Steady State and Dynamic Response of Voltage-Operated Membrane Gates
Abstract
:1. Introduction
2. Electrokinetic Pump and Valve Operation: Functioning Principle
3. Materials and Methods
3.1. Chemicals
3.2. Electrochemical Flow-Cell
4. Results and Discussion
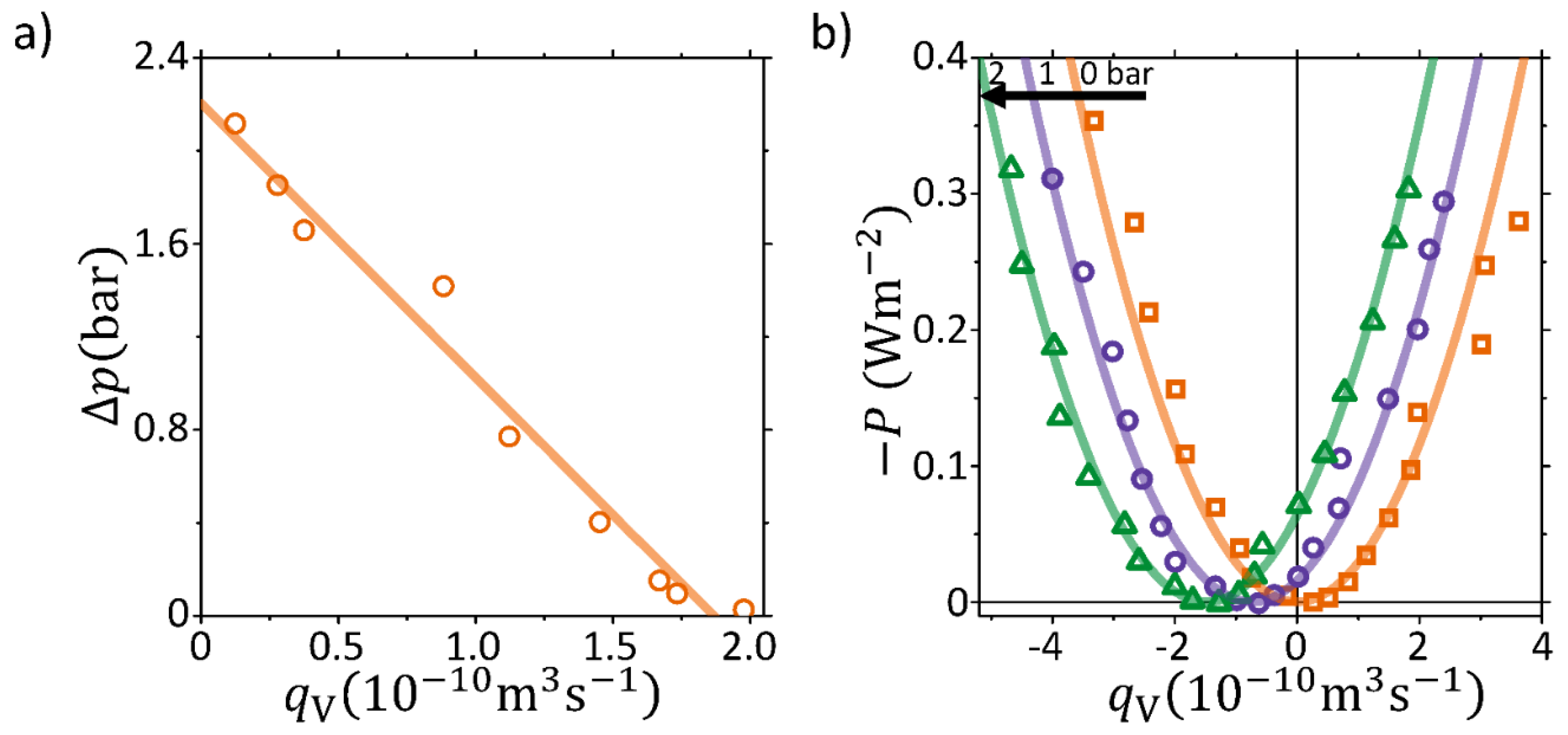
4.1. Pump and Valve Operation
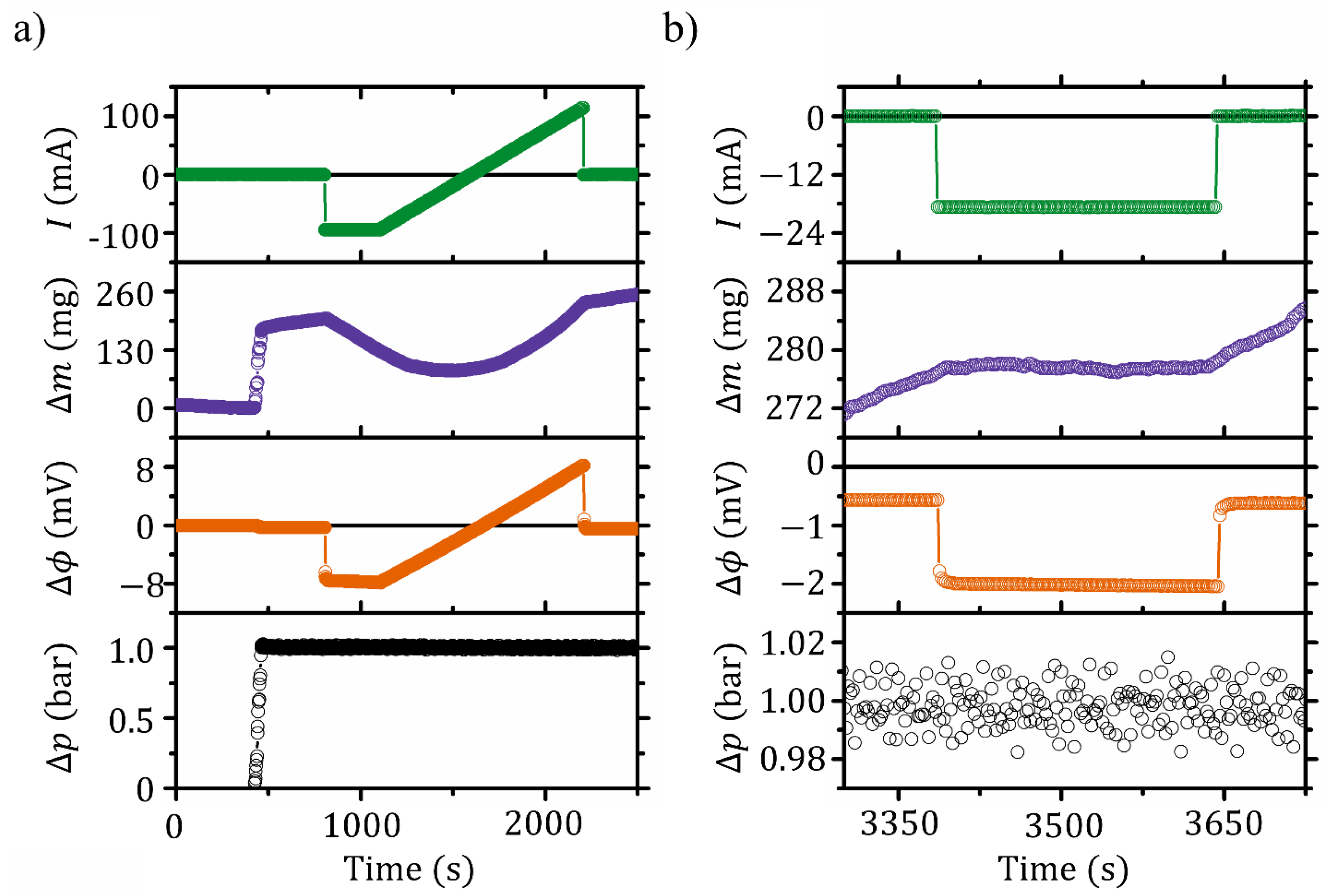
4.2. Dynamic Response
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cooper, K.; Jakobsson, E.; Wolynes, P. The theory of ion transport through membrane channels. Prog. Biophys. Mol. Biol. 1985, 46, 51–96. [Google Scholar] [CrossRef]
- Hediger, M.A.; Romero, M.F.; Peng, J.B.; Rolfs, A.; Takanaga, H.; Bruford, E.A. The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteins. Pflügers Arch. 2004, 447, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Hille, B. (Ed.) The Molecular Cell Biology, 3rd ed.; WH Freeman: New York, NY, USA, 1995. [Google Scholar]
- Hille, B. (Ed.) Ion Channels of Excitable Membranes, 3rd ed.; Sinauer Associates Inc.: Sunderland, MA, USA, 2001. [Google Scholar]
- Holt, J.K.; Park, H.G.; Wang, Y.; Stadermann, M.; Artyukhin, A.B.; Grigoropoulos, C.P.; Noy, A.; Bakajin, O. Fast Mass Transport Through Sub-2-Nanometer Carbon Nanotubes. Science 2006, 312, 1034–1037. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Crooks, R.M. Single Carbon Nanotube Membranes a Well Defined Model for Studying Mass Transport through Nanoporous Materials. J. Am. Chem. Soc. 2000, 122, 12340–12345. [Google Scholar] [CrossRef]
- Regan, B.; Aloni, S.; Ritchie, R.; Dahmen, U.; Zettl, A. Carbon nanotubes as nanoscale mass conveyors. Nature 2004, 428, 924–927. [Google Scholar] [CrossRef] [PubMed]
- Buchsbaum, S.F.; Nguyen, G.; Howorka, S.; Siwy, Z.S. DNA-Modified Polymer Pores Allow pH- and Voltage-Gated Control of Channel Flux. J. Am. Chem. Soc. 2014, 136, 9902–9905. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Guo, W.; Mao, Y.; Hou, X.; Xue, J.; Xia, H.; Wang, L.; Song, Y.; Ji, H.; Ouyang, Q.; et al. Gating of Single Synthetic Nanopores by Proton-Driven DNA Molecular Motors. J. Am. Chem. Soc. 2008, 130, 8345–8350. