiri, N. Filtration Behaviorsin Constant Rate Microfiltration with Cyclic Backwashing of Coagulated Sewage Secondary Effluent. Sep. Sci. Technol. 2003, 38, 951–966. [Google Scholar] [CrossRef]
- Van den Berg, G.B.; Smolders, C.A. Flux Decline in Ultrafiltration Processes. Desalination 1990, 77, 101–133. [Google Scholar] [CrossRef]
- Yuan, W.; Kocic, A.; Zydney, A.L. Analysis of Humic Acid Fouling During Microfiltration using a Pore Blockage Cake Filtration Model. J. Membr. Sci. 2002, 198, 51–62. [Google Scholar] [CrossRef]
- Wang, Z.W.; Wu, Z.C.; Tang, S.J. Extracellular polymeric substances (EPS) properties and their effects on membrane fouling in a submerged membrane bioreactor. Water Res. 2009, 43, 2504–2512. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Liang, H.; Wang, Z.; Wang, H.; Yu, H.; Li, G. Ultrafiltration membrane fouling by extracellular organic matters (EOM) of Microcystis aeruginosa in stationary phase: Influences of interfacial characteristics of foulants and fouling mechanisms. Water Res. 2012, 46, 1490–1500. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Wu, Y.; Li, X.; Wei, B.; Li, S.; Wang, X. Toxic effects of different types of zinc oxide nanoparticles on algae, plants, invertebrates, vertebrates and microorganisms. Chemosphere 2018, 193, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, F.; Huang, X.; Geng, W.; Wen, X. Using inorganic coagulants to control membrane fouling in a submerged membrane bioreactor. Desalination 2006, 197, 124–136. [Google Scholar] [CrossRef]
- Lee, S.A.; Fane, A.G. The effect of floc size and structure on specific cake resistance and compressibility in dead-end microfiltration. Sep. Purif. Technol. 2003, 38, 869–887. [Google Scholar] [CrossRef]
- Tabatabai, S.A.A.; Schippers, J.; Kennedy, M.D. Effect of coagulation on fouling potential and removal of algal organic matter in ultrafiltration pretreatment to seawater reverse osmosis. Water Res. 2014, 59, 283–294. [Google Scholar] [CrossRef] [PubMed]



| Parameters | ||
|---|---|---|
| Cell density (cells/mL) | Cyanobacteria | 262,000 |
| Chlorophyta | 116 | |
| Diatom | 318 | |
| Other algae | - | |
| Total algae | 262,434 | |
| Algal species | Dominant species | Microcystis |
| Subdominant species | Synedra | |
| pH | 7.8–8.1 | |
| Suspended matter (mg/L) | AVG. 525.0 | |
| Turbidity (NTU) | AVG. 443.0 | |
| Characteristic Items | Properties |
|---|---|
| Membrane material | Silicon carbide |
| Effective filtration area (m2) | 0.00652 |
| Membrane type | Flat type |
| Pores if the MF membrane (µm) | 0.1 |
| Clean water permeability (LMH */bar) | 5000 LMH/bar at 20 °C |
| Dosage | Specific Cake Resistance, α (m/kg) | Compressible Cake Index, n |
|---|---|---|
| 0 | 1.62 × 1013 | 0.50 |
| 50 | 1.07 × 1013 | 0.35 |
| 100 | 7.27 × 1012 | 0.35 |
| 150 | 3.81 × 1012 | 0.33 |
| 200 | 2.98 × 1012 | 0.29 |
| 250 | 2.23 × 1012 | 0.36 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.; Kim, P.; Kim, H.G.; Kim, J. Membrane Fouling Mechanisms in Combined Microfiltration-Coagulation of Algal Rich Water Applying Ceramic Membranes. Membranes 2019, 9, 33. https://doi.org/10.3390/membranes9020033
Park K, Kim P, Kim HG, Kim J. Membrane Fouling Mechanisms in Combined Microfiltration-Coagulation of Algal Rich Water Applying Ceramic Membranes. Membranes. 2019; 9(2):33. https://doi.org/10.3390/membranes9020033
Chicago/Turabian StylePark, Kitae, Pooreum Kim, Hyoung Gun Kim, and JiHoon Kim. 2019. "Membrane Fouling Mechanisms in Combined Microfiltration-Coagulation of Algal Rich Water Applying Ceramic Membranes" Membranes 9, no. 2: 33. https://doi.org/10.3390/membranes9020033
APA StylePark, K., Kim, P., Kim, H. G., & Kim, J. (2019). Membrane Fouling Mechanisms in Combined Microfiltration-Coagulation of Algal Rich Water Applying Ceramic Membranes. Membranes, 9(2), 33. https://doi.org/10.3390/membranes9020033