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Peters, P.B.; van Roij, R.; Bazant, M.Z.; Biesheuvel, P.M. Analysis of electrolyte transport through charged nanopores. Phys. Rev. E 2016, 93, 053108. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, M.B.; Bentien, A.; Tedesco, M.; Catalano, J. Counter-ion transport number and membrane potential in working membrane systems. J. Colloid Interface Sci. 2017, 504, 800–813. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Santiago, J.G. A planar electroosmotic micropump. J. Microelectromech. Syst. 2002, 11, 672–683. [Google Scholar] [CrossRef]
- Yao, S.; Santiago, J.G. Porous glass electroosmotic pumps: Theory. J. Colloid Interface Sci. 2003, 268, 133–142. [Google Scholar] [CrossRef]
- Min, J.Y.; Hasselbrink, E.; Kim, S.J. On the efficiency of electrokinetic pumping of liquids through nanoscale channels. Sens. Actuators B Chem. 2004, 98, 368–377. [Google Scholar] [CrossRef]
- Griffiths, S.K.; Nilson, R.H. The efficiency of electrokinetic pumping at a condition of maximum work. Electrophoresis 2005, 26, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Catalano, J.; Bentien, A.; Østedgaard Munck, D.N.; Kjelstrup, S. Efficiency of electrochemical gas compression, pumping and power generation in membranes. J. Membr. Sci. 2015, 478, 37–48. [Google Scholar] [CrossRef]
- Bentien, A.; Okada, T.; Kjelstrup, S. Evaluation of Nanoporous Polymer Membranes for Electrokinetic Energy Conversion in Power Applications. J. Phys. Chem. C 2013, 117, 1582–1588. [Google Scholar] [CrossRef]
- Burgreen, D.; Nakache, F.R. Electrokinetic Flow in Ultrafine Capillary Slits. J. Phys. Chem. 1964, 68, 1084–1091. [Google Scholar] [CrossRef]
- Burgreen, D.; Nakache, F.R. Efficiency of Pumping and Power Generation in Ultrafine Electrokinetic System. J. Appl. Mech. 1965, 32, 675–679. [Google Scholar] [CrossRef]
- Morrison, F.A.; Osterle, J.F. Electrokinetic Energy Conversion in Ultrafine Capillaries. J. Chem. Phys. 1965, 43, 2111–2115. [Google Scholar] [CrossRef]
- Gross, R.J.; Osterle, J.F. Membrane Transport Characteristics of Ultrafine Capillaries. J. Chem. Phys. 1968, 49, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Stein, D. Slip-enhanced electrokinetic energy conversion in nanofluidic channels. Nanotechnology 2008, 19, 195707. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, D. High Energy Conversion Efficiency in Nanofluidic Channels. Nano Lett. 2012, 12, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, A.; Bhattacharjee, S.; Kostiuk, L. High-power electrokinetic energy conversion in a glass microchannel array. Lab Chip 2012, 12, 4033–4036. [Google Scholar] [CrossRef] [PubMed]
- Olthuis, W.; Schippers, B.; Eijkel, J.; van den Berg, A. Energy from streaming current and potential. Sens. Actuators B Chem. 2005, 111–112, 385–389. [Google Scholar] [CrossRef]
- Mansouri, A.; Kostiuk, L.W.; Bhattacharjee, S. Streaming Current Measurements in a Glass Microchannel Array. J. Phys. Chem. C 2008, 112, 16192–16195. [Google Scholar] [CrossRef]
- Mansouri, A.; Bhattacharjee, S.; Kostiuk, L.W. Electrokinetic Energy Conversion by Microchannel Array: Electrical Analogy, Experiments, and Electrode Polarization. J. Phys. Chem. C 2014, 118, 24310–24324. [Google Scholar] [CrossRef]
- Kilsgaard, B.S.; Haldrup, S.; Catalano, J.; Bentien, A. High figure of merit for electrokinetic energy conversion in Nafion membranes. J. Power Sources 2014, 247, 235–242. [Google Scholar] [CrossRef]
- Catalano, J.; Bentien, A. Influence of temperature on the electrokinetic properties and power generation efficiency of Nafion® 117 membranes. J. Power Sources 2014, 262, 192–200. [Google Scholar] [CrossRef]
- Haldrup, S.; Catalano, J.; Hansen, M.R.; Wagner, M.; Jensen, G.V.; Pedersen, J.S.; Bentien, A. High Electrokinetic Energy Conversion Efficiency in Charged Nanoporous Nitrocellulose/Sulfonated Polystyrene Membranes. Nano Lett. 2015, 15, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Haldrup, S.; Catalano, J.; Hinge, M.; Jensen, G.V.; Pedersen, J.S.; Bentien, A. Tailoring Membrane Nanostructure and Charge Density for High Electrokinetic Energy Conversion Efficiency. ACS Nano 2016, 10, 2415–2423. [Google Scholar] [CrossRef] [PubMed]
- Østedgaard Munck, D.N.; Catalano, J.; Kristensen, M.B.; Bentien, A. Membrane-based electrokinetic energy conversion. Mater. Today Energy 2017, 5, 118–125. [Google Scholar] [CrossRef]
- Fair, J.C.; Osterle, J.F. Reverse Electrodialysis in Charged Capillary Membranes. J. Chem. Phys. 1971, 54, 3307–3316. [Google Scholar] [CrossRef]
- Catalano, J.; Hamelers, H.V.M.; Bentien, A.; Biesheuvel, P.M. Revisiting Morrison and Osterle 1965: The efficiency of membrane-based electrokinetic energy conversion. J. Phys. Condens. Matter 2016, 28, 324001. [Google Scholar] [CrossRef] [PubMed]
- Østedgaard Munck, D.N.; Catalano, J.; Kristensen, M.B.; Bentien, A. Data on flow cell optimization for membrane-based electrokinetic energy conversion. Data Brief 2017, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Martí-Calatayud, M.; Schneider, S.; Wessling, M. On the rejection and reversibility of fouling in ultrafiltration as assessed by hydraulic impedance spectroscopy. J. Membr. Sci. 2018, 564, 532–542. [Google Scholar] [CrossRef]
- Groves, J.N.; Sears, A.R. Alternating streaming current measurements. J. Colloid Interface Sci. 1975, 53, 83–89. [Google Scholar] [CrossRef]
- Catalano, J.; Biesheuvel, P.M. AC-driven electro-osmotic flow in charged nanopores. EPL (Europhys. Lett.) 2018, 123, 58006. [Google Scholar] [CrossRef]
- Cwirko, E.H.; Carbonell, R.G. Interpretation of transport coefficients in Nafion using a parallel pore model. J. Membr. Sci. 1992, 67, 227–247. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Østedgaard-Munck, D.N.; Catalano, J.; Bentien, A. Steady State and Dynamic Response of Voltage-Operated Membrane Gates. Membranes 2019, 9, 34. https://doi.org/10.3390/membranes9030034
Østedgaard-Munck DN, Catalano J, Bentien A. Steady State and Dynamic Response of Voltage-Operated Membrane Gates. Membranes. 2019; 9(3):34. https://doi.org/10.3390/membranes9030034
Chicago/Turabian StyleØstedgaard-Munck, David Nicolas, Jacopo Catalano, and Anders Bentien. 2019. "Steady State and Dynamic Response of Voltage-Operated Membrane Gates" Membranes 9, no. 3: 34. https://doi.org/10.3390/membranes9030034
APA StyleØstedgaard-Munck, D. N., Catalano, J., & Bentien, A. (2019). Steady State and Dynamic Response of Voltage-Operated Membrane Gates. Membranes, 9(3), 34. https://doi.org/10.3390/membranes9030034





